Simple Summary
Tetraspanins are a family of molecules abundantly expressed on the surface of normal or tumor cells. They have been implicated in recruiting or sequestering key molecular regulators of malignancy of a variety of human cancers, including breast and lung cancers, glioblastoma and leukemia. Yet, how their actions take place remains mysterious due to a lack of traditional platform for molecular interactions. The current review digs into this mystery by examining findings from recent studies of multiple tetraspanins, particularly CD151. The molecular basis for differential impact of tetraspanins on tumor development, progression, and spreading to secondary sites is highlighted, and the complexity and plasticity of their control over tumor cell activities and interaction with their surroundings is discussed. Finally, an outlook is provided regarding tetraspanins as candidate biomarkers and targets for the diagnosis and treatment of human cancer.
Abstract
As a family of integral membrane proteins, tetraspanins have been functionally linked to a wide spectrum of human cancers, ranging from breast, colon, lung, ovarian, prostate, and skin carcinomas to glioblastoma. CD151 is one such prominent member of the tetraspanin family recently suggested to mediate tumor development, growth, and progression in oncogenic context- and cell lineage-dependent manners. In the current review, we summarize recent advances in mechanistic understanding of the function and signaling of integrin-associated CD151 and other tetraspanins in multiple cancer types. We also highlight emerging genetic and epigenetic evidence on the intrinsic links between tetraspanins, the epithelial-mesenchymal transition (EMT), cancer stem cells (CSCs), and the Wnt/β-catenin pathway, as well as the dynamics of exosome and cellular metabolism. Finally, we discuss the implications of the highly plastic nature and epigenetic susceptibility of CD151 expression, function, and signaling for clinical diagnosis and therapeutic intervention for human cancer.
1. Introduction
Tetraspanins are a family of integral membrane proteins widely expressed in human tissues and are linked to normal developmental and physiological processes, immunity, and pathologies of many human diseases, including cancer [1,2,3,4,5,6,7]. Structurally, these 20 50 kDa molecules are featured by the presence of highly conserved amino acid residues in each of their extracellular small and large loops, which are connected to four lipid-interacting transmembrane domains and two short cytoplasmic tails [8,9,10]. Despite their high structural similarity, tetraspanins seem to differ both in their functions and signal transduction. Notably, a single point mutation or deletion of the CD151 or CD37 gene leads to kidney and skin malfunctions or defective immunity in both humans and mice, while the targeted deletion or alternative splicing of tetraspanin CD9, CD37, CD53, CD81, and CD82 (singularly or in combination) profoundly impairs function or maturation of B and T lymphocytes and myeloid lineage cells or tissue metabolism in vertebrates [4,7,11,12,13,14,15,16,17,18,19]. Aberrant expression of tetraspanins have been observed in tumor tissues across a wide spectrum of cancer types, ranging from breast, colon, ovarian and prostate cancers to glioblastoma and leukemia [1,20,21]. A series of in vitro and in vivo and clinical studies have highlighted strong roles of these integral membrane proteins in tumor growth, metabolism, angiogenesis, and metastatic dissemination [22,23,24,25,26,27]. Additionally, within the tetraspanin family, CD151 has long been regarded as a potent driver for cell adhesion and behaviors, and tumor onset, growth, angiogenesis and metastatic dissemination, and cancer relapse or resistance to current chemo- and targeted therapies [1,28,29,30,31,32].
Intriguingly, there is growing evidence that many tetraspanins such as CD151 and CD82 can negatively or positively influence tumor development and metastasis in oncogenic context- and cell lineage-dependent manners, where they regulate strength of cell-cell junctions, signal transduction of the Wnt pathway, the epithelial-mesenchymal transition (EMT), maintenance of cancer stem cells (CSCs), and dynamics of exosomes (Table 1) [20,21,28,33,34,35]. This emerging paradigm presents a challenge to conceptualize functional and signaling roles of the tetraspanin family in human cancer, and their utilities as biomarkers and drug targets for cancer diagnosis and treatment. In the current review, we will summarize this dilemma for tetraspanin molecules, particularly CD151, and discuss their implications for conceptualizing the role of tetraspanins in human cancer at the cellular, signaling, and epigenetic levels. In addition, our review will be concentrated on the evolving role of CD151 and associated protein complexes in breast, ovarian and prostate cancers, and glioblastoma, as well as their potential for clinical application.

Table 1.
A glance of tumor-promoting and suppressing roles of tetraspanin molecules across human epithelia-origin cancers *.
2. The Complex Role of CD151 in Tumor Metastasis
2.1. Early Studies and Present Views on the Pro-Metastatic Role of CD151
The link between CD151 and human cancer was initially implicated following discovery of its co-translation with laminin-binding α3β1 integrin in human carcinoma cells or modulation of αIIbβ3 function in platelets [6,22,68,69]. The hard evidence of a role of CD151 in cancer, however, came from detection of elevated CD151 expression in metastatic tumor cells and the inhibition of cell motility by an anti-CD151 monoclonal antibody [5]. This pro-metastatic role was subsequently confirmed by a large body of cell line-based in vitro studies in multiple cancer types and has been substantiated by its pro-invasive and pro-angiogenic functions [1,70,71]. More recently, we and others have detected a marked decrease in the formation of pulmonary metastases in breast cancer upon CD151 deletion or knockdown in MMTV-ErbB2 or MMTV-PyMT transgenic or xenograft model [28,32,38]. In line with this evidence, CD151 expression appears elevated at the mRNA level in metastatic tumors and is associated with poor clinical outcomes in the ErbB2+ breast cancer subtype [32]. Collectively, these in vitro and in vivo investigations, along with clinical analyses, underpin CD151 as a premier player in human cancer metastasis.
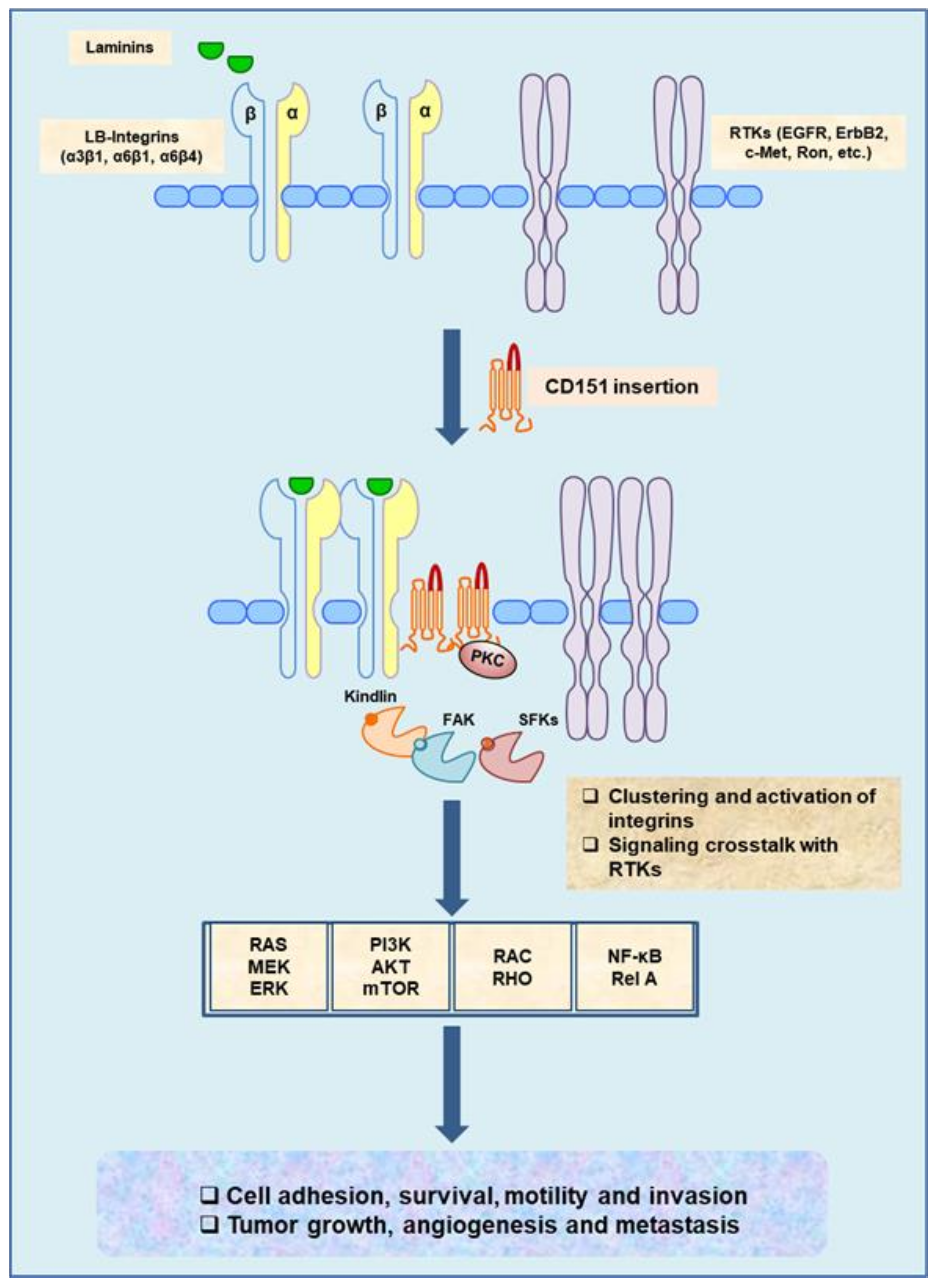
Mechanistically, downregulation or removal of CD151 markedly impairs integrin-dependent tumor cell adhesion, motility, and invasion [21,72]. Studies from our groups and others have also implicated that the pro-metastatic function of CD151 is in part tied to its impact on tumor cell survival or their resistance to anoikis [21,32,71]. Presence of CD151 molecules in tumor cells appears to facilitate the clustering/activation of laminin-binding (LB) integrins (α3β1, α6β1 and α6β4) on the cell surface, which in turn activates SFKs/FAK-, JAK/STAT3-, RAS/MEK- and NF-kB-dependent signaling pathways, cytoskeleton remodeling, and diverse cellular activities and behaviors [32,38,44,71,73]. Interestingly, in breast cancer cells exhibiting a strong promoting role of CD151, receptor tyrosine kinases (RTKs) (e.g., EGFR, ErbB2, c-Met and Ron) or K-Ras are frequently activated because of gene amplification/overexpression or mutations (Figure 1) [1].
Figure 1.
The pro-metastatic function and signaling pathways driven by CD151/laminin-binding (LB) integrin complexes and their crosstalk with oncogenic receptor tyrosine kinases (RTKs) in cancer cells and endothelial cells. The role of CD151 illustrated in a gain-of-function manner is based on observations from a series of studies with gene-targeted or shRNA knockdown effect in mouse models or cancer cell lines.
