Psychotherapy with Music Intervention Improves Anxiety, Depression and the Redox Status in Breast Cancer Patients Undergoing Radiotherapy: A Randomized Controlled Clinical Trial
Abstract
Simple Summary
Abstract
1. Introduction
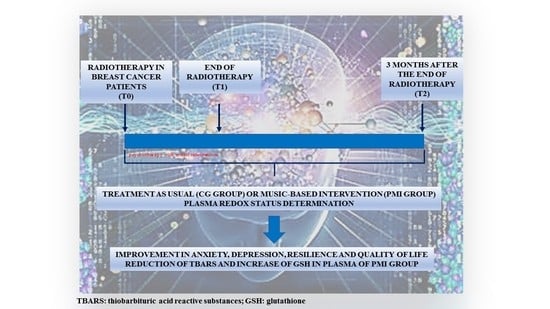
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
Outcome and Estimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Hall, S.; Rudrawar, S.; Zunk, M.; Bernaitis, N.; Arora, D.; McDermott, C.M.; Anoopkumar-Dukie, S. Protection against Radiotherapy-Induced Toxicity. Antioxidants 2016, 5, 22. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Terrazzino, S.; La Mattina, P.; Masini, L.; Caltavuturo, T.; Gambaro, G.; Canonico, P.L.; Genazzani, A.A.; Krengli, M. Common variants of eNOS and XRCC1 genes may predict acute skin toxicity in breast cancer patients receiving radiotherapy after breast conserving surgery. Radiother. Oncol. 2012, 103, 199–205. [Google Scholar] [CrossRef]
- Terrazzino, S.; Cargnin, S.; DeAntonio, L.; Pisani, C.; Masini, L.; Canonico, P.L.; Genazzani, A.A.; Krengli, M. Impact of ATM rs1801516 on late skin reactions of radiotherapy for breast cancer: Evidences from a cohort study and a trial sequential meta-analysis. PLoS ONE 2019, 14, e0225685. [Google Scholar] [CrossRef]
- Liu, C.; Lin, Q.; Yun, Z. Cellular and Molecular Mechanisms Underlying Oxygen-Dependent Radiosensitivity. Radiat. Res. 2015, 183, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Laskaratou, D.A.; Frey, B.; Candéias, S.M.; Gaipl, U.S.; Lumniczky, K.; Georgakilas, A.G. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 2016, 5, 12–33. [Google Scholar] [CrossRef]
- Xiao, C.; Miller, A.H.; Felger, J.; Mister, D.; Liu, T.; Torres, M.A. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol. Med. 2017, 47, 1733–1743. [Google Scholar] [CrossRef]
- Bouchard, L.C.; Antoni, M.H.; Blomberg, B.B.; Stagl, J.M.; Gudenkauf, L.M.; Jutagir, D.R.; Diaz, A.; Lechner, S.; Glück, S.; Derhagopian, R.P.; et al. Postsurgical Depressive Symptoms and Proinflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosom. Med. 2016, 78, 26–37. [Google Scholar] [CrossRef]
- Pitman, A.; Suleman, S.; Hyde, N.; Hodgkiss, A. Depression and anxiety in patients with cancer. BMJ 2018, 361, k1415. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Boehm, K.; Cramer, H.; Staroszynski, T.; Ostermann, T. Arts Therapies for Anxiety, Depression, and Quality of Life in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2014, 2014, 103297. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Buxton, S.; Sheffield, D. The effect of creative psychological interventions on psychological outcomes for adult cancer patients: A systematic review of randomised controlled trials. Psychooncology 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; DuPont-Reyes, M.J.; Rn, L.G.B.