Simple Summary
Head and neck squamous cell carcinomas (HNSCCs) are highly heterogeneous human malignancies associated with genetic and environmental factors. In HNSCCs, cancer stem cells (CSCs) provide the plasticity for cancer cell progression, metastasis, therapeutic resistance, and recurrence. During carcinogenesis, microRNAs (miRNAs) play important roles in regulating the maintenance and acquisition of cancer stem cell features. Therefore, in this review, we summarize the roles of miRNAs in regulating the cancer stemness of HNSCCs to provide potential therapeutic applications.
Abstract
Head and neck squamous cell carcinomas (HNSCCs) are epithelial malignancies with 5-year overall survival rates of approximately 40–50%. Emerging evidence indicates that a small population of cells in HNSCC patients, named cancer stem cells (CSCs), play vital roles in the processes of tumor initiation, progression, metastasis, immune evasion, chemo-/radioresistance, and recurrence. The acquisition of stem-like properties of cancer cells further provides cellular plasticity for stress adaptation and contributes to therapeutic resistance, resulting in a worse clinical outcome. Thus, targeting cancer stemness is fundamental for cancer treatment. MicroRNAs (miRNAs) are known to regulate stem cell features in the development and tissue regeneration through a miRNA–target interactive network. In HNSCCs, miRNAs act as tumor suppressors and/or oncogenes to modulate cancer stemness and therapeutic efficacy by regulating the CSC-specific tumor microenvironment (TME) and signaling pathways, such as epithelial-to-mesenchymal transition (EMT), Wnt/β-catenin signaling, and epidermal growth factor receptor (EGFR) or insulin-like growth factor 1 receptor (IGF1R) signaling pathways. Owing to a deeper understanding of disease-relevant miRNAs and advances in in vivo delivery systems, the administration of miRNA-based therapeutics is feasible and safe in humans, with encouraging efficacy results in early-phase clinical trials. In this review, we summarize the present findings to better understand the mechanical actions of miRNAs in maintaining CSCs and acquiring the stem-like features of cancer cells during HNSCC pathogenesis.
1. Introduction
Cancer is responsible for about 30% of all premature deaths from non-communicable diseases (NCDs) in adults aged approximately 30–69 years [1]. In 2018, there were 18.1 million people who suffered from cancer worldwide, and 9.6 million of them died from cancer (around one in six deaths globally). In addition, 354,864 (2% of all cancer sites) of new cases were of the lip and oral cavity, accounting for 177,384 (1.9% of all cancer sites) deaths [1,2]. Head and neck squamous cell carcinomas (HNSCCs) are epithelial malignancies located in the oral cavity, nasal cavity, pharynx (nasopharynx, oropharynx, and hypopharynx), and larynx [3,4]. HNSCC subtypes include oral SCCs (OSCCs), laryngeal SCCs (LSCCs), nasopharyngeal carcinomas (NPCs), and oropharyngeal SCCs (OPSCCs) [5,6]. It should be noted that with tongue squamous cell carcinomas, an OSCC includes the anterior two-thirds of the tongue (anterior to the circumvallate papillae) and an OPSCC consists of the base (or posterior one-third) of the tongue [7]. The incidence of HNSCC continues to rise and is anticipated to increase by 30% with 1.08 million new cases annually by 2030 [2,8,9]. Risk factors for the malignant incidence of HNSCCs include tobacco use, alcohol abuse, and human papillomavirus (HPV) infection [3,6]. Signs and symptoms can manifest as a lesion in the nose, mouth, or throat; a lump or neck mass; and ear discomfort; and functional abnormalities such as difficulty swallowing and/or chewing are often found in the later stages of these diseases [3]. The tumor, node, and metastasis (TNM) staging system is used for clinical staging and as a basis for treatment choice [10]. Therapeutic approaches include surgery, radiotherapy, chemoradiotherapy, a combination of surgery with radiotherapy or chemotherapy, and a combination of surgery with adjuvant chemotherapy and radiotherapy. Chemoradiotherapy may be taken as adjuvant therapy in advanced stages [11]. Unfortunately, despite several treatment options being available, outcomes of HNSCC treatment remain poor, patients generally develop resistance, and, as a result, the five-year overall survival rates of HNSCC patients are approximately 40–50% [6,12]. Advanced approaches have been developed by applying immunotherapy or combined immunotherapy treatment to treat resistant and recurrent cases [13].
The major obstacle in cancer therapy is tumor heterogeneity. Cancer stem cells (CSCs) are small populations of cancer cells and are well-known for their association with cancer resistance, relapse, tumorigenesis, and poor clinical outcomes in HNSCCs, which has promoted the development of novel and effective therapeutic protocols for better clinical outcomes [5,14]. Therefore, targeting CSCs has become an attractive approach for potential strategies to treat HNSCCs [15,16]. The abnormal activation of signaling cassettes, genetic and epigenetic modification, and microRNA (miR or miRNA) regulation are central regulators of CSC malignancy [17,18,19]. miRNAs work as hub regulators to modulate cell functions by binding to multiple 3′-untranslated regions (3′-UTRs) of target messenger RNAs (mRNAs) and cause the translational inhibition and/or degradation of transcripts [19,20,21]. Therefore, in this review, we address the roles of miRNAs in regulating the cancer stemness of HNSCCs.
2. CSCs and Cancer Stemness
CSCs are a minor population of cells within tumor tissues with a tumor-initiating capacity [22] and stem-like features, including self-renewal [23,24] and asymmetric cell division (ACD) [25]. Under chemotherapy, the cycling rates of CSCs slow and they enter the G0 phase in order to survive, accounting for therapeutic heterogeneity [26,27,28]. Cancer patients with higher stem cell signatures present poorly differentiated histological properties and are associated with a worse clinical outcome [29]. CSCs are heterogeneous populations. In colorectal cancer (CRC) tissues, prominin-1 (CD133) is the first molecular marker used to isolate colorectal cancer stem cells (CRCSCs) [30,31]. The epithelial-specific antigen (ESA)(+)/CD44 molecule and Indian blood group (CD44)(+) CRCSCs are associated with tumor recurrence after chemotherapy [32]. Dipeptidyl peptidase 4 (CD26)(+) CRCSCs enriched from CD133(+)/CD44(+) cells drive tumor metastasis [33]. Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5)(+) CRCSCs are considered to be responsible for liver metastasis [34]. CD24 molecule (CD24) and activated leukocyte cell adhesion molecule (CD166) surface antigens are often combined with CD44 or CD133 for the identification and separation of CRCSCs [35]. In HNSCC, CSCs are grouped in accordance with the expression of surface markers such as CD44+ and aldehyde dehydrogenase 1+ (ALDH1+). CD44 mediates the adhesion, migration, and metastasis of CSCs [36], while ALDH1 ameliorate oxidative stress under therapeutic regimens such as platinum, taxanes, and oxazaphosphorine [37,38].
Despite the fact that the origins of CSCs have been linked to genetic mutation, epigenetic alterations, and unrestrained signaling pathways for the normal stem cells and progenitor cells [39,40], CSC properties would be induced or maintained by inflammatory mediators. Inflammatory cytokines and chemokines secreted by CSCs, including interleukin (IL)-1, IL-4, IL-6, and IL-8, sustain CSC niches in an autocrine manner [41,42,43,44]. Besides, the expression of IL-8 promotes the migratory and tube-forming capacities of endothelial cells [44]. IL-6 is also involved in cancer metastasis [45]. IL-6 activates Janus kinase 1 (JAK1) and phosphorylates programmed death–ligand 1(PD-L1) and promotes PD-L1 protein stability [46]. CSCs also enhance PD-L1 expression to escape immune surveillance, thereby enriching the CSC subpopulation [47,48,49]. In addition to secretory proteins, CSCs create an immunosuppressive, pro-tumoral microenvironment by releasing CSC exosomes for cancer progression [50,51].
To target CSCs, researchers have focused on deciphering how cancer cells acquire stemness properties. The major mechanisms involve the expression of genes associated with early development and aberrant intracellular signaling activation. Activation of stemness regulators sustains the stemness properties of HNSCCs, including the MYC proto-oncogene, bHLH transcription factor (MYC), sex-determining region Y-box 2 (SOX2), Nanog homeobox (NANOG), Krüppel-like factor 4 (KLF4), octamer-binding transcription factor 4 (OCT4), high-mobility group AT-hook 2 (HMGA2), cytokines, and epithelial-to-mesenchymal transition (EMT) transcription factors (EMT-TFs) [52,53,54,55,56,57,58]. On the other hand, abnormal signaling activation in Notch, Wnt(wingless)/β-catenin, transforming growth factor-β (TGF-β), Janus-activated kinase/signal transducer and activator of transcription (JAK/STAT), nuclear factor-κB (NF-κB), and the sonic hedgehog (SHH) pathway maintains cancer stemness [59,60,61,62,63,64]. Therefore, the rationale for identifying combinatorial therapeutic strategies combating CSC is intriguing.
3. miRNAs
miRNAs are non-coding (nc) RNA components with approximately 21–23 nucleotides that bind to and repress complementary mRNA targets [21,65]. Previously, ncRNAs were only considered to be evolutionary junk, but emerging evidence has indicated that miRNAs have important cellular functional roles and act as post-translational regulators [21,65,66,67]. miRNAs control around 30% of human genes, and about half of those genes are tumor associated or situated in vulnerable loci [68,69,70]; other studies have even suggested that miRNAs can regulate the expression of more than 60% of human genes [71,72]. miRNA expression is modulated by several mechanisms, such as transcriptional control, epigenetic modulation, and post-transcriptional regulation [67,73,74]. On the other hand, the biogenesis of miRNAs can mainly be divided into six steps: (1) RNA polymerase II transcribes miRNA genes into primary (pri)-miRNAs in the nucleus [75], (2) intermediate precursor (pre)-miRNA is released by pri-miRNA after being processed by the Drosha/DiGeorge syndrome critical region 8 (DGCR8) complex [75,76], (3) pre-miRNA bonds to exportin-5 (Exp5)/ras-related nuclear protein (Ran)-guanosine 5′-triphosphate (GTP) complex and is transferred to the cytoplasm [77], (4) the Dicer/(HIV-1 transactivating response (TAR)) RNA-binding protein (TRBP)/PACT complex turns pre-miRNA into double-stranded (ds) RNA in the cytoplasm [78,79,80], (5) the miRNA duplex is released into single strands by helicase [81], and (6) the miRNA-induced silencing complex (RISC) is bound to the 3′-UTRs of target mRNAs via the seed region of miRNA and subsequently triggers inhibition of the translation or degradation of target mRNAs [82]. The seed region of an miRNA (also known as the seed sequence) is a short, conserved sequence at nucleotides 2–8 at the 5′ end of the miRNA [83,84,85]. Therefore, the miRNA target prediction tools rely on an algorithm with the thermodynamics-based modeling of RNA, i.e., RNA duplex interactions with comparative sequence analysis to evaluate the seed region matching to the mRNAs [86].