Part of the pro-metastatic role of CD151 may be linked to its regulation of tumor angiogenesis and microenvironments, as it supports function and integrity of vascular endothelial cells and infiltration of tumor-associated macrophages [25,28,48,74,75]. Consistent with these observations, CD151 deletion markedly decreases expression of several key myeloid cell-associated hallmark genes in mammary tumors, including CD36 and MMP2 [28]. Such notion is also supported by recent findings on the role of CD151-associated α6β4 integrin in macrophages [76]. Additionally, CD151 is implicated to propel tumor progression by regulating integrity and trafficking of exosomes produced in tumor-associated fibroblasts and the Wnt pathway [30,77,78]. In some cancer types, the pro-metastatic role of CD151 appears to be achieved through strong synergy with other tetraspanins (e.g., TSPAN8) [25]. Hence, the impact of CD151 on cancer metastasis largely stems from regulation of tumor cell behaviors and survival, and their microenvironments.
2.2. Emerging Evidence of an Anti-Metastatic Role
Unexpectedly, several recent studies have implicated CD151 as a suppressor of tumor metastasis, particularly in ovarian and prostate cancers [46,47,49,79]. A parallel scenario has also been raised for other tetraspanins, such as CD9 and CD82, traditionally regarded as bona fide metastasis suppressors (Table 1). This is a sharp deviation from the well-established paradigm of CD151 being pro-metastatic and CD9/CD82 being anti-metastatic [1]. In fact, the current view of the pro-metastatic role of CD151 in prostate cancer was largely drawn from studies with the endocrine subtype-related Tramp model, and analyses with the PC-3 cell line which is regarded as oncogene-targeting squamous epithelial cells [35,36,46,80]. In contrast, the studies suggesting an anti-metastatic role of CD151 were performed with the oncogene-targeting cells falling into the differentiated epithelial cell category, where CD151 is abundantly expressed at the cell-cell junction [46,47]. In such context, loss of CD151 at cell-cell junctions leads to induction of EMT or a highly motile/invasive mesenchymal cellular phenotype featuring altered expression of typical epithelial (e.g., E-cadherin) and mesenchymal makers (e.g., vimentin, fibronectin and transcription factors Slug/Snail, etc.), enhanced tumor cell motility and invasiveness, as well as strong extracellular matrix (ECM)- arginine-glycine-aspartate (RGD) motif-binding integrin interactions [31,81]. Consistent with this line of observations, data from histological and genomic analyses show that a fraction of human breast and prostate carcinomas may arise from PTEN or E-cadherin mutations or loss in differentiated epithelial cells [46]. It may also be linked to activation of the non-canonical Wnt pathway [28,46,49]. Furthermore, this paradigm may be originated from the cell lineage- and the oncogenic context-dependent role of CD151, highly reminiscent of its associated laminin-binding integrin α3β1 or α6β4 integrin, which seem to vary with cancer subtype or oncogenic context (inactivation/loss of tumor suppressors p53 and SMAD4 versus activation of Ras oncogene) [82,83]. More surprisingly, an opposing scenario appears to occur in a group of tetraspanins traditionally regarded as metastasis suppressors, including CD9 and CD82, in which they seem to dampen or sequester activation or signal transduction of another class of growth factors or receptors, such as the membrane-bound TGF-α or TGF-β Type II receptor [84,85].
3. A Oncogenic Context- and Cell Lineage-Dependent Role of CD151 in Tumor Growth and Metabolism
Aside from being a key player in tumor metastasis, CD151 has long been regarded as being pro-tumorigenic in multiple cancer types, particularly in breast and skin cancers [28,29,32,86]. Again, this unidirectional view has recently been challenged by data from a series of in vivo studies with CD151 gene-targeted mice, transgenic animal models, and clinical analyses [32,39,71,86]. Because of the strong clinical implications, here we will discuss this twist by centering around recent studies of CD151 function in solid tumors, particularly breast and prostate carcinomas.
3.1. Being Pro-Tumorigenic
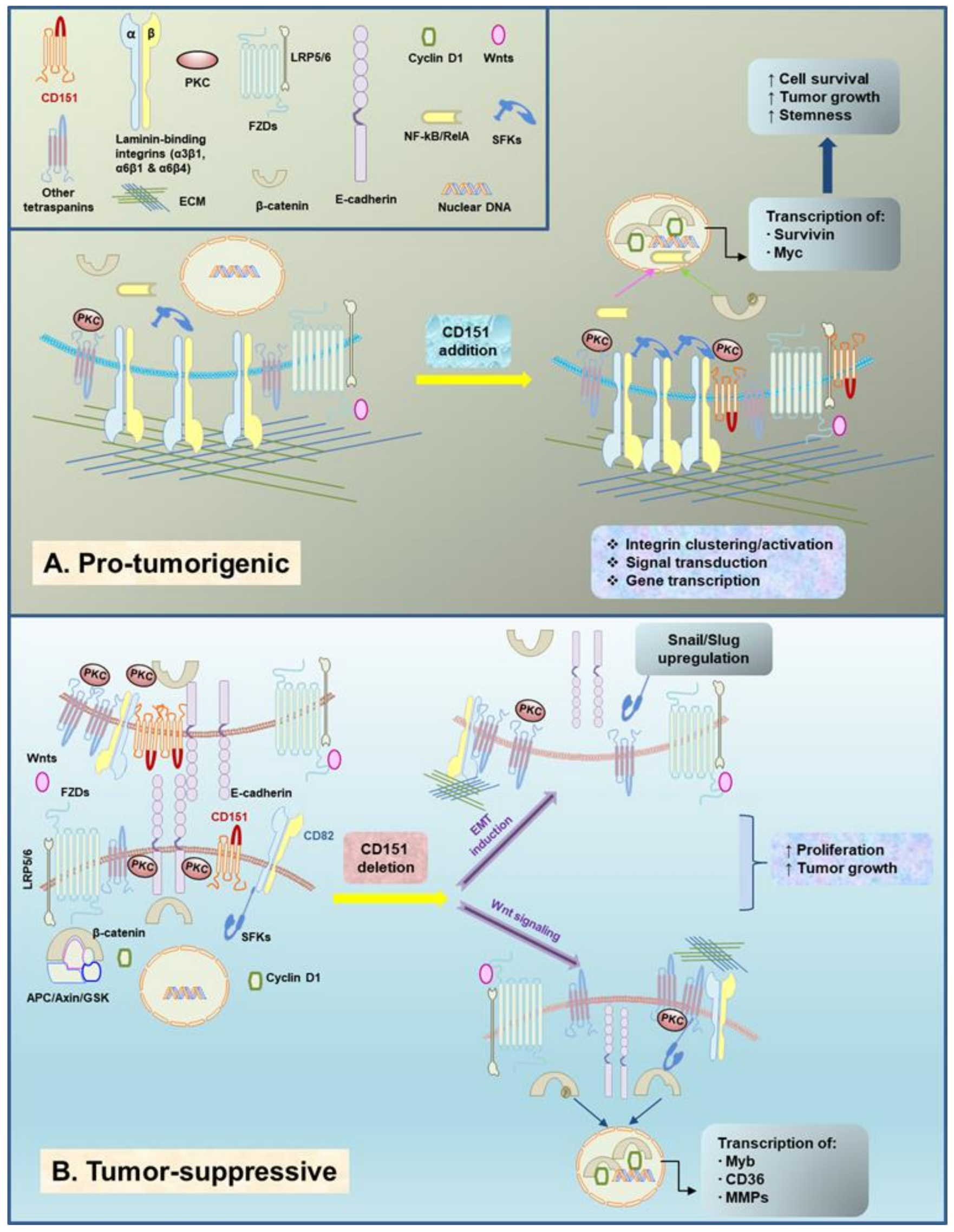
As one of the most common cancer types among women worldwide, breast cancer is a highly heterogeneous disease. Based on histological and genetic alterations, breast cancer is grossly categorized into four major subtypes: estrogen receptor (ER)-negative, including ErbB2+ and triple-negative, and ER-positive (Luminal A and Luminal B) [87]. The malignancy of these breast tumors is largely driven by activation of the PI3K/Akt and RAS/MEK/ERK pathways and loss of key tumor suppressors (p53, BRCA1/2, PTEN, RB, etc.) [87,88,89,90]. In the case of CD151, a series of in vitro and in vivo studies reveal a strong inhibitory effect of CD151 downregulation or deletion on tumor onset and growth in either ErbB2+ or basal-like subtypes [29,32,38,71,86]. Collectively speaking, there is robust evidence that CD151 is pro-tumorigenic in the context of signaling driven by overexpression/amplification of RTKs or by the oncogenic activation of their downstream effector pathways (e.g., RAS/ERK1/2 and PI3K/AKT, as well as TGF-β- or Wnt/β-catenin-mediated pathways) (Figure 1 and Figure 2A).
Figure 2.
Emerging evidence on the context-dependent crosstalk between tetraspanin CD151 and the Wnt signaling pathway in breast cancer. (A) Hypothetical molecular basis for the cooperative role of CD151/laminin-binding integrin complexes and the Wnt/ pathway in basal cell-origin breast cancer. (B) A working model for CD151-mediated suppression of the Wnt/β-catenin signaling, and associated changes in transcription factors Snail and Slug, cell-cell adhesion and EMT in luminal epithelial cell-origin breast cancer.
Another developing theme on CD151 in breast cancer is its promoting role in development and growth of basal-like mammary tumors driven by the oncogenic Wnt1 pathway [28]. As a key downstream effector of the canonical Wnt pathway, transcription factor Myc has long been implicated in regulation of the pro-tumorigenic role of CD151 in gliomas [43]. Consistent with this notion, we and others have observed a strong tumor-promoting role of CD151 in glioblastoma [40,42]. Intriguingly, Myc, a key effector downstream of an array of oncogenic pathways, including RAS/Erk and PI3/Akt /mTOR, also serves as a master driver of cell metabolism in diverse cancer types, particularly for nutrients glutamine and glucose [43,90,91,92]. Following this link, we speculate that CD151 might promote tumor growth in breast cancer largely through regulation of c-Myc-driven cellular metabolism. So far, this notion is supported by recent studies of the role of tetraspanins CD9 and CD81 in metabolism of glutamate and lipids in normal or tumor tissues [8,11,27].
Importantly, CD151 appears to be a key player in maintenance of tumor-initiating cells in breast, prostate, and pancreatic cancers, that is, CSCs, as CD151-null/deficient ER+ tumor cells seem unable to sustain the population under in vitro culture [28,37,65,93]. This line of observation is consistent with the critical role of CD151-associated α6 integrin in human cancer stem cells across a wide range of human cancer types, particularly breast cancer and glioblastoma [94,95]. They are also of clinical importance, as CSCs are regarded as front runners for candidate therapeutic targets given their crucial roles in drug resistance and disease recurrence in human cancers [96,97].