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA A Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhou, K.-N.; Yan, H.; Wang, D.-L.; Zhang, Y.-P. Effects of music therapy on anxiety of patients with breast cancer after radical mastectomy: A randomized clinical trial. J. Adv. Nurs. 2011, 68, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Haun, M.; Mainous, R.O.; Looney, S.W. Effect of Music on Anxiety of Women Awaiting Breast Biopsy. Behav. Med. 2001, 27, 127–132. [Google Scholar] [CrossRef]
- Hanser, S.B.; Bauer-Wu, S.; Kubicek, L.; Healey, M.; Manola, J.; Hernandez, M.; Bunnell, C. Effects of a Music Therapy Intervention on Quality of Life and Distress in Women with Metastatic Breast Cancer. J. Soc. Integr. Oncol. 2006, 4, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Xu, L.; Zhang, J.; Liu, W.; Zhu, J. Role of Arts Therapy in Patients with Breast and Gynecological Cancers: A Systematic Review and Meta-Analysis. J. Palliat. Med. 2021, 24, 443–452. [Google Scholar] [CrossRef]
- Gramaglia, C.; Gambaro, E.; Vecchi, C.; Licandro, D.; Raina, G.; Pisani, C.; Burgio, V.; Farruggio, S.; Rolla, R.; DeAntonio, L.; et al. Outcomes of music therapy interventions in cancer patients—A review of the literature. Crit. Rev. Oncol. 2019, 138, 241–254. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Robb, S.L.; Burns, D.S.; Carpenter, J.S. Reporting guidelines for music-based interventions. J. Health Psychol. 2011, 16, 342–352. [Google Scholar] [CrossRef]
- Spielberger, C.D. Inventario per L’ansia di “Stato” e di “Tratto”, Nuova Versione Italiana Dello S.T.A.I.-Forma Y; Organizzazione Speciali: Florence, Italy, 1989. [Google Scholar]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II, Beck Depression Inventory; Pearson: London, UK, 1996. [Google Scholar]
- Friborg, O.; Hjemdal, O.; Rosenvinge, J.H.; Martinussen, M. A new rating scale for adult resilience: What are the central protective resource behind healthy adjustment? Int. J. Methods Psychiatr. Res. 2003, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Grossini, E.; Farruggio, S.; Pierelli, D.; Bolzani, V.; Rossi, L.; Pollesello, P.; Monaco, C. Levosimendan Improves Oxidative Balance in Cardiogenic Shock/Low Cardiac Output Patients. J. Clin. Med. 2020, 9, 373. [Google Scholar] [CrossRef]
- Bulfone, T.; Quattrin, R.; Zanotti, R.; Regattin, L.; Brusaferro, S. Effectiveness of Music Therapy for Anxiety Reduction in Women With Breast Cancer in Chemotherapy Treatment. Holist. Nurs. Pract. 2009, 23, 238–242. [Google Scholar] [CrossRef]
- Bradt, J.; Dileo, C.; Magill, L.; Teague, A. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst. Rev. 2016, 8, CD006911. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U. The Anxiety- and Pain-Reducing Effects of Music Interventions: A Systematic Review. AORN J. 2008, 87, 780–807. [Google Scholar] [CrossRef]