The squamous epithelium covering the oral mucosa and skin depends on epithelial stem cells for tissue renewal [87]. In the oral mucosa, the basal cell layer harbors the self-renewing stem cells and their immediate descendants, the transient amplifying progenitor cells, to produce expanded terminally differentiating cells [88]. The terminally differentiating cells then leave the basal layer and form the outer layers to maintain the oral mucosa integrity. Therefore, stem cells and the proper controls between the phase transition of stem cells and differentiating cells are critical to maintaining tissue homeostasis. Evidence has shown that miRNA expression patterns control the epithelium stem cells’ characteristics. For example, Peng et al. indicated that the miR-103/107 family is highly expressed in the stem-cell-enriched limbal epithelium. The miR-103/107 family regulates and integrates these stem cell characteristics, thereby sustaining tissue maintenance and regeneration [89]. Moreover, studies have also indicated that the epidermal-specific deletion of enzymes responsible for miRNA maturation, such as DICER, Drosha, and DGCR8, severely impairs the homeostasis and morphogenesis of the epidermis [90,91,92,93]. These results indicate that miRNA expression is critical for the proper development of the epidermis and oral mucosa. Moreover, emerging evidence also highlights the importance of miRNAs in regulating carcinogenesis and CSCs. Better characterizations of miRNAs in regulating the stemness features of CSCs will contribute to better cancer treatment strategies (Figure 1).
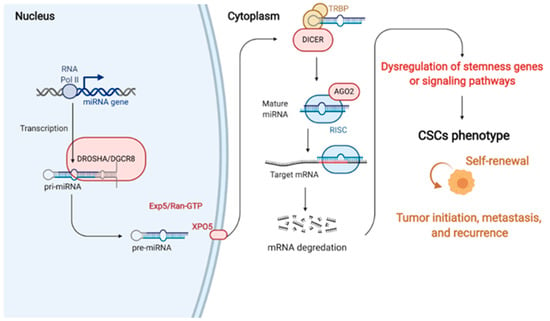
Figure 1.
The generations and roles of miRNAs in cancer stem cell (CSC) regulation. In miRNA generation, miRNA genes are commonly transcribed by RNA Pol II in the nucleus. This transcription, which is cleaved by Drosha, produces a multiprotein complex with the DGCR8 protein. Cleavage induces pre-miRNA binding to the nuclear export factor EXO5 and then transported from the nucleus to the cytoplasm. In the cytoplasm, Dicer (another RNase III), which forms a complex with the double-stranded RNA-binding protein TRBP, cuts out the hairpin and produces an RNA duplex with approximately 22 nucleotides. The RNA duplex is dissociated to a single strand via AGO2 mediation, incorporated into the RISC, and binds to 3′UTR of the target mRNA to suppress the gene expression. Therefore, miRNAs can regulate cancer stemness properties and phenotypes by targeting the critical genes that control the activation of signaling pathways, transcriptional factors, and secreted factors. RNA Pol II, RNA polymerase II; pri-miRNAs, primary miRNAs; pre-miRNAs, precursor miRNAs; EXO5, exportin 5; AGO2, argonaute 2; RISC, miRNA-induced silencing complex; mRNA, messenger RNA; miRNA, microRNA (created with Biorender.com (accessed on 30 January 2021)).
4. miRNAs in Regulating Cancer Stemness
CSCs maintain and acquire stemness features through complex mechanisms, including abnormal activation of oncogenes, cytokines, signaling pathways, and EMT-TFs, as mentioned in Section 2 above. Studies indicated that miRNAs that regulate cancer stemness mainly depend on post-translational regulation to modulate activation of those stemness-related factors. Several studies have proven that abnormal miRNA expressions can act as oncogenes, tumor suppressors, or dual-role regulators [94,95]. All of these data highlight the potential for targeting miRNAs to eradicate CSCs, and researchers are working on anti-miRNA drugs and are searching for diagnostic miRNAs [96,97,98]. miRNAs have been applied as biomarkers to determine cancer prognoses and diagnoses due to their stability [99,100,101]. Xia et al. indicated that various tumor mutational burden levels had different miRNA expression patterns in HNSCC patients [102], and correlations between miRNA prognostic values as applied to HNSCCs have generated significant interest among researchers [103,104,105].
4.1. miRNAs as Oncomirs
As oncomirs, miRNAs can act as oncogenic miRNAs that promote biological processes such as proliferation, migration, angiogenesis, invasion, EMT, and stemness [106,107,108,109,110,111]. Oncomirs regulate cancer stemness through targeting their downstream targets which results in activation of stemness-related factors and signaling pathways. Therefore, oncomirs were shown to enhance tumor initiation and progression by modifying CSC properties such as self-renewal, tumorigenesis, drug resistance, and signaling pathways in cancer [112,113,114,115].
Several mechanisms for the oncogenicity of HNSCCs can be affected by miRNA presence. For example, miR-125a enhances the proliferation, migration, invasion, and stemness maintenance in cancer cells via suppressing p53 expression [116]. The overexpression of p53 makes cell viability significantly decrease and induces cell cycle arrest at the G0/G1 phase [116]. miR-134 suppresses E-cadherin expression and promotes OSCC cell progression through targeting programmed cell death 7 (PDCD7) [117]. E-cadherin can suppress cancer stemness by regulating the expressions of pluripotent genes (MYC, NESTIN, POU5F1, and SOX2) via the activation of Wnt/β-catenin signaling [118]. On the other hand, by suppressing the expression of the WW domain-containing oxidoreductase (WWOX) gene, miR-134 can trigger oncogenicity and metastasis in HNSCCs [119]. WWOX is a tumor stemness suppressor that reduces the self-renewal ability of CSCs, differentiation potential, in vivo tumorigenic capability, and multidrug resistance [120]. Consistently, the downregulation of WWOX was indicated to induce EMT, enhance stemness, and increase chemoresistance in breast cancer [121]. miR-1246 confers tumorigenicity and affects cancer stemness in OSCC through suppressing cyclin-G2 (CCNG2) [122] CCNG2 has been shown to suppress EMT by disrupting Wnt/β-catenin signaling [123], which has been proven to be involved in the migration and invasion of OSCCs [124].
Protocadherins are cell–cell adhesion molecules. The loss of protocadherins may contribute to cancer development not only by altering cell–cell adhesion but also by enhancing proliferation and EMT via activating the Wnt signaling pathway [125,126]. With LSCC, Giefing et al. showed that protocadherin 17 (PCDH-17) acts as a tumor suppressor gene [127]. Inhibition of miR-196b can suppress cell proliferation, migration, and invasion abilities but promote apoptosis by targeting PCDH-17 in LSCC cells [128]. Moreover, LSCC patients with low expression of miR-196b and high expression of PCDH-17 were shown to have an increase in the 5-year survival rates [128]. miR-19a and miR-424 inhibit the TGF-β type III receptor (TGFBR3), also known as β-glycan, which results in promoting the EMT of tongue squamous carcinoma cells [108]. Other studies have also indicated that miR-19a promotes migration and EMT in gastric cancer, CRC, and lung cancer [129,130,131].
Mitogen-activated protein kinase (MAPK) signaling cascades are critical signal pathways related to EMT, which promotes cancer cell progression and metastasis in CSCs [132]. miR-106A-5p facilitates a malignant phenotype by acting as an autophagic suppressor through targeting BTG anti-proliferation factor 3 (BTG3) and activates autophagy-regulating MAPK signaling in NPC [133]. MYC target 1 (MYCT1), a direct target gene of MYC, is a novel candidate tumor suppressor gene cloned from LSCC [134,135]. MYCT1 protein suppresses miR-629-3p expression by reducing specificity protein 1 (SP1) expression. SP1 is also a TF for miR-629-3p, and its suppression enhances the expression of miR-629-3p’s downstream target, epithelial splicing regulatory protein 2 (ESRP2). Taken together, MYCT1 protein suppresses the EMT of laryngeal cancer via the SP1/miR-629-3p/ESRP2 pathway [136] Previous studies have shown that oral CSCs switch from expressing the CD44-variant form (CD44v) to expressing the standard form (CD44s) during the acquisition of cisplatin resistance, which results in EMT induction [137] During the process, CD44s induces miR-629-3p expression, which inhibits apoptotic cell death under cisplatin treatment conditions and promotes cell migration in HNSCCs [138]. Therefore, miR-629-3p serves as a therapeutic target to reverse chemotherapy resistance. Altogether, the miRNAs as oncomirs that regulate the stemness process of HNSCC are summarized in Table 1.

Table 1.
miRNAs as oncomirs that are involved in the stemness of head and neck squamous cell carcinomas (HNSCCs).
4.2. miRNAs as Tumor Suppressors
In contrast, tumor suppressor miRNAs were found to suppress activation of stemness factors, thereby decreasing CSC populations and tumor progression. Studies indicated that expressions of tumor suppressor miRNAs were commonly reduced in tumor samples. Conversely, their corresponding oncogenic downstream targets were activated, thereby activating stemness factors and enhancing the ability of cancer cells to acquire stemness features.
4.2.1. miRNAs in HNSCC
The miRNA let-7 family controls normal cellular development and differentiation, and a reduction in let-7 contributes to carcinogenesis via the upregulation of oncogenic downstream targets and stemness properties [99]. Therefore, members of the let-7 family are considered to be tumor suppressors for various cancers [139]. Ten members of the human let-7 family have been identified, i.e., let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i, miR-98, and miR-202, which share the same seed region sequence [140,141]. Expressions of let-7 family members decrease in HNSCCs patients, and among them, let-7i has been shown to most significantly suppress the expression of the chromatin modifier AT-rich interacting domain 3B (ARID3B). By suppressing let-7i expression, cells enhance ARID3B expression and acquire stemness features by activating embryonic SC (ESC)-specific genes such as POU5F1, NANOG, and SOX2 via histone modifications [142]. The study also indicated that the EMT factor twist family bHLH transcription factor 1 (Twist1) cooperates with B lymphoma Mo-MLV insertion region 1 (BMI1), suppresses let-7i expression, and contributes to stem-like properties, thus enabling mesenchymal movements [143]. In OSCC of the tongue, ALDH1+ cells with cancer stemness characteristics show decreased expression of let-7a and high expressions of NANOG and POU5F1. let-7a overexpression in ALDH1+ cells further inhibited tumor formation and metastasis in vivo, suggesting that the let-7a gene plays an important role in modulating tumorigenesis stemness of HNSCC cells [144].