3.2. Being Tumor-Suppressive
In contrast to the role in ER− breast cancer (basal-like and ErbB2+) summarized above, CD151 appears to be tumor suppressive in some cases, for example, during the development and growth of ER+ mammary tumors in the mouse mammary tumor virus (MMTV) promoter-driven Wnt1 oncogene model (Figure 2B) [28]. In fact, this unexpected observation is consistent with our prior analysis of the impact of CD151 deletion on mammary luminal progenitor cells (CD24high CD49flow population), where CD151 seems involved in maintenance of quiescence of mammary progenitor cells and the associated impact on mammary gland development [93]. Additionally, it is supported by analyses of CD151 and α3β1 integrin expression in tumor biopsies of lobular or inflammatory breast cancer or colon and ovarian cancer patients [33,49,50,93]. More surprisingly, removing one or two CD151 alleles seems to have nearly an equivalent effect on tumor growth [28], thus illuminating the haploinsufficient nature of CD151 gene and a strong player in human cancer.
Furthermore, the unique aspect of the tumor-suppressive role of CD151 is its intrinsic association with the dynamics of the EMT phenotype, a hallmark trait for the progression of epithelial-origin tumors [32,49]. It has long been advocated that CD151 is a crucial contributor of cell-cell adhesion in immortalized epithelial or carcinoma cells [98,99]. In line with this notion, downregulation or loss of CD151 expression not only weakens such junctional structures, but leads to activation of the transcription factors Snail and Slug and associated EMT-like phenotype (Figure 2B) [28,49]. In line with this functional impact, the downregulation or deletion of CD151 in normal luminal progenitor cells or related breast cancer cell lines or mammary gland is accompanied by the upregulation of fibronectin expression and Slug [93]. While this function has been linked to activation of protein kinase C (PKC) and Cdc42, it remains controversial in terms of integrin involvement [46,47].
One of the noticeable observations from our study with the MMTV-Wnt model was the absence of tumor-initiating cells in CD151-deficient ER+ mammary tumors [28]. This phenomenon is highly unexpected, since the canonical Wnt pathway or transcription factors Snail and Slug or associated EMT have long been regarded as major drivers of the metastatic progression of ER+ breast tumors [94,100,101]. This may also represent another layer of complexity of CD151 action in human ER+ breast cancer.
4. Molecular Basis for Functional and Signaling Versatility of CD151 and Other Tetraspanins
Mechanistically, the complex role of CD151 in human cancer is intimately linked to diversity of its laterally associated molecular partners on the cell surface, besides heterogeneity in its subcellular localization in tumor cells. Despite lack of extracellular ligands or classical domains/motifs for intracellular protein-protein interactions, tetraspanins are capable of carrying out a variety of functional and signaling roles through at least three distinct types of molecular interactions: (1) Imposing a lateral impact on the activation of their cell surface partners; (2) Recruiting signaling molecules via self-association-based micro- or nano-domains; (3) Long-range impact via regulation of secretory vesicles [73,102,103,104,105]. To date, there is a consensus that tetraspanin molecules, together with their molecular partners, form a nano-scale protein complex or molecular network on the plasma membrane, termed as tetraspanin-enriched microdomain (TEM) [3,8,10,102,106].
In CD151-based TEM, there exists at least two distinct pools of tetraspanin molecules on the cell surface. One pool contains large-sized transmembrane protein or receptors through protein-protein interactions, such as LB-integrins interacting with CD151 through their extracellular domains [10]. Another pool, based on our prior biochemical and antibody-based analyses, consists of self-associated or interspecies aggregates of CD151 and other tetraspanins, where they seem localized at the periphery of TEM [103,105,107]. The targeting and stability of this pool of molecules is highly dependent on the palmitoylation of its membrane-proximal cysteine residues and N-terminal cytoplasmic tail [103,104]. Interestingly, the integrin-absent pool of molecular aggregates of CD151, which presumably corresponds to the so-called integrin-free CD151 fraction, seems to have a regulatory role in cell-cell contact and tumor resistance to therapeutic agents [47,108]. More recently, we and others have noted that some of these molecular interactions varies with cell lineage, that is the cell-of-origin or differentiation state of oncogene-targeting cells, as well as oncogenic context (RAS vs. mutation or PTEN mutation) [28,47,49]. Here, we will examine such advances in the context of both pro- and anti-tumorigenic roles of tetraspanins in human cancers.
4.1. Hijacking Function and Signaling of Single Transmembrane-Containing Receptor or Protein Partner
Although many tetraspanins are regarded as key mediators of tumor development and progression, their actions have been connected to at least two classes of cell surface molecules: CD151-associated heterodimeric adhesion receptors for laminins, α3β1, α6β1, and α6β4 integrins, and the CD9/CD81/CD82-binding Ig-G-containing proteins such as EWI-2 and EWI-F. In the case of CD151, its pro-tumorigenic role is largely carried out through regulation of function and signaling of laminin-binding integrins (Figure 2A) [32,71]. Studies from our group and others indicate that the pro-metastatic role of CD151 in human basal-like and ErbB2 breast cancer subtypes is achieved largely through regulating α6 integrin-dependent cell motility, invasion and survival [45,50]. In particular, CD151 contributes to the lateral clustering in cis of α6 integrins through direct extracellular domain linkages, which in turn enhances clustering/activation of these adhesion receptors and subsequent changes in cell-ECM adhesion, cytoskeleton remodeling, and signal transduction [71,73,109].
The tumor-promoting role of CD151 may also be carried out through regulation of α3β1 integrin-dependent cell-ECM adhesion, migration, survival, and signaling [50,110,111,112]. Such impact, however, seems largely restricted to tumor cells with basal cell lineage or a mesenchymal phenotype [28,71,113]. In contrast, in tumor cells with strong epithelial cell characteristics, CD151, like α3β1 integrin, is more engaged in the maintenance of cell-cell contact through basolateral distribution, conferring an anti-tumor role in an integrin-dependent manner or through interacting non-integrin partners or self-association/clustering [47,49,114]. In this case, CD151 seems to repress tumor cell growth by counteracting EMT in multiple cancer types, including breast, ovarian and prostate [28,46,49,115]. In fact, this scenario highly resembles the well-established established tumor-suppressive role of tetraspanins CD82, CD9, and TSPAN8 in epithelial-origin cancers, whereby they regulate E-cadherin/β-catenin complex-dependent cell-cell adhesion [45,50,99,116,117,118]. For CD9 and CD82, this function appears to take place through EWI proteins [10,20,21,58]. Interestingly, these tetraspanins are capable of suppressing metastasis of mesenchymal cell-origin melanoma by sequestering activation of TGF-β type II receptor [85]. Hence, tetraspanins impact tumor growth and progression largely through regulation of function and signaling of their major partners in a parasitic manner, regardless of being pro- or anti-tumorigenic.
4.2. Recruiting Signaling Molecules via the Tetraspanin Self-Associated Membrane Microdomain
Another important mode for tetraspanin-mediated tumorigenesis is to relay PKC-dependent signaling through formation of tetraspanin-enriched microdomain (TEM) [3,8,10]. Based on biochemical and microscopy-based studies, this type of interaction may involve palmitoylation of multiple membrane-proximal cysteine residues in both N- and C-terminals of tetraspanin molecules, which provide key support for clustering or oligomerization of these molecules on the cell surface (Figure 2) [102,119,120]. Evidence from extensive biochemical and microscopy studies have firmly established that the primary function of TEM is to recruit PKC to TEM-associated protein complexes, molecular aggregates or cluster-like structures on the cell surface [98,120]. In case of CD151, TEM recruits PKC-α to phosphorylate laminin-binding (LB) α3, α6 or β4 integrins, while CD53-mediated TEM drives recruitment of PKC-β to BCR complexes in B cells, which in turn leads to signal transduction [71,73,109]. In this context, the tumor-suppressive role of integrin-free CD151 in prostate cancer [47] may be regarded as recruiting the PKC-like signaling molecules to strengthen cell-cell adhesion through oligomerized CD151 molecules. Consistent with this notion, compared to immortalized mammary basal epithelial cells (MCF-10A), luminal epithelia cells (MCF-7) exhibit relatively poor expression of laminins and LB integrins, while having strong E-cadherin expression and cell-cell interactions [71,103]. A similar scenario can be said for the so-called integrin-free CD151 in prostate cancer cells [47]. Moreover, the TEM assembly may involve multiple intracellular membrane compartments, such as endoplasmic reticulum, Golgi, lysosomal, endosomal and multivesicular bodies, which have been extensively described by multiple reviews [10,20,21].
Another remarkable advance in our understanding of TEM is that it can be visualized as nanometer-sized molecular aggregates on the cell surface using high-resolution microscopy [102,119]. These imaging-based findings are consistent with the observation of molecular composition of TEM from our prior biochemical analyses [71,103,104,114]. This type of approach is of particular value to delineate key molecular components or interactions in TEM across various cancer types, ultimately accelerating our mechanistic understanding of the crucial role of CD151 and other tetraspanins in tumorigenesis and metastasis.
4.3. The Long-Range Effect via Regulation of Exosome Formation, Trafficking and Function
Besides TEM, tetraspanins are capable of impacting tumorigenic and metastatic processes through regulation of secretory vesicles named as exosomes [30,121,122]. As a class of extracellular vesicles, exosomes are <200 nm in diameter and formed by cells through invagination of endosomal and plasma membranes. Exosomes are highly enriched in tetraspanins, particularly CD63 and CD151, in addition to diverse intracellular components, including diverse RNA species, metabolites, and proteases [10,121,123]. Given the nature and signaling capabilities of TEMs, it is of no surprise that many tetraspanins are regarded as key contributors to a variety of exosome-associated functional roles, ranging from tumor cell migration, angiogenesis and signal transduction to expression of critical cancer genes and tumor metabolism through control of micro-RNA or non-coding RNA or metabolite pools [67,124,125]. In case of CD151, it is suggested to act in concert with TSPAN8 to drive exosome production in both tumor and endothelial cells, thereby facilitating tumor metastasis [30,118]. However, the mechanism for tetraspanin-dependent regulation of exosomes could be far more complex than originally thought, as some tetraspanins seem to negatively regulate activity and integrity of key exosome-producing machinery, that is, the endosomal sorting complexes required for transport (ESCRT) [126]. With variation in extent of palmitoylation and glycosylation between tetraspanins, they may mediate exosome functions by modulation of their lipid composition [41]. Finally, Wnt signaling has long been known for having a long-range effect on tumor immune microenvironments [77]. Given the strong link between CD151 and exosomes, we speculate that the significant role of CD151 in Wnt-induced mammary tumorigenesis may be partially achieved through regulation of exosome-mediated delivery of Wnt ligands [28].