- Lyman, G.H.; Greenlee, H.; Bohlke, K.; Bao, T.; DeMichele, A.M.; Deng, G.E.; Fouladbakhsh, J.M.; Gil, B.; Hershman, D.L.; Mansfield, S.; et al. Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 2647–2655. [Google Scholar] [CrossRef]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of Life, Fertility Concerns, and Behavioral Health Outcomes in Younger Breast Cancer Survivors: A Systematic Review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.C.; Paladini, M.S.; Riva, M.A.; Molteni, R. Oxidation-reduction mechanisms in psychiatric disorders: A novel target for pharmacological intervention. Pharmacol. Ther. 2020, 210, 107520. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Hanna, M.; Chang, S.-L.; Jacob, S.; Têtu, B.; Diorio, C. Evaluation of Antioxidant Intakes in Relation to Inflammatory Markers Expression Within the Normal Breast Tissue of Breast Cancer Patients. Integr. Cancer Ther. 2016, 16, 485–495. [Google Scholar] [CrossRef]
- Arathi, B.P.; Raghavendra-Rao Sowmya, P.; Kuriakose, G.C.; Shilpa, S.; Shwetha, H.J.; Kumar, S.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Fractionation and Characterization of Lycopene-Oxidation Products by LC-MS/MS (ESI)+: Elucidation of the Chemopreventive Potency of Oxidized Lycopene in Breast-Cancer Cell Lines. J. Agric. Food Chem. 2018, 66, 11362–11371. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.; Chadha, M.; Torres, B.N.; Lee, J.K.; Hylton, D.; Loewy, J.V.; Harrison, L.B. The Impact of Music Therapy on Anxiety in Cancer Patients Undergoing Simulation for Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 103–110. [Google Scholar] [CrossRef] [PubMed]


| Sample Feature | CG Group (n = 29) | PMI Group (n = 26) | p-Value |
|---|---|---|---|
| Civil Status | |||
| Married | 14 (48.2) | 19 (73.0) | |
| Unmarried | 15 (51.8) | 7 (27.0) | 0.06 |
| Education | |||
| Lower-middle | 18 (62.0) | 15 (57.7) | |
| Upper | 11 (38.0) | 11 (42.3) | 0.74 |
| Employment | |||
| Non employed/retired | 25 (86.2) | 23 (88.4) | |
| Employed | 4 (13.8) | 3 (11.6) | 0.80 |
| Previous Psychotherapy/Musical Practice | 0 | 0 | - |
| Age at Enrollment | 66.6 (10.9) | 63.0 (10.7) | 0.22 |
| Smoking a | |||
| Yes | 4 (13.8) | 3 (11.5) | 1.00 |
| No | 25 (86.2) | 23 (88.5) | |
| Stage a | |||
| Tis | 4 (13.8) | 6 (23.1) | 0.67 |
| T1 | 22 (75.9) | 18 (69.2) | |
| T2 | 3 (10.3) | 2 (7.7) | |
| Histologic Type a | |||
| Ductal carcinoma | 25 (86.2) | 22 (84.6) | 1.00 |
| Lobular carcinoma | 4 (13.8) | 4 (15.4) | |
| Radiotherapy Duration b (days) | 26.0 (5.5) | 28.4 (4.9) | 0.10 |
| STAI TOT | CG Group Mean (SD) (n = 29) | PMI Group Mean (SD) (n = 26) | F (p-Value) |
|---|---|---|---|