Moreover, radioresistance poses a major challenge in HNSCC treatment, in which CSCs are relatively radioresistant owing to different intrinsic and extrinsic factors [145]. Evidence has indicated that miRNAs might regulate not only stemness properties but also radiotherapy response. For example, let-7c contributes to oral cancer stemness and radio/chemoresistance through suppressing CXCL8 (IL-8) [146]. Similarly, CXCL8 was identified as a direct target of miR-203, and the reduction in miR-203 promoted radioresistance by activating IL-8/AKT serine/threonine kinase 1 (AKT) signaling in NPC cells [147]. The low expression of miR-203 was also showed to enhance EMT and result in intrinsic radioresistance of HNSCC, which could enable identification and treatment modification of radioresistant tumors [148]. miR-520b attenuates cell mobility via EMT suppression and suppressed spheroid cell formation, as well as reduced expressions of multiple stemness regulators (Nestin, Twist1, NANOG, OCT4) through targeting suppression of CD44 in HNSCC cells [149]. Moreover, miR-520b also sensitized cells to therapeutic drugs and irradiation through targeting CD44 [149]. CD44 is an adhesion molecule expressed in CSCs, which interacts with a glutamate–cystine transporter and controls the intracellular level of reduced glutathione (GSH). Therefore, CSCs with high CD44 expression show an enhanced capacity for GSH synthesis, resulting in higher reactive oxygen species (ROS) defense and radiotherapy resistance [150,151]. Therefore, miR-520b suppresses CD44 and not only inhibits cancer stemness and multiple malignant properties but also sensitizes cells to chemoradiotherapy [149].
miR-101 acts as a potent tumor suppressor, and its downregulation is associated with oral carcinomas [152]. In HNSCCs, low expressions of miR-101 upregulate the oncogene Zeste homolog 2 (EZH2), which subsequently downregulates another tumor suppressor gene rap1GAP, which promotes HNSCC progression. EZH2 is a histone methyltransferase that belongs to the polycomb repressive complex 2 (PRC2) family that facilitates the trimethylation of H3K27 on the rap1GAP promoter to suppress its activation [153,154]. EZH2 can regulate cancer stemness by mediating the NOTCH1 activator and signaling to promote the initiation and growth of SCs [155]. EZH2 was shown to promote cell migration, invasion, and metastasis, and EMT, thereby enhancing cellular plasticity for oral tongue squamous cell carcinomas [156,157]. The miR-29 family is also significantly downregulated in HNSCC patients [158]. Moreover, miR-29b suppresses DNA methyltransferase 3 beta (DNMT3B), resulting in inhibited EMT and promoted invasiveness of HNSCC cell lines through restoring E-cadherin expression by the demethylation of the promoter region [159].
miR-204-5p is a tumor suppressor in HNSCCs, which inhibits tumor growth, metastasis, and stemness by suppressing the signal transducer and activator of transcription 3 (STAT3) signaling and EMT via targeting SNAI2, SUZ12, HDAC1, and JAK2 [160]. STAT3 is a critical regulator of CSCs because of its relationship with EMT as one of the major proposed mechanisms for generating CSCs. It also plays a critical role in the angiogenesis and regulation of the tumor microenvironment (TME), which provides signals for differentiation or proliferation, especially through its involvement in the inflammatory NF-κB pathway [161]. miR-124 was observed to target STAT3 to repress tumor growth and metastasis in NPCs [162]. miR-365-3p targets the ETS homologous factor (EHF), a keratin 16 (KRT16) transcription factor, thereby suppressing KRT16 expression. The decrease in KRT16 further enhances the lysosomal degradation of β5-integrin and c-Met, leading to inhibition of their downstream Src/STAT3 signaling. In OSCC cells, miR-365-3p decreases migration, invasion, metastasis, cancer stemness, and chemoresistance via inhibiting Src/STAT3 signaling [163].
4.2.2. miRNAs in OSCC
In OSCC, let-7d was shown to function as a negative regulator of EMT and exhibited chemoresistant properties and silencing of enhanced mesenchymal, stem-like, and chemoresistant traits through suppressing TWIST1 and Snail family transcriptional repressor 1 (SNAI1) expression [164]. miR-98 acts as a tumor suppressor, which reduces tumor cell growth and metastasis through targeting the insulin-like growth factor 1 receptor (IGF1R) in OSCCs [165]. IGF1R is critical in the human embryonic niche for self-renewal and SC expansion and regulates SC maintenance in normal tissue processes [166,167]. Moreover, the IGF1R pathway is critical for EMT induction/maintenance and the expansion of cancer stem-like cells [167,168,169,170]. In HNSCCs, Leong et al. indicated increased epidermal growth factor (EGF) receptor (EGFR) and IGF1R expressions and phosphorylation, which increased the activation of downstream pathways in ALDH1+ cells compared to ALDH- cells. Importantly, treatment with EGFR and IGF1R inhibitors reduced SC fractions, implying that the IGF1R is critical for maintaining HNSCC CSCs [171].
miR-139-5p overexpression inhibits OSCC cell proliferation, in vitro mobility of OSCC, and the expression of WNT-responsive MYC, CCND1, and BCL2 through suppressing CXC chemokine receptor 4 (CXCR4) [172]. MYC-related signaling regulates CSC chemotherapeutic resistance and CRC organoids [173]. In addition, miR-139 triggers the apoptosis of an oral cancer cell line, Tca8113 cells, through the Akt signaling pathway [174]. Another study suggested that miR-139-5p suppresses the tumorigenesis process and OSCC cell mobility by targeting homeobox (HOX)-A9 (HOXA9) [175]. HOX genes can encode master regulatory TFs that regulate SCs during development in various cancers; HOX4 and HOXA9 were observed to upregulate expression of the SC marker ALDH1 and increase SC self-renewal [176]. Similarly, miR-495 was observed to suppress EMT, proliferation, migration, and invasion and promote the apoptosis of CSCs by inhibiting the HOXC6-mediated TGF-β signaling pathway in OSCCs [177]. Other studies have also indicated that miR-495 significantly inhibits cell proliferation, migration, invasion, and EMT through the miR-495/IGF1/AKT signaling axis or by targeting NOTCH1 in OSCCs [178,179].
The miR-34 family contains three members, miR-34a, miR-34b, and miR-34c, clustered on two different chromosomal loci on chromosomes 1p36.22 (Mir34a) and 11q23.1 (Mir34b/c) [180,181]. In OSCCs and OPSCCs, miR-34a is described as a regulator of SCs [182]. miR-34a was observed to be downregulated in HNSCC tumors and cell lines [183]. Sun et al. observed that CSC enrichment by a spheroid culture showed significant downregulation of miR-34a expression. Furthermore, the restoration of miR-34a significantly inhibited EMT formation of the CSC phenotype and functionally reduced clonogenic and invasive capacities in HNSCC cell lines [184]. During the EMT process, cancer cells acquire the ability for tumor metastasis, invasion, drug resistance, and recurrence, which are associated with CSC functions. Gregory et al. first indicated that miR-205 and the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141, and miR-429) suppressed the EMT by targeting zinc finger E-box binding homeobox 1 (ZEB1) and Smad-interacting protein 1 (SIP1, also known as ZEB2) in breast cancer [185]. Similarly, the miR-200 family was indicated to enhance EMT through a reciprocal feedback loop between the miR-200 family and ZEB1 in HNSCCs [186,187]. Recent emerging evidence has indicated that the EMT process might not simply be divided into a dichotomous system but may actually be an EMT spectrum. The epithelial/mesenchymal (E/M) hybrid status provides plasticity for cells with mixed E and M characteristics [188]. Lu et al. devised a unique model of miRNA-based coupled chimeric modules to elucidate the core regulatory network that underlies the hybrid E/M status. In that model system, two double-negative feedback loops of miR-34/SNAI1 and the miR-200/ZEB mutually regulate the E and M phenotypes and the hybrid phenotype. miR-200/ZEB was indicated to act as the decision-making module for cancer cells to undergo partial or complete EMT [189,190].
miR-22 inhibits phosphatidylinositol 3-kinase (PI3K)/Akt/NF-κB signaling via downregulating activators such as S100A8, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), which implies a tumor suppressor role of miR-22 in tongue squamous cell carcinoma [191]. PI3K is well known as a regulator for stemness-related signaling, including RAS/mitogen-activated protein kinase (MAPK) [192,193], NF-κB [194,195], Wnt/β-catenin [196,197], and TGF-β [198,199,200,201,202]. The NF-κB pathway maintains stemness by regulating many tumor-promoting inflammation-related cytokines, like tumor necrosis factor (TNF)-α [203], IL-1 [204], IL-6 [205,206], monocyte chemoattractant protein 1 (MCP1) [207], cytochrome oxidase subunit 2 (COX2) [203], and inducible nitric oxide synthase (iNOS) [203,208]. Simultaneously, the NF-κB pathway downregulates the expression of matrix metalloproteinases (MMPs) to increase tumor cell invasion [209]. miR-22 also targets the expression of node-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) and suppresses OSCC cell growth, migration, and invasion [210]. The NLRP3 inflammasome was associated with the carcinogenesis and CSC self-renewal activation in HNSCC patients with upregulated expression of BMI1, ALDH1, and CD44 [211]. The overexpression of miR-22 results in reduced cell viability and an increase in the OSCC cell apoptotic rate by targeting the Wnt/β-catenin signaling pathway [212]. Qiu et al. indicated that the downregulation of miR-22 would result in the upregulation of CD147 in tongue squamous cell carcinomas [213]. CD147 is also known as an extracellular MMP inducer, which promotes tumor initiation and progression through NF-κB signaling and also mediates the TGF-β1-induced EMT in HNSCC cells [214,215]. Therefore, CD147 might be a potential prognostic and treatment biomarker for HNSCCs.