Overall, compared to traditional cell surface molecules or receptors, the impact of CD151 and other tetraspanins on tumor growth and metastasis is more closely linked to regulation of multi-component protein complexes on the cell surface, extracellular vesicles and tumor microenvironments.
4.4. Decoding the Myth of the Crosstalk Between Tetrspanins and the Wnt Pathway in Cancer Cells
Our recent in vivo study suggests additional complexity of tetraspanin function in cancer cells, particularly in the context of oncogenic activation of the Wnt-dependent pathway (Figure 2). As one of the widely activated oncogenic pathways, Wnt signaling involves interactions between extracellular ligands (Wnts) and multi-component protein complexes on the cell surface composed of seven transmembrane-spanning Frizzled 1-7 and Type I transmembrane co-receptors (e.g., LRP5/6) [127]. Importantly, many of these components, along with their downstream effectors (i.e., Axin, DVL, β-catenin, and TCF7-L2) or mediators (e.g., RNF43) are frequently overexpressed or downregulated in human cancers, particularly those of epithelial cell origin [128,129,130]. The Wnt pathway can also be constitutively activated through genetic mutations or deletions of their protein destruction complexes such as by APC mutation, accompanied by translocation and elevated transcriptional activity of β-catenin [131]. Additionally, the Wnt pathway has been strongly implicated in tumor recurrence and progression in multiple cancer types [127,132,133,134].
Our recent study shows that upon CD151 deletion, there was more than 10-fold increase in the level of nuclear β-catenin as well as the strong cytosolic presence of E-cadherin in mammary tumors [117]. These molecular and signaling changes ultimately bolster transcriptional activation of pro-proliferative genes and tumor growth [135,136]. Also, the genes that regulate cell proliferation, survival, and metabolism (e.g., Cyclin D1 and Myb) are strongly affected [127]. Additionally, expression of the genes involved in regulation of the stability of E-cadherin/β-catenin complexes appeared suppressed, strengthening the intrinsic role of CD151 in cell-cell adhesion [34,116,117,137]. In contrast, CD151 disruption markedly blunts survival of epithelial basal cell-derived tumor cells [28], consistent with the impact of CD151 knockdown on MDA-MB-231 cells [38,71]. Importantly, these emerging observations on CD151 are in line with the current paradigm over the crosstalk between other tetraspanins, including TSPAN8, TSPAN5, and CD82, and the Wnt/β-catenin pathway [1,71,109,138]. Meanwhile, expression of α3 integrin in luminal cells appear unaffected, implicating that the tumor-suppressive role of CD151 in breast, prostate and ovarian cancers are attributed to the combined action of integrin- and self-associated CD151 molecules. As a result, our studies argue that CD151 is a suppressor of ER+ breast cancer and prostate cancer, as they frequently arise from oncogenic targeting of luminal or well-differentiated epithelial cells, rather than basal epithelial or progenitor cells.
5. Control of Expression of CD151 and Other Tetraspanins at Multiple Levels
There is evidence that in contrast to traditional oncogenes or tumor suppressors, the role of tetraspanins including CD151 during carcinogenesis and metastasis is achieved largely through altered expression level and associated impact on activation state and signaling strength of their associated receptors or protein complexes in tumor cells. Notably, very few tetraspanins exhibit functional loss/gain due to genetic alterations (mutations, amplifications, or deletions) [1,20,54,60]. In this sense, the impact of tetraspanins on cancer development and progression may primarily stem from their deregulated expression, subcellular distribution, and molecular partners. However, there is accumulating evidence that expression of tetraspanins seems more regulated at transcriptional and epigenetic levels, largely reminiscent of deregulation of classical tumor suppressors, such as PTEN or BRCA1/2 genes [89].
Thus far, DNA hypermethylation in cancer cells has been documented for at least 6 members of the tetraspanin family, including CD9, CD81, CD82, CD151, TSPAN1, TSPAN3, and Uroplakin [46,63,139,140,141,142,143]. Largely occurring in their promoter regions [139,142], DNA hypermethylation ultimately leads to decreased mRNA and/or protein levels of tetraspanins in tumor cells or tissues [10,144,145,146,147]. Interestingly, this type of regulation appears common in prostate, colon, and ovarian carcinomas, as well as in neuroblastomas and glioblastomas [148,149]. The precise mediators behind DNA methylation of tetraspanin genes however, remain to be identified. Based on recent studies, this type of regulation may be particularly evident in cancer driven by MYC-N amplification and activation of inflammation-oriented NF-κB-driven pro-survival network [92], as well as activation of RTK- or TGF-β receptor-mediated oncogenic pathways [71,85,86,150,151].
The deregulated expression of tetraspanins during tumorigenesis and metastasis may also be attributed to dynamics of their associated protein partners at the co-translational level [82,83,152]. In the case of CD151, its downregulation in tumor cells may be associated with decreased protein expression of α3β1 and α6β4 integrins [32]. There may be a similar scenario for the decreased expression of CD9/CD81-associated EWI proteins in aggressive melanoma [85,140,142,153]. Additionally, there is evidence that tetraspanin expression is regulated through proteasome- or microRNA-mediated biochemical processes [1,151]. Combined, the functional plasticity of tetraspanins during tumorigenesis and metastasis is, at least in part, achieved by tight regulation of their expression at epigenetic, transcriptional, and translational levels, and through protein degradation machinery [154].
6. Clinical Significance of Deregulation of CD151 and Its Associated Network
Even though CD151 has a complex role in human carcinomas, the tight link between CD151 expression and tumor relapse provide a unique window for pursuing CD151 as a drug target [1,31]. This link is also strongly supported by its role in the differentiation of mammary progenitor cells (maintenance of quiescence) in CD151-targeted mice and the MMTV-Wnt model [155,156]. Additionally, CD151 and CD9 are tightly associated with activities of tumor-initiating cells or CSCs [27,28,37]. Moreover, this potential targeting is bolstered by the wide recognition of the role of CD151-associated α6 integrin in survival or activity of CSCs and may be attributed to the intimate crosstalk between CD151-α6 integrin complexes and the RAS/MEK/ERK pathway in basal epithelia cells [28,82]. In this regard, our observed effect of CD151 deletion is consistent with the role of α6 integrin/CD49f-based CSCs in the MMTV-Wnt1 model described by the Perou group [96]. Because CD151 is associated with recurrence of basal-like breast cancer [31], it will be of interest to determine whether the CSC-associated role of CD151 is recapitulated by use of more clinically relevant PDX model of breast cancer under taxane-based regimens known to foster CSCs [157]. This notion is also supported by the evidence that altered CD151 promotes cancer cell resistance to targeted (anti-ErbB receptors) and chemo- therapies in multiple cancer types [32,40,86,108]. Meanwhile, a number of tetraspanins have been shown to be key players in hematopoietic stem/progenitor cells [15,93,158]. Conversely, the strong role of tetraspanins, such as CD151 and CD37, in cell differentiation could serve as a basis for development of lineage-based targeting, in a manner similar to the antibody targeting of CD20 [159]. In case of CD37, because of its restricted expression and strong biological role in mature B lymphocytes, it has been clinically targeted with monoclonal antibody for leukemia treatment. The efficacy of this approach, however, appears challenged by emerging incidence of mutations at the critical domain or deletion of this gene in biopsies in some patient populations [16].
Meanwhile, the prevalence of DNA methylation in the promoter regions of tetraspanins provides a unique opportunity for the evaluation of tumor progression, as their regulation frequently correlates with the onset of metastases in multiple cancer types. Such data may also provide complimentary support for carcinogenesis in the context of tetraspanin-enriched exosomes in patient body fluids [54]. Tetraspanins are highly regarded as potential therapeutic targets, as they support activities and signaling of cancer cells during angiogenesis and dissimilation to secondary sites of primary tumors [1]. In fact, a number of function-blocking monoclonal antibodies against tetraspanins are under investigation for their anti-tumor efficacy [5]. Also, some tetraspanins have been chosen for launching the chimeric antigen receptor T cell (CAR-T)-based anti-cancer therapy [160]. Meanwhile, the impact of tetraspanins on EMT, CSCs and other malignant processes is susceptible to the epigenetic regulation [34,45,49,98,99,117,118,137,161,162,163]. Thus, targeting DNA hypermethylation through chemical inhibitors of DNA methyltransferases, such as 5-Aza-CdR or HDAC inhibition via Trichostatin A, may provide a means to restore expression of many tetraspanins and their anti-tumor functions in various carcinomas, such as CD82 and CD151 in prostate cancer and CD81 in neuroblastomas or glioblastomas [148,149,163,164,165].
7. Conclusions
The role of CD151 and other tetraspanins during tumor growth and progression has long been postulated to be manifested through regulation of tumor cell adhesion, survival, migration, and invasion in an integrin-dependent manner. Especially for CD151, it is regarded as a key adaptor for activation and signal transduction of multiple LB-binding integrins on the cell surface [32,39,45,50,71]. Over the past decade, CD151 is increasingly appreciated as a functionally versatile molecule and driver of human epithelial-origin cancers whereby it regulates cell-cell junctions, proliferation, EMT, metabolism and CSCs, as well as tumor microenvironments [54]. These diverse functions highlight a key basis for the crucial role of CD151 across a wide spectrum of human cancer [1,3]. Additionally, the presence of CD151 in exosomes derived from tumors or their microenvironments suggests a new mechanism for its role in tumor relapse and drug resistance [41,48,54]. There is also evidence on the cell lineage- and oncogenic context-dependent roles of CD151 and other tetraspanins [28,29,48,50,71,93,166]. However, the underlying genetic, epigenetic and metabolic mechanisms remain to be defined. With emerging powerful genomic, proteomic, and metabolomic tools, we will be able to delineate the versatile role of CD151 and other tetraspanins across different stages of cancer development and progression, progression, as well as launch a new line of biomarkers and drug targets for the clinical management of this aggressive disease.