| T0 | 85.7 (22.0) | 82.8 (18.2) | Time effect: 7.73 (0.001) |
| T1 | 80.3 (20.7) | 81.0 (17.6) | Group effect: 3.96 (0.052) |
| T2 | 83.5 (17.8) | 62.3 (16.5) | Interaction group × time: 9.33 (0.0003) |
| Outcome | CG Group Mean (SD) (n = 29) | PMI Group Mean (SD) (n = 26) | F (p-Value) |
|---|---|---|---|
| STAI I | |||
| T0 | 43.4 (12.1) | 41.8 (10.7) | Time effect: 5.07 (0.01) |
| T1 | 39.9 (11.3) | 41.7 (9.2) | Group effect: 1.91 (0.173) |
| T2 | 41.9 (9.0) | 33.0 (8.1) | Interaction group × time: 7.87 (0.001) |
| STAI II | |||
| T0 | 42.3 (10.7) | 41.0 (9.3) | Time effect: 7.46 (0.001) |
| T1 | 40.4 (11.0) | 39.3 (9.7) | Group effect: 5.52 (0.023) |
| T2 | 41.6 (10.3) | 29.4 (10.3) | Interaction group × time: 7.71 (0.0012) |
| MADRS | |||
| T0 | 10.2 (8.2) | 8.6 (5.7) | Time effect: 5.39 (0.0074) |
| T1 | 7.4 (6.5) | 6.4 (3.5) | Group effect: 3.27 (0.076) |
| T2 | 8.1 (5.4) | 4.5 (3.4) | Interaction group × time: 2.07 (0.136) |
| BDI | |||
| T0 | 12.2 (10.7) | 10.2 (6.6) | Time effect: 3.39 (0.041) |
| T1 | 9.5 (6.4) | 6.6 (5.5) | Group effect: 4.93 (0.031) |
| T2 | 11.0 (5.8) | 5.9 (6.9) | Interaction group × time: 1.37 (0.263) |
| GSH (µM) | |||
| T0 | 3.6 (1.7) | 4.0 (1.6) | Time effect: 13.77 (<0.0001) |
| T1 | 4.6 (2.7) | 4.8 (2.0) | Group effect: 1.52 (0.223) |
| T2 | 4.8 (2.0) | 6.0 (3.8) | Interaction group × time: 0.62 (0.542) |
| TBARS (µM) | |||
| T0 | 1.9 (1.2) | 2.2 (1.7) | Time effect: 5.83 (0.005) |
| T1 | 1.8 (0.9) | 1.5 (0.8) | Group effect: 0.08 (0.782) |
| T2 | 1.4 (0.7) | 1.2 (0.4) | Interaction group × time: 0.94 (0.395) |
| Outcome | CG Group Mean (SD) (n = 29) | PMI Group Mean (SD) (n = 26) | F (p-Value) |
|---|---|---|---|
| RSA PS | |||
| T0 | 18.3 (1.6) | 17.7 (2.3) | Time effect: 0.60 (0.553) |
| T1 | 18.0 (2.3) | 18.1 (1.8) | Group effect: 3.11 (0.084) |
| T2 | 16.3 (2.7) | 19.0 (2.3) | Interaction group × time: 7.51 (0.001) |
| RSA PF | |||
| T0 | 11.5 (2.5) | 11.4 (2.6) | Time effect: 0.60 (0.551) |
| T1 | 11.2 (2.4) | 11.7 (2.8) | Group effect: 3.70 (0.060) |
| T2 | 10.3 (2.0) | 13.2 (3.3) | Interaction group × time: 9.08 (0.0004) |
| RSA SS | |||
| T0 | 11.2 (2.8) | 10.9 (2.9) | Time effect: 1.19 (0.313) |
| T1 | 10.9 (2.4) | 11.3 (3.0) | Group effect: 1.99 (0.164) |
| T2 | 10.4 (2.0) | 13.0 (4.2) | Interaction group × time: 4.76 (0.012) |
| RSA SC | |||
| T0 | 17.0 (3.6) | 15.4 (2.6) | Time effect: 0.03 (0.969) |
| T1 | 16.3 (3.8) | 16 (2.5) | Group effect: 0.06 (0.806) |
| T2 | 14.9 (3.6) | 17.3 (3.0) | Interaction group × time: 8.12 (0.001) |
| RSA FC | |||
| T0 | 20.5 (2.0) | 21.0 (2.8) | Time effect: 1.43 (0.249) |
| T1 | 19.6 (3.6) | 21.5 (2.5) | Group effect: 12.01 (0.001) |
| T2 | 17.6 (4.4) | 22.1 (4.1) | Interaction group × time: 6.42 (0.003) |
| RSA SR | |||
| T0 | 18.2 (1.9) | 19.0 (2.4) | Time effect: 0.02 (0.981) |