4.2.3. miRNAs in LSCC
In LSCC, miR-98 was shown significantly reduced in both clinical specimens and cell lines, and miR-98 directly targeted HMGA2-POSTN signaling and then suppressed cell migration, metastasis, invasion, and EMT-TFs of SNAI1 and Twist1, as well as SC-like features [216]. Moreover, miR-101 inhibited tumorigenesis progression by regulating the Wnt/β-catenin signaling pathway by directly targeting cyclin-dependent kinase 8 (CDK8) in LSCC [217]. CDK8 plays an important role in regulating biological processes at the transcription level in the Wnt/β-catenin signaling pathway, and it is considered a CRC oncogene [218,219].
4.2.4. miRNAs in NPC
miR-139-5p inhibits the proliferation, invasion, and migration of human NPC cells by modulating EMT [220]. EMT enhances cancer cell motility and dissemination, which led to the concept of migrating CSCs as the basis of metastasis [221]. Findings have demonstrated a direct molecular link between EMT and stemness, where EMT activators such as Twist1 can co-induce EMT and stemness properties, thereby linking the EMT and CSC concepts [188]. EMT plays an important role in tumor metastasis and recurrence, and thus it is closely related to CSC functions [222,223]. Moreover, miR-139-5p reduces cisplatin resistance in NPC cells [220].
miR-488-3p activates the p53 pathway through suppressing zinc finger and BTB domain-containing protein 2 (ZBTB2), a reader of unmethylated DNA that regulates embryonic stem cell differentiation, thereby inhibiting proliferation and inducing apoptosis in esophageal SCCs [224,225]. p53 is able to suppress CD44, which is a CSC marker and suppresses cellular plasticity [226]. In NPCs, miR-372 promotes radiosensitivity by activating the p53 signaling pathway via the inhibition of PDZ-binding kinase (PBK) [227]. Moreover, p53 represses EMT by mediating miR-200c expression, which causes the inhibition the translation of ZEB1 and BMI1 [228]. By downregulating ATF3 expression, miRNA-488 suppresses cell invasion and EMT in tongue squamous cell carcinoma cells [229]. Taken together, miRNAs as tumor suppressors that regulate the stemness process are summarized in Table 2.

Table 2.
miRNAs as tumor suppressors that are involved in the stemness of head and neck squamous cell carcinomas (HNSCCs).
4.3. miRNAs as Pleiotropic Functions
Some miRNAs play dual roles in oncogenes and tumor suppression, depending on the specific cell/tissue context. This reflects the complexity of the miRNA–target regulatory network. For example, miR-107 was observed to antagonize and degrade let-7. miR-107 suppressed let-7 expression and activated downstream oncoprotein expressions such as HMGA2 and RAS and enhanced the tumorigenic and metastatic potential of cancer cells [230,231]. In HNSCCs, a miR-107 increment was found in patients with lymph node metastasis, suggesting an oncogenic role for miR-107 [232]; however, miR-107 was indicated to suppress the proliferation, invasion, and colony formation of cells in LSCCs via inhibiting the voltage-gated calcium channel subunit α2δ1 (α2δ1) (encoded by CACNA2D1) [233]. In non-small-cell lung cancer (NSCLC), α2δ1 also enhances radioresistance in cancer stem-like cells by enhancing the efficiency of DNA damage repair [234]. Those results indicate the pleiotropic functions of miR107 in HNSCCs.
miRNAs also mediated the regulation of cytokines/chemokines and the TME that modulates the CSC signaling pathway and sustains the CSC niche for acquiring and maintaining CSC features. For example, downregulation of miR-9, miR-542-3p, and miR-34a, and significant upregulation of miR-21 were shown in CD44-positive CSCs with increased IL-6 and IL-8 expressions via targeting of the CD44v6/NANOG/phosphatase and tensin homolog (PTEN) axis in oral cancer [235]. miR-9 acts as a tumor suppressor microRNA in HNSCC, and its role seems to be mediated through CXCR4 suppression [236]. Studies have indicated that miR-9 overexpression results in decreased cellular proliferation and inhibited colony formation in soft agars when targeting CXCR4 in HNSCC cells [236]. Conversely, another study has also indicated that miR-9 was expressed at high levels in patients with recurrent HNSCCs [237]. Similar results were also shown for breast cancer with miR-9 acting as a tumor suppressor in breast cancer proliferation in the early stage of breast cancer, while, with a higher malignancy, miR-9 plays an opposite role in the metastatic process [238]. Thus, miR-9 was suggested to have dual roles in carcinogenesis. Taken together, miRNAs with pleiotropic functions in HNSCCs are summarized in Table 3.

Table 3.
miRNAs with pleiotropic functions that are involved in the stemness of head and neck squamous cell carcinomas (HNSCCs).
5. Conclusions
miRNAs can function as cancer suppressors or oncogenes, or even exhibit dual roles during cancer development, depending on the different cancer types or tumorigenesis stage. miRNAs are critical to tumor initiation, progression, metastasis, EMT, and chemoresistance via regulating CSC functions. miRNAs regulate important EMT-TFs and signaling pathways and modulate the TME to sustain and enhance cancer stemness. Therefore, targeting CSCs through miRNA manipulation provides a therapeutic opportunity for managing metastatic diseases. Moreover, with an understanding of miRNAs during tumorigenesis, we can take advantage of miRNA stability and use it as a diagnostic marker for primary diagnoses and patient follow-ups. We can also monitor miRNA changes to predict therapeutic responses as a non-invasive detection method. Recent studies have indicated that exosomal miRNAs can be better sources of biomarkers due to their advantages in terms of their quantity, quality, and stability [239]. Ludwig et al. indicated that miR-205-5p was exclusively expressed in HPV(+) exosomes, whereas miR-1972 was only detected in HPV(−) exosomes. These miRNAs emerge as potential discriminating HPV-associated biomarkers [240] Intriguingly, human papillomavirus 16 (HPV16) infection has been indicated to enhance CSC properties, including ALDH1 activity, migration/invasion, and CSC-related factor expression, and enhances tumor growth OSCC cells [241]. Whether tumor-derived exosomes (TEX)-miRNAs are also involved in regulating the recipient cell stemness is unclear. In contrast to the extensive studies for cellular miRNAs in regulating cancer stemness, TEX-miRNA knowledge is relatively limited.
Moreover, Huang et al. indicated that only 5.63% of miRNAs were detected in both cells and TEX, which implies that cells can selectively pack certain miRNAs into exosomes in OSCC cells [242]. Meanwhile, exosomes can be released by various cell types, such as cancer-associated fibroblasts (CAFs) [243], dendritic cells [244], B cells [245], T cells [246], and tumor cells [247]. For example, Li et al. indicated that miR-34a-5p was significantly reduced in CAF-derived exosomes in OSCC patients. CAF transfers miR-34a-5p-devoid exosomes to OSCC cells and results in promoting the proliferation and motility of OSCC cells by upregulating the downstream target AXL (encoding AXL receptor tyrosine kinase). Therefore, the miR-34a-5p/AXL axis promotes the proliferation, metastasis, and EMT of oral cancer cells through the AKT/glycogen synthase kinase (GSK)-3β (GSK-3β)/β-catenin/SNAI1 signaling cascade [248]. Consistently, the cellular miR-34a significantly inhibited EMT formation of the CSC phenotype in HNSCC cell lines [184]. Hence, the sources and biological functions of exosomal miRNAs warrant further research before using them for screening and surveillance.
Among the tumor suppressor microRNAs of HNSCCs, miR-34 is the only one that has been used in a clinical trial applied to treat primary liver cancer, small-cell lung carcinomas, lymphomas, multiple myelomas, and renal cell carcinomas. In 2013, the first microRNA-associated therapeutic drug was tested in a clinical trial (NCT01829971), MRX34, a special amphoteric lipid nanoparticle filled with miR-34 mimics. Although this phase I study provided a dose-dependent modulation of relevant target genes that provide a proof-of-concept for MRX34 application for cancer therapy, severe adverse events were reported in five patients in terms of experiencing serious immune responses [249,250]. Hence, leading up to the MRX34 phase 2 clinical trials, NCT02862145 for melanomas has been withdrawn [251]. Other clinical trials have mainly focused on observational studies to explore the prognostic value of miRNAs in HNSCCs, for example, the prognostic value of miR-29b in the tissue, blood, and saliva in oral squamous cell carcinomas (NCT02009852). The miR-29 family has been used to investigate Twist1-mediated cancer metastasis in HNSCCs (NCT01927354) (Table 4). Further research is warranted to determine the molecular functions and mechanisms of cellular or exosomal miRNAs, as well as their potential as miRNA-based diagnostics and therapeutics for HNSCCs.

Table 4.
List of miRNA-related clinical trials in head and neck squamous cell carcinomas (HNSCCs).