Author Contributions
Conceptualization, S.E., H.H., Y.P., B.P.Z. and X.H.Y.; writing—original draft preparation, S.E., H.H. and X.H.Y.; writing—review and editing, S.E. and X.H.Y.; visualization, S.E., H.H. and X.H.Y.; supervision, X.H.Y.; funding acquisition, X.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported in part by pilot project funding from National Institutes of Health COBRE grant #5P20GM121327-03, as well as support from the University of Kentucky Department of Pharmacology and Nutritional Sciences (to X.H.Y.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CSC | Cancer Stem Cell |
| ECM | Extracellular Matrix |
| EMT | Epithelial-Mesenchymal Transition |
| ER | Estrogen Receptor |
| ESCRT | Endosomal Sorting Complexes Required for Transport |
| LB | Laminin-Binding |
| PKC | Protein Kinase C |
| RTK | Tyrosine Kinase Receptor |
| SFKs | Src Family Kinases |
| TEM | Tetraspanin-Enriched Microdomain |
References
- Hemler, M.E. Tetraspanin proteins promote multiple cancer stages. Nat. Rev. Cancer 2014, 14, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Vences-Catalan, F.; Rajapaksa, R.; Srivastava, M.K.; Marabelle, A.; Kuo, C.C.; Levy, R.; Levy, S. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 2015, 75, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E. The complexity of tetraspanins. Biochem. Soc. Trans. 2011, 39, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Karamatic Crew, V.; Burton, N.; Kagan, A.; Green, C.A.; Levene, C.; Flinter, F.; Brady, R.L.; Daniels, G.; Anstee, D.J. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 2004, 104, 2217–2223. [Google Scholar] [CrossRef]
- Testa, J.E.; Brooks, P.C.; Lin, J.M.; Quigley, J.P. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999, 59, 3812–3820. [Google Scholar]
- Fitter, S.; Tetaz, T.J.; Berndt, M.C.; Ashman, L.K. Molecular-Cloning of Cdna-Encoding a Novel Platelet-Endothelial Cell Tetra-Span Antigen, Peta-3. Blood 1995, 86, 1348–1355. [Google Scholar] [CrossRef]
- Schick, M.R.; Levy, S. The TAPA-1 molecule is associated on the surface of B cells with HLA-DR molecules. J. Immunol. 1993, 151, 4090–4097. [Google Scholar]
- Zimmerman, B.; Kelly, B.; McMillan, B.J.; Seegar, T.C.M.; Dror, R.O.; Kruse, A.C.; Blacklow, S.C. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell 2016, 167, 1041–1051. [Google Scholar] [CrossRef]
- Umeda, R.; Satouh, Y.; Takemoto, M.; Nakada-Nakura, Y.; Liu, K.; Yokoyama, T.; Shirouzu, M.; Iwata, S.; Nomura, N.; Sato, K.; et al. Structural insights into tetraspanin CD9 function. Nat. Commun. 2020, 11, 1606. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577.e520. [Google Scholar] [CrossRef]
- Sachs, N.; Kreft, M.; van den Bergh Weerman, M.A.; Beynon, A.J.; Peters, T.A.; Weening, J.J.; Sonnenberg, A. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 2006, 175, 33–39. [Google Scholar] [CrossRef]
- Vahidnezhad, H.; Youssefian, L.; Saeidian, A.H.; Mahmoudi, H.; Touati, A.; Abiri, M.; Kajbafzadeh, A.M.; Aristodemou, S.; Liu, L.; McGrath, J.A.; et al. Recessive mutation in tetraspanin CD151 causes Kindler syndrome-like epidermolysis bullosa with multi-systemic manifestations including nephropathy. Matrix Biol. 2018, 66, 22–33. [Google Scholar] [CrossRef]
- Spring, F.A.; Griffiths, R.E.; Mankelow, T.J.; Agnew, C.; Parsons, S.F.; Chasis, J.A.; Anstee, D.J. Tetraspanins CD81 and CD82 facilitate alpha4beta1-mediated adhesion of human erythroblasts to vascular cell adhesion molecule-1. PLoS ONE 2013, 8, e62654. [Google Scholar] [CrossRef]
- Bergsma, A.; Ganguly, S.S.; Dick, D.; Williams, B.O.; Miranti, C.K. Global deletion of tetraspanin CD82 attenuates bone growth and enhances bone marrow adipogenesis. Bone 2018, 113, 105–113. [Google Scholar] [CrossRef]
- Elfrink, S.; de Winde, C.M.; van den Brand, M.; Berendsen, M.; Roemer, M.G.M.; Arnold, F.; Janssen, L.; van der Schaaf, A.; Jansen, E.; Groenen, P.; et al. High frequency of inactivating tetraspanin C D37 mutations in diffuse large B-cell lymphoma at immune-privileged sites. Blood 2019, 134, 946–950. [Google Scholar] [CrossRef]
- Hochheimer, N.; Sies, R.; Aschenbrenner, A.C.; Schneider, D.; Lang, T. Classes of non-conventional tetraspanins defined by alternative splicing. Sci. Rep. 2019, 9, 14075. [Google Scholar] [CrossRef]
- Saiz, M.L.; Rocha-Perugini, V.; Sanchez-Madrid, F. Tetraspanins as Organizers of Antigen-Presenting Cell Function. Front. Immunol. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Yeung, L.; Hickey, M.J.; Wright, M.D. The Many and Varied Roles of Tetraspanins in Immune Cell Recruitment and Migration. Front. Immunol. 2018, 9, 1644. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef]
- Zoller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef]
- Yauch, R.L.; Berditchevski, F.; Harler, M.B.; Reichner, J.; Hemler, M.E. Highly stoichiometric, stable, and specific association of integrin alpha 3 beta 1 with CD151 provides a major link to phosphatidylinositol 4 kinase, and may regulate cell migration. Mol. Biol. Cell 1998, 9, 2751–2765. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Alfranca, A.; Cabanas, C.; Marazuela, M.; Tejedor, R.; Ursa, M.A.; Ashman, L.K.; de Landazuri, M.O.; Sanchez-Madrid, F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha 3 beta 1 integrin localized at endothelial lateral junctions. J. Cell Biol. 1998, 141, 791–804. [Google Scholar] [CrossRef]
- Sincock, P.M.; Fitter, S.; Parton, R.G.; Berndt, M.C.; Gamble, J.R.; Ashman, L.K. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J. Cell Sci. 1999, 112, 833–844. [Google Scholar] [PubMed]
- Zhao, K.; Erb, U.; Hackert, T.; Zoller, M.; Yue, S. Distorted leukocyte migration, angiogenesis, wound repair and metastasis in Tspan8 and Tspan8/CD151 double knockout mice indicate complementary activities of Tspan8 and CD51. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Kazarov, A.R.; Butterfield, C.E.; Hopkins, B.D.; Benjamin, L.E.; Kaipainen, A.; Hemler, M.E. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 2007, 109, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.M.; Ferreira, R.M.M.; Almagro, J.; Evan, T.; Legrave, N.; Zaw Thin, M.; Frith, D.; Carvalho, J.; Barry, D.J.; Snijders, A.P.; et al. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat. Cell Biol. 2019, 21, 1425–1435. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Han, R.; Deng, X.; Shi, J.; Huang, H.; Hamad, N.; McCaughley, A.; Liu, J.; Wang, C.; et al. Deletion of tetraspanin CD151 alters the Wnt oncogene-induced mammary tumorigenesis: A cell type-linked function and signaling. Neoplasia 2019, 21, 1151–1163. [Google Scholar] [CrossRef]
- Roselli, S.; Kahl, R.G.; Copeland, B.T.; Naylor, M.J.; Weidenhofer, J.; Muller, W.J.; Ashman, L.K. Deletion of Cd151 reduces mammary tumorigenesis in the MMTV/PyMT mouse model. BMC Cancer 2014, 14, 509. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.; Hackert, T.; Pitzer, C.; Zoller, M. Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression. J. Exp. Clin. Cancer Res. 2018, 37, 312. [Google Scholar] [CrossRef]
- Kwon, M.J.; Park, S.; Choi, J.Y.; Oh, E.; Kim, Y.J.; Park, Y.H.; Cho, E.Y.; Nam, S.J.; Im, Y.H.; Shin, Y.K.; et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br. J. Cancer 2012, 106, 923–930. [Google Scholar] [CrossRef]
- Deng, X.; Li, Q.; Hoff, J.; Novak, M.; Yang, H.; Jin, H.; Erfani, S.F.; Sharma, C.; Zhou, P.; Rabinovitz, I.; et al. Integrin-associated CD151 drives ErbB2-evoked mammary tumor onset and metastasis. Neoplasia 2012, 14, 678–689. [Google Scholar] [CrossRef]
- Chien, C.W.; Lin, S.C.; Lai, Y.Y.; Lin, B.W.; Lee, J.C.; Tsai, S.J. Regulation of CD151 by hypoxia controls cell adhesion and metastasis in colorectal cancer. Clin. Cancer Res. 2008, 14, 8043–8051. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.; Kim, Y.M.; Lee, H. The metastasis suppressor CD82/KAI1 represses the TGF-beta 1 and Wnt signalings inducing epithelial-to-mesenchymal transition linked to invasiveness of prostate cancer cells. Prostate 2019, 79, 1400–1411. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Tian, T.V.; Mas, A.; Pecoraro, M.; Batlle-Morera, L.; Noya, L.; Martin-Caballero, J.; Ruberte, J.; Keyes, W.M. DeltaNp63alpha promotes adhesion of metastatic prostate cancer cells to the bone through regulation of CD82. Oncogene 2017, 36, 4381–4392. [Google Scholar] [CrossRef]
- Copeland, B.T.; Bowman, M.J.; Ashman, L.K. Genetic ablation of the tetraspanin CD151 reduces spontaneous metastatic spread of prostate cancer in the TRAMP model. Mol. Cancer Res. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Rajasekhar, V.K.; Studer, L.; Gerald, W.; Socci, N.D.; Scher, H.I. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat. Commun. 2011, 2, 162. [Google Scholar] [CrossRef]
- Sadej, R.; Romanska, H.; Kavanagh, D.; Baldwin, G.; Takahashi, T.; Kalia, N.; Berditchevski, F. Tetraspanin CD151 Regulates Transforming Growth Factor beta Signaling: Implication in Tumor Metastasis. Cancer Res. 2010, 70, 6059–6070. [Google Scholar] [CrossRef]
- Novitskaya, V.; Romanska, H.; Kordek, R.; Potemski, P.; Kusinska, R.; Parsons, M.; Odintsova, E.; Berditchevski, F. Integrin alpha3beta1-CD151 complex regulates dimerization of ErbB2 via RhoA. Oncogene 2014, 33, 2779–2789. [Google Scholar] [CrossRef]