| T1 | 18.2 (3.7) | 19.0 (1.8) | Group effect: 7.28 (0.009) |
| T2 | 17.0 (3.8) | 20.0 (3.1) | Interaction group × time: 2.99 (0.059) |
| RSA TOT | |||
| T0 | 112.7 (15.9) | 116.5 (14.9) | Time effect: 39.55 (<0.0001) |
| T1 | 110.4 (23.2) | 116.0 (17.8) | Group effect: 12.57 (0.001) |
| T2 | 86.6 (12.8) | 104.6 (16.3) | Interaction group × time: 5.20 (0.009) |
| SF 36 PF | |||
| T0 | 79.5 (26.1) | 78.8 (30.6) | Time effect: 1.49 (0.235) |
| T1 | 75.9 (24.2) | 84.8 (20.5) | Group effect: 1.30 (0.260) |
| T2 | 71.5 (25.0) | 85.2 (20.7) | Interaction group × time: 8.41 (0.0007) |
| SF 36 RP | |||
| T0 | 53.4 (46.1) | 49.0 (44.4) | Time effect: 5.73 (0.006) |
| T1 | 56.0 (40.0) | 64.6 (33.2) | Group effect: 0.20 (0.656) |
| T2 | 56.9 (38.0) | 66.4 (31.5) | Interaction group × time: 2.78 (0.071) |
| SF 36 BP | |||
| T0 | 75.5 (22.4) | 70.2 (26.2) | Time effect: 0.96 (0.389) |
| T1 | 71.9 (20.3) | 77.2 (20.7) | Group effect: 0.30 (0.585) |
| T2 | 70.8 (21.9) | 80.0 (19.5) | Interaction group × time: 7.93 (0.001) |
| SF 36 GH | |||
| T0 | 63.9 (15.9) | 61.7 (17.1) | Time effect: 2.51 (0.091) |
| T1 | 60.5 (15.0) | 72.3 (11.5) | Group effect: 4.91 (0.031) |
| T2 | 59.3 (16.8) | 73.8 (11.6) | Interaction group × time: 10.87 (0.0001) |
| SF 36 VT | |||
| T0 | 64.3 (20.3) | 61.0 (18.2) | Time effect: 4.16 (0.021) |
| T1 | 61.4 (19.7) | 72.8 (11.4) | Group effect: 3.26 (0.076) |
| T2 | 59.9 (20.8) | 75.5 (12.0) | Interaction group × time: 13.89 (<0.0001) |
| SF 36 SF | |||
| T0 | 69.6 (17.0) | 61.8 (14.4) | Time effect: 1.46 (0.242) |
| T1 | 61.0 (13.2) | 76.3 (12.2) | Group effect: 6.79 (0.012) |
| T2 | 59.3 (14.1) | 77.4 (10.6) | Interaction group × time: 33.25 (<0.0001) |
| SF 36 RE | |||
| T0 | 66.4 (33.4) | 67.5 (29.1) | Time effect: 0.58 (0.562) |
| T1 | 59.6 (24.1) | 79.1 (22.1) | Group effect: 4.09 (0.048) |
| T2 | 59.6 (24.7) | 79.4 (20.6) | Interaction group × time: 8.53 (0.0006) |
| SF 36 MH | |||
| T0 | 69.8 (20.5) | 65.6 (17.8) | Time effect: 1.31 (0.279) |
| T1 | 63.9 (21.4) | 75.5 (16.1) | Group effect: 2.21 (0.143) |
| T2 | 63.6 (21.5) | 77.7 (16.0) | Interaction group × time: 13.51 (<0.0001) |
| IL-6 (pg/mL) | |||
| T0 | 6.3 (1.8) | 6.0 (2.1) | Time effect: 0.43 (0.652) |
| T1 | 6.0 (1.6) | 6.2 (2.3) | Group effect: 0.37 (0.545) |
| T2 | 6.2 (1.9) | 5.5 (2.4) | Interaction group × time: 0.43 (0.652) |
| TNF α (pg/mL) | |||
| T0 | 5.4 (3.1) | 4.5 (3.0) | Time effect: 2.82 (0.068) |
| T1 | 3.2 (2.4) | 4.6 (3.0) | Group effect: 0.00 (0.953) |
| T2 | 3.4 (2.1) | 3.2 (3.1) | Interaction group × time: 1.72 (0.189) |
| ß CAROTENE (nM) | |||
| T0 | 624.3 (361.3) | 871.8 (495.2) | Time effect: 4.23 (0.020) |
| T1 | 721.8 (387.5) | 931 (598.2) | Group effect: 3.45 (0.069) |
| T2 | 869.6 (708.0) | 1136.4 (1033.9) | Interaction group × time: 0.12 (0.891) |