Author Contributions
M.F., W.-L.H., and T.-T.L. wrote and revised the paper with assistance from P.-Y.C. and T.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Yen Tjing Ling Medical Foundation in Taiwan and the Cancer Progression Research Center, National Yang Ming Chiao Tung University, from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE). We appreciate the funding from the Ministry of Science and Technology (109-2320-B-010-021 to W.-L.H.; MOST 109-2636-B-038-001 and MOST 110-2636-B-038-005 to T.-T.L.) and Taipei Medical University (TMU108-AE1-B25 to T.-T.L), the Yen Tjing Ling Medical Foundation (CI-109-11 to W.-L.H.), and the Ministry of Health and Welfare, Center of Excellence for Cancer Research (MOHW107-TDU-B-211-114019, 109 CRC-T208, and 110 CRC-T208 to W.-L.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Simon, S. Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019; pp. 1–71. [Google Scholar]
- Dale, O.T.; Pring, M.; Davies, A.; Leary, S.; Ingarfield, K.; Toms, S.; Waterboer, T.; Pawlita, M.; Ness, A.R.; Thomas, S.J. Squamous cell carcinoma of the nasal cavity: A descriptive analysis of cases from the head and neck 5000 study. Clin. Otolaryngol. 2019, 44, 961–967. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Argirion, I.; Zarins, K.R.; Defever, K.; Suwanrungruang, K.; Chang, J.T.; Pongnikorn, D.; Chitapanarux, I.; Sriplung, H.; Vatanasapt, P.; Rozek, L.S. Temporal Changes in Head and Neck Cancer Incidence in Thailand Suggest Changing Oropharyngeal Epidemiology in the Region. J. Glob. Oncol. 2019, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Sethi, S.; Lu, M.; Kapke, A.; Benninger, M.S.; Worsham, M.J. Patient and tumor factors at diagnosis in a multi-ethnic primary head and neck squamous cell carcinoma cohort. J. Surg. Oncol. 2008, 99, 104–108. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, Y.; Feng, M.; Xue, X.; Fan, Y. A novel seven-miRNA prognostic model to predict overall survival in head and neck squamous cell carcinoma patients. Mol. Med. Rep. 2019, 20, 4340–4348. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zheng, X.; Yu, J.; Ma, H.; Hua, C.; Gao, R. EGFR enhances the stemness and progression of oral cancer through inhibiting autophagic degradation of SOX2. Cancer Med. 2019, 9, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, J.H.; Longmire, M.; Wang, H.; Kohrt, H.E.; Chang, H.Y.; Sunwoo, J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell–Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin. Cancer Res. 2016, 22, 3571–3581. [Google Scholar] [CrossRef]
- Chen, D.; Wu, M.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y.; Dong, J.; et al. Targeting BMI1 + Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell 2017, 20, 621–634.e6. [Google Scholar] [CrossRef]
- Giudice, F.S.; Jr, D.S.P.; Nör, J.E.; Squarize, C.H.; Castilho, R.M. Inhibition of Histone Deacetylase Impacts Cancer Stem Cells and Induces Epithelial-Mesenchyme Transition of Head and Neck Cancer. PLoS ONE 2013, 8, e58672. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; Zhong, L.; Wen, Y.; Ye, Q.; Cao, D.; Li, P.; Liu, Y. Construction of an 11-microRNA-based signature and a prognostic nomogram to predict the overall survival of head and neck squamous cell carcinoma patients. BMC Genom. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yata, K.; Beder, L.B.; Tamagawa, S.; Hotomi, M.; Hirohashi, Y.; Grenman, R.; Yamanaka, N. MicroRNA expression profiles of cancer stem cells in head and neck squamous cell carcinoma. Int. J. Oncol. 2015, 47, 1249–1256. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nat. Cell Biol. 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Ishizawa, K.; Rasheed, Z.A.; Karisch, R.; Wang, Q.; Kowalski, J.; Susky, E.; Pereira, K.; Karamboulas, C.; Moghal, N.; Rajeshkumar, N.; et al. Tumor-Initiating Cells Are Rare in Many Human Tumors. Cell Stem Cell 2010, 7, 279–282. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Kreso, A.; Jamieson, C.H. Cancer Stem Cells and Self-renewal. Clin. Cancer Res. 2010, 16, 3113–3120. [Google Scholar] [CrossRef]
- Chen, R.; Nishimura, M.C.; Bumbaca, S.M.; Kharbanda, S.; Forrest, W.F.; Kasman, I.M.; Greve, J.M.; Soriano, R.H.; Gilmour, L.L.; Rivers, C.S.; et al. A Hierarchy of Self-Renewing Tumor-Initiating Cell Types in Glioblastoma. Cancer Cell 2010, 17, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.D.; Kwon, Y.T. Molecular mechanisms controlling asymmetric and symmetric self-renewal of cancer stem cells. J. Anal. Sci. Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, J.; Haiech, J.; Kilhoffer, M.-C.; Zeniou, M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Cheung, T.H.T.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Merlos-Suárez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Céspedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Muñoz, P.; et al. The Intestinal Stem Cell Signature Identifies Colorectal Cancer Stem Cells and Predicts Disease Relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nat. Cell Biol. 2006, 445, 106–110. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Dylla, S.J.; Beviglia, L.; Park, I.-K.; Chartier, C.; Raval, J.; Ngan, L.; Pickell, K.; Aguilar, J.; Lazetic, S.; Smith-Berdan, S.; et al. Colorectal Cancer Stem Cells Are Enriched in Xenogeneic Tumors Following Chemotherapy. PLoS ONE 2008, 3, e2428. [Google Scholar] [CrossRef]
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A Subpopulation of CD26+ Cancer Stem Cells with Metastatic Capacity in Human Colorectal Cancer. Cell Stem Cell 2010, 6, 603–615. [Google Scholar] [CrossRef]
- De Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Francipane, M.G.; Medema, J.P.; Stassi, G. Colon Cancer Stem Cells: Promise of Targeted Therapy. Gastroenterology 2010, 138, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Trapasso, S. Role of CD44 as a marker of cancer stem cells in head and neck cancer. Biol. Targets Ther. 2012, 6, 379–383. [Google Scholar] [CrossRef][Green Version]
- Landen, C.N.; Goodman, B.; Katre, A.A.; Steg, A.D.; Nick, A.M.; Stone, R.L.; Miller, L.D.; Mejia, P.V.; Jennings, N.B.; Gershenson, D.M.; et al. Targeting Aldehyde Dehydrogenase Cancer Stem Cells in Ovarian Cancer. Mol. Cancer Ther. 2010, 9, 3186–3199. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilicstress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Korkaya, H.; Liu, S.; Wicha, M.S. Regulation of Cancer Stem Cells by Cytokine Networks: Attacking Cancer’s Inflammatory Roots: Figure 1. Clin. Cancer Res. 2011, 17, 6125–6129. [Google Scholar] [CrossRef]
- Todaro, M.; Alea, M.P.; Di Stefano, A.B.; Cammareri, P.; Vermeulen, L.; Iovino, F.; Tripodo, C.; Russo, A.; Gulotta, G.; Medema, J.P.; et al. Colon Cancer Stem Cells Dictate Tumor Growth and Resist Cell Death by Production of Interleukin-4. Cell Stem Cell 2007, 1, 389–402. [Google Scholar] [CrossRef]
- Samanta, D.; Gilkes, D.M.; Chaturvedi, P.; Xiang, L.; Semenza, G.L. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E5429–E5438. [Google Scholar] [CrossRef]
- Hwang, W.; Yang, M.; Tsai, M.; Lan, H.; Su, S.; Chang, S.; Teng, H.; Yang, S.; Lan, Y.; Chiou, S. SNAIL Regulates Interleukin-8 Expression, Stem Cell–Like Activity, and Tumorigenicity of Human Colorectal Carcinoma Cells. Gastroenterology 2011, 141, 279–291.e5. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Kim, G.-I.; Davis, A.; Malik, F.; Henry, N.L.; Ithimakin, S.; Quraishi, A.A.; Tawakkol, N.; D’Angelo, R.; Paulson, A.K.; et al. Activation of an IL6 Inflammatory Loop Mediates Trastuzumab Resistance in HER2+ Breast Cancer by Expanding the Cancer Stem Cell Population. Mol. Cell 2012, 47, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-C.; Li, C.-W.; Xia, W.; Hsu, J.-M.; Lee, H.-H.; Cha, J.-H.; Wang, H.-L.; Yang, W.-H.; Yen, E.-Y.; Chang, W.-C.; et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J. Clin. Investig. 2019, 129, 3324–3338. [Google Scholar] [CrossRef] [PubMed]
- Reiman, J.M.; Knutson, K.L.; Radisky, D.C. Immune Promotion of Epithelial-mesenchymal Transition and Generation of Breast Cancer Stem Cells: Figure 1. Cancer Res. 2010, 70, 3005–3008. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Xia, W.; Hsu, Y.-H.; Chan, L.-C.; Yu, W.-H.; Cha, J.-H.; Chen, C.-T.; Liao, H.-W.; Kuo, C.-W.; Khoo, K.-H.; et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Liao, T.-T.; Lin, C.-C.; Jiang, J.-K.; Yang, S.-H.; Teng, H.-W.; Yang, M.-H. Harnessing stemness and PD-L1 expression by AT-rich interaction domain-containing protein 3B in colorectal cancer. Theranostics 2020, 10, 6095–6112. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Hwang, W.-L.; Lan, H.-Y.; Cheng, W.-C.; Huang, S.-C.; Yang, M.-H. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J. Hematol. Oncol. 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Maherali, N.; Sridharan, R.; Xie, W.; Utikal, J.; Eminli, S.; Arnold, K.; Stadtfeld, M.; Yachechko, R.; Tchieu, J.; Jaenisch, R.; et al. Directly Reprogrammed Fibroblasts Show Global Epigenetic Remodeling and Widespread Tissue Contribution. Cell Stem Cell 2007, 1, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nat. Cell Biol. 2007, 448, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Zha, L.; Kong, D.; Liao, G.; Li, H. HMGA2 regulates epithelial-mesenchymal transition and the acquisition of tumor stem cell properties through TWIST1 in gastric cancer. Oncol. Rep. 2016, 37, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hong, H.S.; Liu, Z.X.; Kim, R.H.; Kang, M.K.; Park, N.-H.; Shin, K.-H. TNFα enhances cancer stem cell-like phenotype via Notch-Hes1 activation in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 424, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kang, J.W.; Song, X.; Kim, B.K.; Yoo, Y.D.; Kwon, Y.T.; Lee, Y.J. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell. Signal. 2013, 25, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-T.; Yang, M.-H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Pelullo, M.; Zema, S.; Nardozza, F.; Checquolo, S.; Screpanti, I.; Bellavia, D. Wnt, Notch, and TGF-β Pathways Impinge on Hedgehog Signaling Complexity: An Open Window on Cancer. Front. Genet. 2019, 10, 711. [Google Scholar] [CrossRef]