- Zhou, P.; Erfani, S.; Liu, Z.; Jia, C.; Chen, Y.; Xu, B.; Deng, X.; Alfaro, J.E.; Chen, L.; Napier, D.; et al. CD151-alpha3beta1 integrin complexes are prognostic markers of glioblastoma and cooperate with EGFR to drive tumor cell motility and invasion. Oncotarget 2015, 6, 29675–29693. [Google Scholar] [CrossRef]
- Malla, R.R.; Pandrangi, S.; Kumari, S.; Gavara, M.M.; Badana, A.K. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia Pac. J. Clin. Oncol. 2018, 14, 383–391. [Google Scholar] [CrossRef]
- Tilghman, J.; Schiapparelli, P.; Lal, B.; Ying, M.; Quinones-Hinojosa, A.; Xia, S.; Laterra, J. Regulation of Glioblastoma Tumor-Propagating Cells by the Integrin Partner Tetraspanin CD151. Neoplasia 2016, 18, 185–198. [Google Scholar] [CrossRef]
- Bredel, M.; Bredel, C.; Juric, D.; Harsh, G.R.; Vogel, H.; Recht, L.D.; Sikic, B.I. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005, 65, 8679–8689. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.H.; Xu, F.; Sharma, C.; Wang, H.X.; Knoblich, K.; Rabinovitz, I.; Granter, S.R.; Hemler, M.E. Tetraspanin CD151 plays a key role in skin squamous cell carcinoma. Oncogene 2013, 32, 1772–1783. [Google Scholar] [CrossRef]
- Johnson, J.L.; Winterwood, N.; DeMali, K.A.; Stipp, C.S. Tetraspanin CD151 regulates RhoA activation and the dynamic stability of carcinoma cell-cell contacts. J. Cell Sci. 2009, 122, 2263–2273. [Google Scholar] [CrossRef]
- Han, R.; Hensley, P.J.; Li, J.; Zhang, Y.; Stark, T.W.; Heller, A.; Qian, H.; Shi, J.; Liu, Z.; Huang, J.A.; et al. Integrin-associated CD151 is a suppressor of prostate cancer progression. Am. J. Transl. Res. 2020, 12, 1428–1442. [Google Scholar]
- Palmer, T.D.; Martinez, C.H.; Vasquez, C.; Hebron, K.E.; Jones-Paris, C.; Arnold, S.A.; Chan, S.M.; Chalasani, V.; Gomez-Lemus, J.A.; Williams, A.K.; et al. Integrin-free tetraspanin CD151 can inhibit tumor cell motility upon clustering and is a clinical indicator of prostate cancer progression. Cancer Res. 2014, 74, 173–187. [Google Scholar] [CrossRef]
- Hayward, S.; Gachehiladze, M.; Badr, N.; Andrijes, R.; Molostvov, G.; Paniushkina, L.; Sopikova, B.; Slobodova, Z.; Mgebrishvili, G.; Sharma, N.; et al. The CD151-midkine pathway regulates the immune microenvironment in inflammatory breast cancer. J. Pathol. 2020, 251, 63–73. [Google Scholar] [CrossRef]
- Baldwin, L.A.; Hoff, J.T.; Lefringhouse, J.; Zhang, M.; Jia, C.; Liu, Z.; Erfani, S.; Jin, H.; Xu, M.; She, Q.B.; et al. CD151-alpha3beta1 integrin complexes suppress ovarian tumor growth by repressing slug-mediated EMT and canonical Wnt signaling. Oncotarget 2014, 5, 12203–12217. [Google Scholar] [CrossRef]
- Romanska, H.M.; Potemski, P.; Krakowska, M.; Mieszkowska, M.; Chaudhri, S.; Kordek, R.; Kubiak, R.; Speirs, V.; Hanby, A.M.; Sadej, R.; et al. Lack of CD151/integrin alpha3beta1 complex is predictive of poor outcome in node-negative lobular breast carcinoma: Opposing roles of CD151 in invasive lobular and ductal breast cancers. Br. J. Cancer 2015, 113, 1350–1357. [Google Scholar] [CrossRef][Green Version]
- Lin, W.; Liu, J.; Chen, J.; Li, J.; Qiu, S.; Ma, J.; Lin, X.; Zhang, L.; Wu, J. Peptides of tetraspanin oncoprotein CD151 trigger active immunity against primary tumour and experimental lung metastasis. EBioMedicine 2019, 49, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Kahl, R.; Brzozowski, J.S.; Jankowski, H.; Naudin, C.; Pariyar, M.; Avery-Kiejda, K.A.; Scarlett, C.J.; Boucheix, C.; Muller, W.J.; et al. Tetraspanin CD9 is Regulated by miR-518f-5p and Functions in Breast Cell Migration and In Vivo Tumor Growth. Cancers 2020, 12, 795. [Google Scholar] [CrossRef]
- Takeda, T.; Hattori, N.; Tokuhara, T.; Nishimura, Y.; Yokoyama, M.; Miyake, M. Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res. 2007, 67, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Jerez, S.; Araya, H.; Thaler, R.; Charlesworth, M.C.; Lopez-Solis, R.; Kalergis, A.M.; Cespedes, P.F.; Dudakovic, A.; Stein, G.S.; van Wijnen, A.J.; et al. Proteomic Analysis of Exosomes and Exosome-Free Conditioned Media From Human Osteosarcoma Cell Lines Reveals Secretion of Proteins Related to Tumor Progression. J. Cell. Biochem. 2017, 118, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, S.; Lee, J.; Shin, H.B.; Yoo, S.M.; Lee, M.S.; Park, J. Suppression of CD81 promotes bladder cancer cell invasion through increased matrix metalloproteinase expression via extracellular signal-regulated kinase phosphorylation. Investig. Clin. Urol. 2019, 60, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, F.; Yao, Y.; Wang, J.; Wei, J.; Wu, Q.; Xiang, S.; Xu, L. Interaction of transforming growth factor-beta-Smads/microRNA-362-3p/CD82 mediated by M2 macrophages promotes the process of epithelial-mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci. 2019, 110, 2507–2519. [Google Scholar] [CrossRef]
- Feng, J.; Huang, C.; Wren, J.D.; Wang, D.W.; Yan, J.; Zhang, J.; Sun, Y.; Han, X.; Zhang, X.A. Tetraspanin CD82: A suppressor of solid tumors and a modulator of membrane heterogeneity. Cancer Metastasis Rev. 2015, 34, 619–633. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, S.; Liu, G.; Wang, H.; Fu, A.; Zhu, H.; Ren, Q.; Wang, B.; Xu, X.; Bai, H.; et al. CD82 suppresses CD44 alternative splicing-dependent melanoma metastasis by mediating U2AF2 ubiquitination and degradation. Oncogene 2016, 35, 5056–5069. [Google Scholar] [CrossRef]
- Huang, C.; Hays, F.A.; Tomasek, J.J.; Benyajati, S.; Zhang, X.A. Tetraspanin CD82 interaction with cholesterol promotes extracellular vesicle-mediated release of ezrin to inhibit tumour cell movement. J. Extracell. Vesicles 2020, 9, 1692417. [Google Scholar] [CrossRef]
- Knoblich, K.; Wang, H.X.; Sharma, C.; Fletcher, A.L.; Turley, S.J.; Hemler, M.E. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits beta-catenin degradation. Cell. Mol. Life Sci. CMLS 2013, 71, 1305–1314. [Google Scholar] [CrossRef]
- Otomo, R.; Otsubo, C.; Matsushima-Hibiya, Y.; Miyazaki, M.; Tashiro, F.; Ichikawa, H.; Kohno, T.; Ochiya, T.; Yokota, J.; Nakagama, H.; et al. TSPAN12 is a critical factor for cancer-fibroblast cell contact-mediated cancer invasion. Proc. Natl. Acad. Sci. USA 2014, 111, 18691–18696. [Google Scholar] [CrossRef]
- Liang, G.; Meng, W.; Huang, X.; Zhu, W.; Yin, C.; Wang, C.; Fassan, M.; Yu, Y.; Kudo, M.; Xiao, S.; et al. miR-196b-5p-mediated downregulation of TSPAN12 and GATA6 promotes tumor progression in non-small cell lung cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 4347–4357. [Google Scholar] [CrossRef]
- Zhu, R.; Gires, O.; Zhu, L.; Liu, J.; Li, J.; Yang, H.; Ju, G.; Huang, J.; Ge, W.; Chen, Y.; et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 2019, 10, 2863. [Google Scholar] [CrossRef]
- Yue, S.; Mu, W.; Erb, U.; Zoller, M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget 2015, 6, 2366–2384. [Google Scholar] [CrossRef]
- El Kharbili, M.; Agaesse, G.; Barbollat-Boutrand, L.; Pommier, R.M.; de la Fouchardiere, A.; Larue, L.; Caramel, J.; Puisieux, A.; Berthier-Vergnes, O.; Masse, I. Tspan8-beta-catenin positive feedback loop promotes melanoma invasion. Oncogene 2019, 38, 3781–3793. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, J.; Xu, T.; Ahmadinejad, N.; Hess, K.; Lin, S.H.; Zhang, J.; Liu, X.; Liu, L.; Ning, B.; et al. Extracellular vesicle tetraspanin-8 level predicts distant metastasis in non-small cell lung cancer after concurrent chemoradiation. Sci. Adv. 2020, 6, eaaz6162. [Google Scholar] [CrossRef]
- Yauch, R.L.; Kazarov, A.R.; Desai, B.; Lee, R.T.; Hemler, M.E. Direct extracellular contact between integrin alpha(3)beta(1) and TM4SF protein CD151. J. Biol. Chem. 2000, 275, 9230–9238. [Google Scholar] [CrossRef]
- Fitter, S.; Sincock, P.M.; Jolliffe, C.N.; Ashman, L.K. Transmembrane 4 superfamily protein CD151 (PETA-3) associates with beta 1 and alpha IIb beta 3 integrins in haemopoietic cell lines and modulates cell-cell adhesion. Biochem. J. 1999, 338, 61–70. [Google Scholar]
- Zijlstra, A.; Lewis, J.; Degryse, B.; Stuhlmann, H.; Quigley, J.P. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell 2008, 13, 221–234. [Google Scholar] [CrossRef]
- Yang, X.H.; Richardson, A.L.; Torres-Arzayus, M.I.; Zhou, P.; Sharma, C.; Kazarov, A.R.; Andzelm, M.M.; Strominger, J.L.; Brown, M.; Hemler, M.E. CD151 accelerates breast cancer by regulating alpha 6 integrin function, signaling, and molecular organization. Cancer Res. 2008, 68, 3204–3213. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Guo, Q.; Xia, B.; Zhang, Y.H.; Giesert, E.E.; Levy, S.; Zheng, J.J.; Zhang, X.A. Tetraspanins regulate the protrusive activities of cell membrane. Biochem. Biophys. Res. Commun. 2011, 415, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Mirchev, R.; Deng, X.; Yacono, P.; Yang, H.L.; Golan, D.E.; Hemler, M.E. CD151 restricts the alpha6 integrin diffusion mode. J. Cell Sci. 2012, 125, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Podsypanina, K.; Li, Y.; Varmus, H.E. Evolution of somatic mutations in mammary tumors in transgenic mice is influenced by the inherited genotype. BMC Med. 2004, 2, 24. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Chen, Y.; Podsypanina, K.; Chamorro, M.; Olshen, A.B.; Desai, K.V.; Tann, A.; Petersen, D.; Green, J.E.; et al. Changes in gene expression during the development of mammary tumors in MMTV-Wnt-1 transgenic mice. Genome Biol. 2005, 6, R84. [Google Scholar] [CrossRef]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P.; et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell Rep. 2019, 27, 1967–1978. [Google Scholar] [CrossRef]