| LYCOPENE (nM) | |||
| T0 | 1201.4 (593.8) | 1411.7 (678.1) | Time effect: 4.76 (0.0125) |
| T1 | 1105.4 (531.6) | 1437.5 (715.6) | Group effect: 0.357 (0.86) |
| T2 | 1693.0 (1215.9) | 1644.0 (922.2) | Interaction group × time: 1.41 (0.254) |
| VITAMIN A (nM) | |||
| T0 | 1984.2 (529.0) | 2081.6 (550.4) | Time effect: 1.32 (0.275) |
| T1 | 1919 (804.7) | 1947.7 (518.5) | Group effect: 0.00 (0.951) |
| T2 | 2047.7 (549.3) | 1948.1 (531.8) | Interaction group × time: 1.59 (0.213) |
| γ TOCOPHEROL (nM) | |||
| T0 | 2088.8 (1459.0) | 2496.3 (1438.4) | Time effect: 2.55 (0.087) |
| T1 | 2119.5 (1147.8) | 2479.0 (1474.2) | Group effect: 2.63 (0.111) |
| T2 | 1590.4 (702.0) | 2287.2 (1401.3) | Interaction group × time: 0.57 (0.568) |
| α TOCOPHEROL (nM) | |||
| T0 | 34161.1 (6834.9) | 34254.3 (6451.9) | Time effect: 0.59 (0.558) |
| T1 | 34071.5 (6230.5) | 34067.2 (6796.5) | Group effect: 0.00 (0.970) |
| T2 | 33383.1 (7854.5) | 33480.7 (7455.3) | Interaction group × time: 0.00 (0.996) |
| REACTIVE C PROTEIN (mg/dL) | |||
| T0 | 0.3 (0.5) | 0.2 (0.2) | Time effect: 1.83 (0.170) |
| T1 | 0.5 (0.9) | 0.3 (0.6) | Group effect: 0.77 (0.384) |
| T2 | 0.3 (0.5) | 0.3 (0.4) | Interaction group × time: 0.55 (0.582) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeppegno, P.; Krengli, M.; Ferrante, D.; Bagnati, M.; Burgio, V.; Farruggio, S.; Rolla, R.; Gramaglia, C.; Grossini, E. Psychotherapy with Music Intervention Improves Anxiety, Depression and the Redox Status in Breast Cancer Patients Undergoing Radiotherapy: A Randomized Controlled Clinical Trial. Cancers 2021, 13, 1752. https://doi.org/10.3390/cancers13081752
Zeppegno P, Krengli M, Ferrante D, Bagnati M, Burgio V, Farruggio S, Rolla R, Gramaglia C, Grossini E. Psychotherapy with Music Intervention Improves Anxiety, Depression and the Redox Status in Breast Cancer Patients Undergoing Radiotherapy: A Randomized Controlled Clinical Trial. Cancers. 2021; 13(8):1752. https://doi.org/10.3390/cancers13081752
Chicago/Turabian StyleZeppegno, Patrizia, Marco Krengli, Daniela Ferrante, Marco Bagnati, Vincenzo Burgio, Serena Farruggio, Roberta Rolla, Carla Gramaglia, and Elena Grossini. 2021. "Psychotherapy with Music Intervention Improves Anxiety, Depression and the Redox Status in Breast Cancer Patients Undergoing Radiotherapy: A Randomized Controlled Clinical Trial" Cancers 13, no. 8: 1752. https://doi.org/10.3390/cancers13081752
APA StyleZeppegno, P., Krengli, M., Ferrante, D., Bagnati, M., Burgio, V., Farruggio, S., Rolla, R., Gramaglia, C., & Grossini, E. (2021). Psychotherapy with Music Intervention Improves Anxiety, Depression and the Redox Status in Breast Cancer Patients Undergoing Radiotherapy: A Randomized Controlled Clinical Trial. Cancers, 13(8), 1752. https://doi.org/10.3390/cancers13081752