- Vermeulen, L.; Melo, F.D.S.E.; Van Der Heijden, M.; Cameron, K.; De Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Yeung, J.; Esposito, M.T.; Gandillet, A.; Zeisig, B.B.; Griessinger, E.; Bonnet, D.; So, C.W.E. β-Catenin Mediates the Establishment and Drug Resistance of MLL Leukemic Stem Cells. Cancer Cell 2010, 18, 606–618. [Google Scholar] [CrossRef]
- Dierks, C.; Beigi, R.; Guo, G.-R.; Zirlik, K.; Stegert, M.R.; Manley, P.; Trussell, C.; Schmitt-Graeff, A.; Landwerlin, K.; Veelken, H.; et al. Expansion of Bcr-Abl-Positive Leukemic Stem Cells Is Dependent on Hedgehog Pathway Activation. Cancer Cell 2008, 14, 238–249. [Google Scholar] [CrossRef]
- Peacock, C.D.; Wang, Q.; Gesell, G.S.; Corcoran-Schwartz, I.M.; Jones, E.; Kim, J.; Devereux, W.L.; Rhodes, J.T.; Huff, C.A.; Beachy, P.A.; et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl. Acad. Sci. USA 2007, 104, 4048–4053. [Google Scholar] [CrossRef] [PubMed]
- A Broderick, J.; Zamore, P.D. MicroRNA therapeutics. Gene Ther. 2011, 18, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.W.; Bruford, E.A. Naming ’junk’: Human non-protein coding RNA (ncRNA) gene nomenclature. Hum. Genom. 2011, 5, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Medvid, R.; Melton, C.; Jaenisch, R.; Blelloch, R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat. Genet. 2007, 39, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Fabbri, M.; Cimmino, A.; Calin, G.A.; Croce, C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006, 12, 580–587. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D. The Pattern of microRNA Binding Site Distribution. Genes 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef]
- Lujambio, A.; Ropero, S.; Ballestar, E.; Fraga, M.F.; Cerrato, C.; Setién, F.; Casado, S.; Suarez-Gauthier, A.; Sanchez-Cespedes, M.; Gitt, A.; et al. Genetic Unmasking of an Epigenetically Silenced microRNA in Human Cancer Cells. Cancer Res. 2007, 67, 1424–1429. [Google Scholar] [CrossRef]
- Obernosterer, G.; Leuschner, P.J.; Alenius, M.; Martinez, J. Post-transcriptional regulation of microRNA expression. RNA 2006, 12, 1161–1167. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nat. Cell Biol. 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; A Kolb, F.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single Processing Center Models for Human Dicer and Bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef]
- Chendrimada, T.P.; Gregory, R.I.; Kumaraswamy, E.; Norman, J.; Cooch, N.; Nishikura, K.; Shiekhattar, R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nat. Cell Biol. 2005, 436, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hur, I.; Park, S.-Y.; Kim, Y.-K.; Suh, M.R.; Kim, V.N. The role of PACT in the RNA silencing pathway. EMBO J. 2006, 25, 522–532. [Google Scholar] [CrossRef]
- Fukuda, T.; Yamagata, K.; Fujiyama, S.; Matsumoto, T.; Koshida, I.; Yoshimura, K.; Mihara, M.; Naitou, M.; Endoh, H.; Nakamura, T.; et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat. Cell Biol. 2007, 9, 604–611. [Google Scholar] [CrossRef]
- Gregory, R.I.; Chendrimada, T.P.; Cooch, N.; Shiekhattar, R. Human RISC Couples MicroRNA Biogenesis and Posttranscriptional Gene Silencing. Cell 2005, 123, 631–640. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Wightman, B.; Burglin, T.R.; Gatto, J.; Arasu, P.; Ruvkun, G. Negative regulatory sequences in the lin-14 3′-untranslated region are necessary to generate a temporal switch during Caenorhabditis elegans development. Genes Dev. 1991, 5, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Shih, I.-H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of Mammalian MicroRNA Targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Iglesias-Bartolome, R.; Callejas-Valera, J.L.; Gutkind, J.S. Control of the epithelial stem cell epigenome: The shaping of epithelial stem cell identity. Curr. Opin. Cell Biol. 2013, 25, 162–169. [Google Scholar] [CrossRef]
- Papagerakis, S.; Pannone, G.; Zheng, L.; About, I.; Taqi, N.; Nguyen, N.P.; Matossian, M.; McAlpin, B.; Santoro, A.; McHugh, J.; et al. Oral epithelial stem cells—Implications in normal development and cancer metastasis. Exp. Cell Res. 2014, 325, 111–129. [Google Scholar] [CrossRef][Green Version]
- Peng, H.; Park, J.K.; Katsnelson, J.; Kaplan, N.; Yang, W.; Getsios, S.; Lavker, R.M. microRNA-103/107 Family Regulates Multiple Epithelial Stem Cell Characteristics. Stem Cells 2015, 33, 1642–1656. [Google Scholar] [CrossRef]
- Andl, T.; Murchison, E.P.; Liu, F.; Zhang, Y.; Yunta-Gonzalez, M.; Tobias, J.W.; Andl, C.D.; Seykora, J.T.; Hannon, G.J.; Millar, S.E. The miRNA-Processing Enzyme Dicer Is Essential for the Morphogenesis and Maintenance of Hair Follicles. Curr. Biol. 2006, 16, 1041–1049. [Google Scholar] [CrossRef]
- Yi, R.; Pasolli, H.A.; Landthaler, M.; Hafner, M.; Ojo, T.; Sheridan, R.; Sander, C.; O’Carroll, D.; Stoffel, M.; Tuschl, T.; et al. DGCR8-dependent microRNA biogenesis is essential for skin development. Proc. Natl. Acad. Sci. USA 2008, 106, 498–502. [Google Scholar] [CrossRef]
- Yi, R.; O’Carroll, D.; A Pasolli, H.; Zhang, Z.; Dietrich, F.S.; Tarakhovsky, A.; Fuchs, E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 2006, 38, 356–362. [Google Scholar] [CrossRef]
- Otsuka-Tanaka, Y.; Oommen, S.; Kawasaki, M.; Kawasaki, K.; Imam, N.; Jalani-Ghazani, F.; Hindges, R.; Sharpe, P.T.; Ohazama, A. Oral Lining Mucosa Development Depends on Mesenchymal microRNAs. J. Dent. Res. 2012, 92, 229–234. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA Signatures in Human Cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.R.; Junejo, K.; Steinhoff, M.; Uddin, S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells 2019, 8, 840. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.C. Inhibition of microRNA with antisense oligonucleotides. Methods 2008, 44, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mercatelli, N.; Coppola, V.; Bonci, D.; Miele, F.; Costantini, A.; Guadagnoli, M.; Bonanno, E.; Muto, G.; Frajese, G.V.; De Maria, R.; et al. The Inhibition of the Highly Expressed Mir-221 and Mir-222 Impairs the Growth of Prostate Carcinoma Xenografts in Mice. PLoS ONE 2008, 3, e4029. [Google Scholar] [CrossRef] [PubMed]
- Van Roosbroeck, K.; Fanini, F.; Setoyama, T.; Ivan, C.; Rodriguez-Aguayo, C.; Fuentes-Mattei, E.; Xiao, L.; Vannini, I.; Redis, R.S.; D’Abundo, L.; et al. Combining Anti-Mir-155 with Chemotherapy for the Treatment of Lung Cancers. Clin. Cancer Res. 2016, 23, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Chirshev, E.; Oberg, K.C.; Ioffe, Y.J.; Unternaehrer, J.J. Let-7as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Transl. Med. 2019, 8, 24. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Guo, H.; Xue, X.; Zhang, Y.; Niu, M.; Cui, J.; Liu, H.; Luo, H.; Yang, D.; et al. Serum Exosomal miR-941 as a promising Oncogenic Biomarker for Laryngeal Squamous Cell Carcinoma. J. Cancer 2020, 11, 5329–5344. [Google Scholar] [CrossRef]
- Troschel, F.M.; Böhly, N.; Borrmann, K.; Braun, T.; Schwickert, A.; Kiesel, L.; Eich, H.T.; Götte, M.; Greve, B. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumor Biol. 2018, 40, 1010428318791887. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Q.; Huang, X.; Yin, X.; Song, J.; Ke, Z.; Duan, X. miRNA-Based Feature Classifier Is Associated with Tumor Mutational Burden in Head and Neck Squamous Cell Carcinoma. BioMed Res. Int. 2020, 2020, 1686480. [Google Scholar] [CrossRef]
- Avilés-Jurado, F.X.; Muñoz, C.; Meler, C.; Flores, J.C.; Gumà, J.; Benaiges, E.; Mora, J.; Camacho, M.; León, X.; Vilaseca, I.; et al. Circulating microRNAs modulating glycolysis as non-invasive prognostic biomarkers of HNSCC. Eur. Arch. Oto-Rhino-Laryngol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Petronacci, C.C.; García, A.G.; Iruegas, E.P.; Mundiña, B.R.; Pouso, A.L.; Sayáns, M.P. Identification of Prognosis Associated microRNAs in HNSCC Subtypes Based on TCGA Dataset. Medicina 2020, 56, 535. [Google Scholar] [CrossRef] [PubMed]
- Ganci, F.; Sacconi, A.; Manciocco, V.; Sperduti, I.; Battaglia, P.; Covello, R.; Muti, P.; Strano, S.; Spriano, G.; Fontemaggi, G.; et al. MicroRNA expression as predictor of local recurrence risk in oral squamous cell carcinoma. Head Neck 2016, 38, E189–E197. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Datta, J.; Lang, J.C.; Teknos, T.N. Down regulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral Oncol. 2014, 50, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, Y.; Jin, Y.; Tai, J.; Zhang, J.; Xiao, X.; Chu, P.; Yu, Y.; Wang, S.C.; Lu, J.; et al. MicroRNA-365a-3p promotes tumor growth and metastasis in laryngeal squamous cell carcinoma. Oncol. Rep. 2016, 35, 2017–2026. [Google Scholar] [CrossRef]
- Li, D.; Liu, K.; Li, Z.; Wang, J.; Wang, X. miR-19a and miR-424 target TGFBR3 to promote epithelial-to-mesenchymal transition and migration of tongue squamous cell carcinoma cells. Cell Adhes. Migr. 2017, 12, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, J.; Sun, L.; Li, X.; Bai, J.; Zhang, H.; Li, T. miR-611 promotes the proliferation, migration and invasion of tongue squamous cell carcinoma cells by targeting FOXN3. Oral Dis. 2019, 25, 1906–1918. [Google Scholar] [CrossRef]