- Kim, Y.C.; Clark, R.J.; Ranheim, E.A.; Alexander, C.M. Wnt1 expression induces short-range and long-range cell recruitments that modify mammary tumor development and are not induced by a cell-autonomous beta-catenin effector. Cancer Res. 2008, 68, 10145–10153. [Google Scholar] [CrossRef]
- Wellenstein, M.D.; Coffelt, S.B.; Duits, D.E.M.; van Miltenburg, M.H.; Slagter, M.; de Rink, I.; Henneman, L.; Kas, S.M.; Prekovic, S.; Hau, C.S.; et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 2019, 572, 538–542. [Google Scholar] [CrossRef]
- Ang, J.; Lijovic, M.; Ashman, L.K.; Kan, K.; Frauman, A.G. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: A new prognostic indicator? Cancer Epidemiol. Biomark. Prev. 2004, 13, 1717–1721. [Google Scholar]
- Ang, J.; Fang, B.L.; Ashman, L.K.; Frauman, A.G. The migration and invasion of human prostate cancer cell lines involves CD151 expression. Oncol. Rep. 2010, 24, 159–1597. [Google Scholar]
- Novitskaya, V.; Romanska, H.; Dawoud, M.; Jones, J.L.; Berditchevski, F. Tetraspanin CD151 Regulates Growth of Mammary Epithelial Cells in Three-Dimensional Extracellular Matrix: Implication for Mammary Ductal Carcinoma In situ. Cancer Res. 2010, 70, 4698–4708. [Google Scholar] [CrossRef]
- Raymond, K.; Kreft, M.; Song, J.Y.; Janssen, H.; Sonnenberg, A. Dual Role of alpha6beta4 integrin in epidermal tumor growth: Tumor-suppressive versus tumor-promoting function. Mol. Biol. Cell 2007, 18, 4210–4221. [Google Scholar] [CrossRef][Green Version]
- Ramovs, V.; Te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57–58, 213–243. [Google Scholar] [CrossRef]
- Shi, W.; Fan, H.; Shum, L.; Derynck, R. The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation. J. Cell Biol. 2000, 148, 591–602. [Google Scholar] [CrossRef]
- Wang, H.X.; Sharma, C.; Knoblich, K.; Granter, S.R.; Hemler, M.E. EWI-2 negatively regulates TGF-beta signaling leading to altered melanoma growth and metastasis. Cell Res. 2015, 25, 370–385. [Google Scholar] [CrossRef]
- Yang, X.H.; Flores, L.M.; Li, Q.; Zhou, P.; Xu, F.; Krop, I.E.; Hemler, M.E. Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res. 2010, 70, 2256–2263. [Google Scholar] [CrossRef]
- Berger, A.C.; Korkut, A.; Kanchi, R.S.; Hegde, A.M.; Lenoir, W.; Liu, W.; Liu, Y.; Fan, H.; Shen, H.; Ravikumar, V.; et al. A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell 2018, 33, 690–705. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.F.; Rueda, O.M.; Vollan, H.K.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef]
- Fallah, Y.; Brundage, J.; Allegakoen, P.; Shajahan-Haq, A.N. MYC-Driven Pathways in Breast Cancer Subtypes. Biomolecules 2017, 7, 53. [Google Scholar] [CrossRef]
- Fabian, J.; Opitz, D.; Althoff, K.; Lodrini, M.; Hero, B.; Volland, R.; Beckers, A.; de Preter, K.; Decock, A.; Patil, N.; et al. MYCN and HDAC5 transcriptionally repress CD9 to trigger invasion and metastasis in neuroblastoma. Oncotarget 2016, 7, 66344–66359. [Google Scholar] [CrossRef]
- Yin, Y.; Deng, X.; Liu, Z.; Baldwin, L.A.; Lefringhouse, J.; Zhang, J.; Hoff, J.T.; Erfani, S.F.; Rucker, E.B., 3rd; O’Connor, K.; et al. CD151 represses mammary gland development by maintaining the niches of progenitor cells. Cell Cycle 2014, 13, 2707–2722. [Google Scholar] [CrossRef]
- Phillips, S.; Prat, A.; Sedic, M.; Proia, T.; Wronski, A.; Mazumdar, S.; Skibinski, A.; Shirley, S.H.; Perou, C.M.; Gill, G.; et al. Cell-State Transitions Regulated by SLUG Are Critical for Tissue Regeneration and Tumor Initiation. Stem Cell Rep. 2014, 2, 633–647. [Google Scholar] [CrossRef]
- Lathia, J.D.; Gallagher, J.; Heddleston, J.M.; Wang, J.; Eyler, C.E.; Macswords, J.; Wu, Q.; Vasanji, A.; McLendon, R.E.; Hjelmeland, A.B.; et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010, 6, 421–432. [Google Scholar] [CrossRef]
- Pfefferle, A.D.; Darr, D.B.; Calhoun, B.C.; Mott, K.R.; Rosen, J.M.; Perou, C.M. The MMTV-Wnt1 murine model produces two phenotypically distinct subtypes of mammary tumors with unique therapeutic responses to an EGFR inhibitor. Dis. Model Mech. 2019, 12. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Visvader, J.E. Insights into the cell of origin in breast cancer and breast cancer stem cells. Asia Pac. J. Clin. Oncol. 2010, 6, 89–97. [Google Scholar] [CrossRef]
- Shigeta, M.; Sanzen, N.; Ozawa, M.; Gu, J.; Hasegawa, H.; Sekiguchi, K. CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J. Cell Biol. 2003, 163, 165–176. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Wang, Z.; Ashman, L.K.; Brady-Kalnay, S.M.; Kreidberg, J.A. Alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J. Cell Biol. 2003, 163, 1351–1362. [Google Scholar] [CrossRef]
- Nassour, M.; Idoux-Gillet, Y.; Selmi, A.; Come, C.; Faraldo, M.L.; Deugnier, M.A.; Savagner, P. Slug controls stem/progenitor cell growth dynamics during mammary gland morphogenesis. PLoS ONE 2012, 7, e53498. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, G.J.; Visvader, J.E. Cell fate takes a slug in BRCA1-associated breast cancer. Breast Cancer Res. 2011, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Zuidscherwoude, M.; Gottfert, F.; Dunlock, V.M.; Figdor, C.G.; van den Bogaart, G.; Spriel, A.B. The tetraspanin web revisited by super-resolution microscopy. Sci. Rep. 2015, 5, 12201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Claas, C.; Kraeft, S.K.; Chen, L.B.; Wang, Z.; Kreidberg, J.A.; Hemler, M.E. Palmitoylation of tetraspanin proteins: Modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol. Biol. Cell 2002, 13, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kovalenko, O.V.; Tang, W.; Claas, C.; Stipp, C.S.; Hemler, M.E. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J. Cell Biol. 2004, 167, 1231–1240. [Google Scholar] [CrossRef]
- Kovalenko, O.V.; Yang, X.; Kolesnikova, T.V.; Hemler, M.E. Evidence for specific tetraspanin homodimers: Inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem. J. 2004, 377, 407–417. [Google Scholar] [CrossRef]
- van Deventer, S.; Arp, A.B.; van Spriel, A.B. Dynamic Plasma Membrane Organization: A Complex Symphony. Trends Cell Biol. 2021, 31, 119–129. [Google Scholar] [CrossRef]
- Sharma, C.; Yang, X.H.; Hemler, M.E. DHHC2 affects palmitoylation, stability, and functions of tetraspanins CD9 and CD151. Mol. Biol. Cell 2008, 19, 3415–3425. [Google Scholar] [CrossRef]
- Hwang, S.; Takimoto, T.; Hemler, M.E. Integrin-independent support of cancer drug resistance by tetraspanin CD151. Cell Mol. Life Sci. 2019, 76, 1595–1604. [Google Scholar] [CrossRef]
- Stipp, C.S. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev. Mol. Med. 2010, 12, e3. [Google Scholar] [CrossRef]
- Mitchell, K.; Svenson, K.B.; Longmate, W.M.; Gkirtzimanaki, K.; Sadej, R.; Wang, X.H.; Zhao, J.H.; Eliopoulos, A.G.; Berditchevski, F.; DiPersio, C.M. Suppression of Integrin alpha 3 beta 1 in Breast Cancer Cells Reduces Cyclooxygenase-2 Gene Expression and Inhibits Tumorigenesis, Invasion, and Cross-Talk to Endothelial Cells. Cancer Res. 2010, 70, 6359–6367. [Google Scholar] [CrossRef]
- Zevian, S.C.; Johnson, J.L.; Winterwood, N.E.; Walters, K.S.; Herndon, M.E.; Henry, M.D.; Stipp, C.S. CD151 promotes alpha3beta1 integrin-dependent organization of carcinoma cell junctions and restrains collective cell invasion. Cancer Biol. Ther. 2015, 16, 1626–1640. [Google Scholar] [CrossRef]
- Roela, R.A.; Brentani, M.M.; Katayama, M.L.; Reis, M.; Federico, M.H. Simultaneous changes in the function and expression of beta 1 integrins during the growth arrest of poorly differentiated colorectal cells (LISP-1). Braz. J. Med. Biol. Res. 2003, 36, 1091–1099. [Google Scholar] [CrossRef]
- Gustafson-Wagner, E.; Stipp, C.S. The CD9/CD81 tetraspanin complex and tetraspanin CD151 regulate alpha3beta1 integrin-dependent tumor cell behaviors by overlapping but distinct mechanisms. PLoS ONE 2013, 8, e61834. [Google Scholar] [CrossRef]
- Yang, X.H.; Kovalenko, O.V.; Kolesnikova, T.V.; Andzelm, M.M.; Rubinstein, E.; Strominger, J.L.; Hemler, M.E. Contrasting effects of EWI proteins, integrins, and protein palmitoylation on cell surface CD9 organization. J. Biol. Chem. 2006, 281, 12976–12985. [Google Scholar] [CrossRef]
- Boudjadi, S.; Beaulieu, J.F. MYC and integrins interplay in colorectal cancer. Oncoscience 2016, 3, 50–51. [Google Scholar] [CrossRef]
- Houle, C.D.; Ding, X.Y.; Foley, J.F.; Afshari, C.A.; Barrett, J.C.; Davis, B.J. Loss of expression and altered localization of KAI1 and CD9 protein are associated with epithelial ovarian cancer progression. Gynecol. Oncol. 2002, 86, 69–78. [Google Scholar] [CrossRef]
- Chigita, S.; Sugiura, T.; Abe, M.; Kobayashi, Y.; Shimoda, M.; Onoda, M.; Shirasuna, K. CD82 inhibits canonical Wnt signalling by controlling the cellular distribution of beta-catenin in carcinoma cells. Int. J. Oncol. 2012, 41, 2021–2028. [Google Scholar] [CrossRef]
- Voglstaetter, M.; Thomsen, A.R.; Nouvel, J.; Koch, A.; Jank, P.; Navarro, E.G.; Gainey-Schleicher, T.; Khanduri, R.; Gross, A.; Rossner, F.; et al. Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles. J. Pathol. 2019, 248, 421–437. [Google Scholar] [CrossRef]