- Yao, X.; Xie, L.; Zeng, Y. MiR-9 Promotes Angiogenesis via Targeting on Sphingosine-1- Phosphate Receptor 1. Front. Cell Dev. Biol. 2020, 8, 755. [Google Scholar] [CrossRef]
- Lages, E. MicroRNAs: Molecular features and role in cancer. Front. Biosci. 2012, 17, 2508–2540. [Google Scholar] [CrossRef]
- Xu, W.; Ji, J.; Xu, Y.; Liu, Y.; Shi, L.; Liu, Y.; Lu, X.; Zhao, Y.; Luo, F.; Wang, B.; et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol. Carcinog. 2014, 54, E148–E161. [Google Scholar] [CrossRef]
- Ceppi, P.; E Peter, M. MicroRNAs regulate both epithelial-to-mesenchymal transition and cancer stem cells. Oncogene 2013, 33, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yao, F.; Xiao, Z.; Sun, Y.; Ma, L. MicroRNAs and metastasis: Small RNAs play big roles. Cancer Metastasis Rev. 2017, 37, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Ito, K.; Ala, U.; Kats, L.; Webster, K.; Sun, S.M.; Jongen-Lavrencic, M.; Manova-Todorova, K.; Teruya-Feldstein, J.; Avigan, D.E.; et al. The Oncogenic MicroRNA miR-22 Targets the TET2 Tumor Suppressor to Promote Hematopoietic Stem Cell Self-Renewal and Transformation. Cell Stem Cell 2013, 13, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ouyang, H.; An, X.; Liu, S. miR-125a is upregulated in cancer stem-like cells derived from TW01 and is responsible for maintaining stemness by inhibiting p53. Oncol. Lett. 2018, 17, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.-Y.; Tu, H.-F.; Yang, C.-C.; Wu, C.-H.; Liu, C.-J.; Chang, K.-W.; Lin, S.-C. miR-134targetsPDCD7to reduce E-cadherin expression and enhance oral cancer progression. Int. J. Cancer 2018, 143, 2892–2904. [Google Scholar] [CrossRef]
- Farmakovskaya, M.; Khromova, N.; Rybko, V.; Dugina, V.; Kopnin, B.; Kopnin, P. E-Cadherin repression increases amount of cancer stem cells in human A549 lung adenocarcinoma and stimulates tumor growth. Cell Cycle 2016, 15, 1084–1092. [Google Scholar] [CrossRef]
- Liu, C.-J.; Shen, W.G.; Peng, S.-Y.; Cheng, H.-W.; Kao, S.-Y.; Lin, S.-C.; Chang, K.-W. miR-134induces oncogenicity and metastasis in head and neck carcinoma through targetingWWOXgene. Int. J. Cancer 2013, 134, 811–821. [Google Scholar] [CrossRef]
- Yan, H.C.; Xu, J.; Fang, L.S.; Qiu, Y.Y.; Lin, X.M.; Huang, H.X.; Han, Q.Y. Ectopic expression of the WWOX gene suppresses stemness of human ovarian cancer stem cells. Oncol. Lett. 2015, 9, 1614–1620. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Liu, J.; Li, P.; Zhou, C.; Liu, P. The downregulation of WWOX induces epithelial–mesenchymal transition and enhances stemness and chemoresistance in breast cancer. Exp. Biol. Med. 2018, 243, 1066–1073. [Google Scholar] [CrossRef]
- Lin, S.-S.; Peng, C.-Y.; Liao, Y.-W.; Chou, M.-Y.; Hsieh, P.-L.; Yu, C.-C. miR-1246 Targets CCNG2 to Enhance Cancer Stemness and Chemoresistance in Oral Carcinomas. Cancers 2018, 10, 272. [Google Scholar] [CrossRef]
- Bernaudo, S.; Salem, M.; Qi, X.; Zhou, W.; Zhang, C.; Yang, W.; Rosman, D.; Deng, Z.; Ye, G.; Yang, B.B.; et al. Cyclin G2 inhibits epithelial-to-mesenchymal transition by disrupting Wnt/β-catenin signaling. Oncogene 2016, 35, 4816–4827. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Yonekawa, A.; Harada, C.; Hamada, M.; Katagiri, W.; Nakazawa, M.; Yura, Y. Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int. J. Oncol. 2010, 37, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Mah, K.M.; Weiner, J.A. Regulation of Wnt signaling by protocadherins. Semin. Cell Dev. Biol. 2017, 69, 158–171. [Google Scholar] [CrossRef] [PubMed]
- DiMeo, T.A.; Anderson, K.; Phadke, P.; Feng, C.; Perou, C.M.; Naber, S.; Kuperwasser, C. A Novel Lung Metastasis Signature Links Wnt Signaling with Cancer Cell Self-Renewal and Epithelial-Mesenchymal Transition in Basal-like Breast Cancer. Cancer Res. 2009, 69, 5364–5373. [Google Scholar] [CrossRef] [PubMed]
- Giefing, M.; Zemke, N.; Brauze, D.; Kostrzewska-Poczekaj, M.; Luczak, M.; Szaumkessel, M.; Pelinska, K.; Kiwerska, K.; Tönnies, H.; Grenman, R.; et al. High resolution ArrayCGH and expression profiling identifies PTPRD and PCDH17/PCH68 as tumor suppressor gene candidates in laryngeal squamous cell carcinoma. Genes Chromosom. Cancer 2010, 50, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Sun, G.; Sun, J.-W. MiR-196b affects the progression and prognosis of human LSCC through targeting PCDH-17. Auris Nasus Larynx 2019, 46, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xu, Z.; Zhang, M.; Zuo, Y. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7286–7296. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Wen, C.; Yang, X.; Song, M.; Chen, J.; Wang, C.; Zhang, B.; Wang, L.; Iwamoto, A.; et al. Hsa-miR-19a is associated with lymph metastasis and mediates the TNF-α induced epithelial-to-mesenchymal transition in colorectal cancer. Sci. Rep. 2015, 5, 13350. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Yan, W.; Yang, J.; Qin, Y.-J.; Lin, X.-L.; Xie, R.-Y.; Wang, S.-C.; Jin, W.; Gao, F.; et al. MicroRNA-19 triggers epithelial–mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab. Investig. 2015, 95, 1056–1070. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Wang, Y.; Wu, H.; Frank, J.A.; Zhang, Z.; Luo, J. Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3605–3617. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, Q.; Gu, M.; Zhang, K.; Xia, T.; Zhang, S.; Chen, W.; Yin, H.; Yao, H.; Fan, Y.; et al. MIR106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma. Autophagy 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Fu, S.; Fu, Y.; Chen, F.; Hu, Y.; Quan, B.; Zhang, J. Overexpression of MYCT1 Inhibits Proliferation and Induces Apoptosis in Human Acute Myeloid Leukemia HL-60 and KG-1a Cells in vitro and in vivo. Front. Pharmacol. 2018, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Guo, Y.; Chen, H.; Xu, Z.-M.; Qiu, G.-B.; Zhong, M.; Sun, K.-L.; Fu, W.-N. MYCT1-TV, A Novel MYCT1 Transcript, Is Regulated by c-Myc and May Participate in Laryngeal Carcinogenesis. PLoS ONE 2011, 6, e25648. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.-J.; Sun, Y.-Y.; Li, Y.-H.; Xu, Z.-M.; Fu, W.-N. MYCT1 inhibits the EMT and migration of laryngeal cancer cells via the SP1/miR-629-3p/ESRP2 pathway. Cell. Signal. 2020, 74, 109709. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Takahashi, R.-U.; Prieto-Vila, M.; Kawamura, Y.; Kondo, S.; Shirota, T.; Ochiya, T. CD44 exerts a functional role during EMT induction in cisplatin-resistant head and neck cancer cells. Oncotarget 2018, 9, 10029–10041. [Google Scholar] [CrossRef]
- Chikuda, J.; Otsuka, K.; Shimomura, I.; Ito, K.; Miyazaki, H.; Takahashi, R.-U.; Nagasaki, M.; Mukudai, Y.; Ochiya, T.; Shimane, T.; et al. CD44s Induces miR-629-3p Expression in Association with Cisplatin Resistance in Head and Neck Cancer Cells. Cancers 2020, 12, 856. [Google Scholar] [CrossRef]
- Büssing, I.; Slack, F.J.; Grosshans, H. let-7 microRNAs in development, stem cells and cancer. Trends Mol. Med. 2008, 14, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Wang, Y.; Wang, X.; Liu, N.; You, Y. Regulation of let-7 and its target oncogenes (Review). Oncol. Lett. 2012, 3, 955–960. [Google Scholar] [CrossRef]
- Liao, T.-T.; Hsu, W.-H.; Ho, C.-H.; Hwang, W.-L.; Lan, H.-Y.; Lo, T.; Chang, C.-C.; Tai, S.-K.; Yang, M.-H. let-7 Modulates Chromatin Configuration and Target Gene Repression through Regulation of the ARID3B Complex. Cell Rep. 2016, 14, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-H.; Lan, H.-Y.; Huang, C.-H.; Tai, S.-K.; Tzeng, C.-H.; Kao, S.-Y.; Wu, K.-J.; Hung, M.-C.; Yang, M.-H. RAC1 activation mediates Twist1-induced cancer cell migration. Nat. Cell Biol. 2012, 14, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Chen, Y.-W.; Chiou, G.-Y.; Tsai, L.-L.; Huang, P.-I.; Chang, C.-Y.; Tseng, L.-M.; Chiou, S.-H.; Yen, S.-H.; Chou, M.-Y.; et al. MicroRNA let-7a represses chemoresistance and tumourigenicity in head and neck cancer via stem-like properties ablation. Oral Oncol. 2011, 47, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Hittelman, W.N.; Liao, Y.; Wang, L.; Milas, L. Are cancer stem cells radioresistant? Future Oncol. 2010, 6, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-Y.; Wang, T.-Y.; Lee, S.-S.; Hsieh, P.-L.; Liao, Y.-W.; Tsai, L.-L.; Fang, C.-Y.; Yu, C.-C.; Hsieh, C.-S. Let-7c restores radiosensitivity and chemosensitivity and impairs stemness in oral cancer cells through inhibiting interleukin-8. J. Oral Pathol. Med. 2018, 47, 590–597. [Google Scholar] [CrossRef]
- Qu, J.-Q.; Yi, H.-M.; Ye, X.; Zhu, J.-F.; Li, L.-N.; Xiao, T.; Yuan, L.; Li, J.-Y.; Wang, Y.-Y.; Feng, J.; et al. MiRNA-203 Reduces Nasopharyngeal Carcinoma Radioresistance by Targeting IL8/AKT Signaling. Mol. Cancer Ther. 2015, 14, 2653–2664. [Google Scholar] [CrossRef]
- De Jong, M.C.; Hoeve, J.J.T.; Grénman, R.; Wessels, L.F.; Kerkhoven, R.M.; Riele, H.T.; Brekel, M.W.M.V.D.; Verheij, M.; Begg, A.C. Pretreatment microRNA Expression Impacting on Epithelial-to-Mesenchymal Transition Predicts Intrinsic Radiosensitivity in Head and Neck Cancer Cell Lines and Patients. Clin. Cancer Res. 2015, 21, 5630–5638. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Cheng, A.-J.; Lee, L.-Y.; You, G.-R.; Li, Y.-L.; Chen, H.-Y.; Chang, J.T. MiR-520b as a novel molecular target for suppressing stemness phenotype of head-neck cancer by inhibiting CD44. Sci. Rep. 2017, 7, 2042. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nat. Cell Biol. 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc− and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Dioguardi, M.; Caloro, G.A.; Laino, L.; Alovisi, M.; Sovereto, D.; Crincoli, V.; Aiuto, R.; Coccia, E.; Troiano, G.; Muzio, L.L. Circulating miR-21 as a Potential Biomarker for the Diagnosis of Oral Cancer: A Systematic Review with Meta-Analysis. Cancers 2020, 12, 936. [Google Scholar] [CrossRef]