- Zuidscherwoude, M.; Dunlock, V.E.; van den Bogaart, G.; van Deventer, S.J.; van der Schaaf, A.; van Oostrum, J.; Goedhart, J.; In’t Hout, J.; Hammerling, G.J.; Tanaka, S.; et al. Tetraspanin microdomains control localized protein kinase C signaling in B cells. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Zhang, X.A.; Bontrager, A.L.; Hemler, M.E. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J. Biol. Chem. 2001, 276, 25005–25013. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zoller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Sung, B.H.; von Lersner, A.; Guerrero, J.; Krystofiak, E.S.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.M.; Weaver, A.M. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 2020, 11, 2092. [Google Scholar] [CrossRef]
- Mu, W.; Provaznik, J.; Hackert, T.; Zoller, M. Tspan8-Tumor Extracellular Vesicle-Induced Endothelial Cell and Fibroblast Remodeling Relies on the Target Cell-Selective Response. Cells 2020, 9, 319. [Google Scholar] [CrossRef]
- Ghossoub, R.; Chery, M.; Audebert, S.; Leblanc, R.; Egea-Jimenez, A.L.; Lembo, F.; Mammar, S.; Le Dez, F.; Camoin, L.; Borg, J.P.; et al. Tetraspanin-6 negatively regulates exosome production. Proc. Natl. Acad. Sci. USA 2020, 117, 5913–5922. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, Q.; Lu, W.; Bu, G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: Implication for breast cancer tumorigenesis. Oncogene 2010, 29, 539–549. [Google Scholar] [CrossRef]
- Prosperi, J.R.; Goss, K.H. A Wnt-ow of opportunity: Targeting the Wnt/beta-catenin pathway in breast cancer. Curr. Drug Targets 2010, 11, 1074–1088. [Google Scholar] [CrossRef]
- Khramtsov, A.I.; Khramtsova, G.F.; Tretiakova, M.; Huo, D.; Olopade, O.I.; Goss, K.H. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010, 176, 2911–2920. [Google Scholar] [CrossRef]
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; Van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.W.; Wang, X.H.; Diehn, M.; Shedden, K.; Chen, G.Y.; Sherlock, G.; Gurney, A.; Lewicki, J.; Clarke, M.F. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells 2008, 26, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Badders, N.M.; Goel, S.; Clark, R.J.; Klos, K.S.; Kim, S.; Bafico, A.; Lindvall, C.; Williams, B.O.; Alexander, C.M. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS ONE 2009, 4, e6594. [Google Scholar] [CrossRef] [PubMed]
- Pohl, S.G.; Brook, N.; Agostino, M.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A. Wnt signaling in triple-negative breast cancer. Oncogenesis 2017, 6, e310. [Google Scholar] [CrossRef]
- Vlahov, N.; Scrace, S.; Soto, M.S.; Grawenda, A.M.; Bradley, L.; Pankova, D.; Papaspyropoulos, A.; Yee, K.S.; Buffa, F.; Goding, C.R.; et al. Alternate RASSF1 Transcripts Control SRC Activity, E-Cadherin Contacts, and YAP-Mediated Invasion. Curr. Biol. 2015, 25, 3019–3034. [Google Scholar] [CrossRef]
- Tobin, N.P.; Harrell, J.C.; Lovrot, J.; Egyhazi Brage, S.; Frostvik Stolt, M.; Carlsson, L.; Einbeigi, Z.; Linderholm, B.; Loman, N.; Malmberg, M.; et al. Molecular subtype and tumor characteristics of breast cancer metastases as assessed by gene expression significantly influence patient post-relapse survival. Ann. Oncol. 2015, 26, 81–88. [Google Scholar] [CrossRef]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of beta-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef]
- Anastas, J.N.; Kulikauskas, R.M.; Tamir, T.; Rizos, H.; Long, G.V.; von Euw, E.M.; Yang, P.T.; Chen, H.W.; Haydu, L.; Toroni, R.A.; et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J. Clin. Invest. 2014, 124, 2877–2890. [Google Scholar] [CrossRef]
- Koh, H.J.; Kim, Y.R.; Kim, J.S.; Yun, J.S.; Kim, S.; Kim, S.Y.; Jang, K.; Yang, C.S. CD82 hypomethylation is essential for tuberculosis pathogenesis via regulation of RUNX1-Rab5/22. Exp. Mol. Med. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Kim, J.H.; Dhanasekaran, S.M.; Prensner, J.R.; Cao, X.; Robinson, D.; Kalyana-Sundaram, S.; Huang, C.; Shankar, S.; Jing, X.; Iyer, M.; et al. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome Res. 2011, 21, 1028–1041. [Google Scholar] [CrossRef]
- Martinez, R.; Martin-Subero, J.I.; Rohde, V.; Kirsch, M.; Alaminos, M.; Fernandez, A.F.; Ropero, S.; Schackert, G.; Esteller, M. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics 2009, 4, 255–264. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, G.Z.; Niles, R.M. Expression and function of CD9 in melanoma cells. Mol. Carcinog. 2010, 49, 85–93. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Hashida, H.; Takabayashi, A.; Tokuhara, T.; Hattori, N.; Taki, T.; Hasegawa, H.; Satoh, S.; Kobayashi, N.; Yamaoka, Y.; Miyake, M. Clinical significance of transmembrane 4 superfamily in colon cancer. Br. J. Cancer 2003, 89, 158–167. [Google Scholar] [CrossRef]
- Jee, B.; Jin, K.; Hahn, J.H.; Song, H.G.; Lee, H. Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway. Exp. Mol. Med. 2003, 35, 30–37. [Google Scholar] [CrossRef]
- White, A.; Lamb, P.W.; Barrett, J.C. Frequent downregulation of the KAI1(CD82) metastasis suppressor protein in human cancer cell lines. Oncogene 1998, 16, 3143–3149. [Google Scholar] [CrossRef]
- Dong, J.T.; Lamb, P.W.; Rinker-Schaeffer, C.W.; Vukanovic, J.; Ichikawa, T.; Isaacs, J.T.; Barrett, J.C. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995, 268, 884–886. [Google Scholar] [CrossRef]
- Durinck, K.; Speleman, F. Epigenetic regulation of neuroblastoma development. Cell Tissue Res. 2018, 372, 309–324. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.S.; Jeoung, D.I.; Kim, Y.M.; Lee, H. Promoter CpG-Site Methylation of the KAI1 Metastasis Suppressor Gene Contributes to Its Epigenetic Repression in Prostate Cancer. Prostate 2017, 77, 350–360. [Google Scholar] [CrossRef]
- Dunn, C.D.; Sulis, M.L.; Ferrando, A.A.; Greenwald, I. A conserved tetraspanin subfamily promotes Notch signaling in Caenorhabditis elegans and in human cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5907–5912. [Google Scholar] [CrossRef]
- Chastagner, P.; Rubinstein, E.; Brou, C. Ligand-activated Notch undergoes DTX4-mediated ubiquitylation and bilateral endocytosis before ADAM10 processing. Sci. Signal 2017, 10. [Google Scholar] [CrossRef]
- Owens, D.M.; Watt, F.M. Influence of beta 1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: Alpha 3 beta 1, but not alpha 2 beta l, suppresses malignant conversion. Cancer Res. 2001, 61, 5248–5254. [Google Scholar]
- Zhao, S.G.; Chen, W.S.; Li, H.; Foye, A.; Zhang, M.; Sjostrom, M.; Aggarwal, R.; Playdle, D.; Liao, A.; Alumkal, J.J.; et al. The DNA methylation landscape of advanced prostate cancer. Nat. Genet. 2020, 52, 778–789. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Farabaugh, S.M.; Ford, H.L. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J. Mammary Gland Biol. Neoplasia 2010, 15, 117–134. [Google Scholar] [CrossRef]
- Schuijers, J.; Clevers, H. Adult mammalian stem cells: The role of Wnt, Lgr5 and R-spondins. EMBO J. 2012, 31, 2685–2696. [Google Scholar] [CrossRef]
- Lenz, H.J.; Kahn, M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014, 105, 1087–1092. [Google Scholar] [CrossRef]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sanchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef]
- Nishioka, C.; Ikezoe, T.; Furihata, M.; Yang, J.; Serada, S.; Naka, T.; Nobumoto, A.; Kataoka, S.; Tsuda, M.; Udaka, K.; et al. CD34(+)/CD38(-) acute myelogenous leukemia cells aberrantly express CD82 which regulates adhesion and survival of leukemia stem cells. Int. J. Cancer 2013, 132, 2006–2019. [Google Scholar] [CrossRef]
- de Winde, C.M.; Elfrink, S.; van Spriel, A.B. Novel Insights into Membrane Targeting of B Cell Lymphoma. Trends Cancer 2017, 3, 442–453. [Google Scholar] [CrossRef]
- Vences-Catalan, F.; Duault, C.; Kuo, C.C.; Rajapaksa, R.; Levy, R.; Levy, S. CD81 as a tumor target. Biochem. Soc. Trans. 2017, 45, 531–535. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.J.; Lee, M.S.; Jin, Y.J.; Jeoung, D.; Kim, Y.M.; Lee, H. The metastasis suppressor CD82/KAI1 inhibits fibronectin adhesion-induced epithelial-to-mesenchymal transition in prostate cancer cells by repressing the associated integrin signaling. Oncotarget 2017, 8, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Tejedor, R.; Rousselle, P.; Sanchez -Madrid, F. Tetraspanins in intercellular adhesion of polarized epithelial cells: Spatial and functional relationship to integrins and cadherins. J. Cell Sci. 2001, 114, 577–587. [Google Scholar] [PubMed]
- Karpf, A.R.; Jones, D.A. Reactivating the expression of methylation silenced genes in human cancer. Oncogene 2002, 21, 5496–5503. [Google Scholar] [CrossRef] [PubMed]
- Falahi, F.; van Kruchten, M.; Martinet, N.; Hospers, G.A.; Rots, M.G. Current and upcoming approaches to exploit the reversibility of epigenetic mutations in breast cancer. Breast Cancer Res. 2014, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Asangani, I.A.; Dommeti, V.L.; Wang, X.; Malik, R.; Cieslik, M.; Yang, R.; Escara-Wilke, J.; Wilder-Romans, K.; Dhanireddy, S.; Engelke, C.; et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014, 510, 278–282. [Google Scholar] [CrossRef]
- Fernandez, S.V.; Robertson, F.M.; Pei, J.; Aburto-Chumpitaz, L.; Mu, Z.; Chu, K.; Alpaugh, R.K.; Huang, Y.; Cao, Y.; Ye, Z.; et al. Inflammatory breast cancer (IBC): Clues for targeted therapies. Breast Cancer Res. Treat. 2013, 140, 23–33. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).