- Banerjee, R.; Mani, R.-S.; Russo, N.; Scanlon, C.S.; Tsodikov, A.; Jing, X.; Cao, Q.; Palanisamy, N.; Metwally, T.; Inglehart, R.C.; et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene 2011, 30, 4339–4349. [Google Scholar] [CrossRef]
- Simon, J.A.; Lange, C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. Mol. Mech. Mutagen. 2008, 647, 21–29. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Moore, H.M.; Li, X.; Toy, K.A.; Huang, W.; Sabel, M.S.; Kidwell, K.M.; Kleer, C.G. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 3098–3103. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Chen, Z.; Huang, H.; Jin, Y.; Kolokythas, A.; Wang, A.; Dai, Y.; Wong, D.T.W.; Zhou, X. Polycomb group protein EZH2-mediated E-cadherin repression promotes metastasis of oral tongue squamous cell carcinoma. Mol. Carcinog. 2011, 52, 229–236. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, Y.; Ma, S.; Chang, H.; Yi, C.; Cao, H.; Gao, Y.; Guo, H.; Hou, J.; Yan, J.; et al. EZH2 promotes invasion and metastasis of laryngeal squamous cells carcinoma via epithelial-mesenchymal transition through H3K27me3. Biochem. Biophys. Res. Commun. 2016, 479, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Nohata, N.; Hanazawa, T.; Kikkawa, N.; Yamamoto, N.; Yoshino, H.; Itesako, T.; Enokida, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin–integrin signalling in head and neck squamous cell carcinoma. Br. J. Cancer 2013, 109, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Hsu, W.-L.; Tseng, Y.-J.; Liu, D.-W.; Weng, C.-F. Involvement of DNMT 3B promotes epithelial-mesenchymal transition and gene expression profile of invasive head and neck squamous cell carcinomas cell lines. BMC Cancer 2016, 16, 431. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yu, P.; Xie, N.; Wu, Y.; Liu, H.; Zhang, M.; Tao, Y.; Wang, W.; Yin, H.; Zou, B.; et al. MicroRNA-204-5p is a tumor suppressor and potential therapeutic target in head and neck squamous cell carcinoma. Theranostics 2020, 10, 1433–1453. [Google Scholar] [CrossRef] [PubMed]
- Galoczova, M.; Coates, P.; Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell. Mol. Biol. Lett. 2018, 23, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Z.; Li, M.; Liu, M.; Bahena, A.; Zhang, Y.; Zhang, Y.; Nambiar, C.; Liu, G. Sulforaphane promotes apoptosis, and inhibits proliferation and self-renewal of nasopharyngeal cancer cells by targeting STAT signal through miRNA-124-3p. Biomed. Pharmacother. 2018, 103, 473–481. [Google Scholar] [CrossRef]
- Huang, W.-C.; Jang, T.-H.; Tung, S.-L.; Yen, T.-C.; Chan, S.-H.; Wang, L.-H. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Chang, C.-J.; Hsu, C.-C.; Tsai, L.-L.; Lu, S.-W.; Huang, H.-S.; Wang, J.-J.; Chou, M.-Y.; Hu, F.-W. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol. Rep. 2011, 26, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, Y.; Lv, H.; Zhou, S.; Sun, Z.; Wang, M. miR-98 suppresses tumor cell growth and metastasis by targeting IGF1R in oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 12252–12259. [Google Scholar] [PubMed]
- Bendall, S.C.; Stewart, M.H.; Menendez, P.; George, D.; Vijayaragavan, K.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Rouleau, A.; Yang, J.; Bossé, M.; et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nat. Cell Biol. 2007, 448, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Farabaugh, S.M.; Boone, D.N.; Lee, A.V. Role of IGF1R in Breast Cancer Subtypes, Stemness, and Lineage Differentiation. Front. Endocrinol. 2015, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xie, D.; Yu, S.-C.; Yang, X.-J.; He, L.-R.; Yang, J.; Ping, Y.-F.; Wang, B.; Yang, L.; Xu, S.-L.; et al. β-Catenin/POU5F1/SOX2 Transcription Factor Complex Mediates IGF-I Receptor Signaling and Predicts Poor Prognosis in Lung Adenocarcinoma. Cancer Res. 2013, 73, 3181–3189. [Google Scholar] [CrossRef]
- Malaguarnera, R.; Belfiore, A. The Emerging Role of Insulin and Insulin-Like Growth Factor Signaling in Cancer Stem Cells. Front. Endocrinol. 2014, 5, 10. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wu, H.-Y.; Gao, J.; Wang, X.-H.; Gao, T.-H.; Zhang, S.-F. IGF1R facilitates epithelial-mesenchymal transition and cancer stem cell properties in neuroblastoma via the STAT3/AKT axis. Cancer Manag. Res. 2019, 11, 5459–5472. [Google Scholar] [CrossRef]
- Leong, H.S.; Chong, F.T.; Sew, P.H.; Lau, D.P.; Wong, B.H.; Teh, B.-T.; Tan, D.S.; Iyer, N.G. Targeting Cancer Stem Cell Plasticity Through Modulation of Epidermal Growth Factor and Insulin-Like Growth Factor Receptor Signaling in Head and Neck Squamous Cell Cancer. Stem Cells Transl. Med. 2014, 3, 1055–1065. [Google Scholar] [CrossRef]
- Jiang, Q.; Cao, Y.; Qiu, Y.; Li, C.; Liu, L.; Xu, G. Progression of squamous cell carcinoma is regulated by miR-139-5p/CXCR4. Front. Biosci. 2020, 25, 1732–1745. [Google Scholar]
- Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Emerging Roles of C-Myc in Cancer Stem Cell-Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target against Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2340. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhu, H.; Chi, C.; Yang, F.; Xu, X. MiRNA-139 regulates oral cancer Tca8113 cells apoptosis through Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 4588–4594. [Google Scholar] [PubMed]
- Wang, K.; Jin, J.; Ma, T.; Zhai, H. MiR-139-5p inhibits the tumorigenesis and progression of oral squamous carcinoma cells by targetingHOXA9. J. Cell. Mol. Med. 2017, 21, 3730–3740. [Google Scholar] [CrossRef] [PubMed]
- Bhatlekar, S.; Viswanathan, V.; Fields, J.Z.; Boman, B.M. Overexpression of HOXA4 and HOXA9 genes promotes self-renewal and contributes to colon cancer stem cell overpopulation. J. Cell. Physiol. 2018, 233, 727–735. [Google Scholar] [CrossRef]
- You, X.; Zhou, Z.; Chen, W.; Wei, X.; Zhou, H.; Luo, W. MicroRNA-495 confers inhibitory effects on cancer stem cells in oral squamous cell carcinoma through the HOXC6-mediated TGF-β signaling pathway. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, L.; Wang, B.; Diao, S.; Jia, R.; Shang, J. MiR-495/IGF-1/AKT Signaling as a Novel Axis Is Involved in the Epithelial-to-Mesenchymal Transition of Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2019, 77, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Wang, Q.; Yang, Y.; Ji, H. MicroRNA-495 targets Notch1 to prohibit cell proliferation and invasion in oral squamous cell carcinoma. Mol. Med. Rep. 2018, 19, 693–702. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, E.J.; Lee, S.; Tan, X.; Liu, X.; Park, S.; Kang, K.; Yoon, J.-S.; Ko, Y.H.; Kurie, J.M.; et al. MiR-34a and miR-34b/c have distinct effects on the suppression of lung adenocarcinomas. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Brito, B.D.L.; Lourenço, S.V.; Damascena, A.S.; Kowalski, L.P.; Soares, F.A.; Coutinho-Camillo, C.M. Expression of stem cell-regulating miRNAs in oral cavity and oropharynx squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 647–654. [Google Scholar] [CrossRef]
- Kumar, B.; Yadav, A.; Lang, J.; Teknos, T.N.; Kumar, P. Dysregulation of MicroRNA-34a Expression in Head and Neck Squamous Cell Carcinoma Promotes Tumor Growth and Tumor Angiogenesis. PLoS ONE 2012, 7, e37601. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, W.; Xu, J.; Kaufmann, A.M.; Albers, A.E. MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro. Int. J. Oncol. 2015, 47, 1339–1350. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, G.; Rao, A.K.D.M.; Manikandan, M.; Rao, H.P.S.; Subbiah, S.; Ilangovan, R.; Murugan, A.K.; Munirajan, A.K. Dysregulation of miR-200 family microRNAs and epithelial-mesenchymal transition markers in oral squamous cell carcinoma. Oncol. Lett. 2017, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, S.; Beder, L.B.; Hotomi, M.; Gunduz, M.; Yata, K.; Grenman, R.; Yamanaka, N. Role of miR-200c/miR-141 in the regulation of epithelial-mesenchymal transition and migration in head and neck squamous cell carcinoma. Int. J. Mol. Med. 2014, 33, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-T.; Yang, M.-H. Hybrid Epithelial/Mesenchymal State in Cancer Metastasis: Clinical Significance and Regulatory Mechanisms. Cells 2020, 9, 623. [Google Scholar] [CrossRef]
- He, P.; Qiu, K.; Jia, Y. Modeling of mesenchymal hybrid epithelial state and phenotypic transitions in EMT and MET processes of cancer cells. Sci. Rep. 2018, 8, 14323. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Jolly, M.K.; Levine, H.; Onuchic, J.N.; Ben-Jacob, E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc. Natl. Acad. Sci. USA 2013, 110, 18144–18149. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, H.; Kong, F.; Ye, J.; Jia, X.; Zhang, Z.; Li, N.; Yin, J.; Zheng, G.; He, Z. miR-22/KAT6B axis is a chemotherapeutic determiner via regulation of PI3k-Akt-NF-kB pathway in tongue squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 164. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef]
- Castellano, E.; Molina-Arcas, M.; Krygowska, A.A.; East, P.; Warne, P.; Nicol, A.; Downward, J. RAS signalling through PI3-Kinase controls cell migration via modulation of Reelin expression. Nat. Commun. 2016, 7, 11245. [Google Scholar] [CrossRef]
- Park, S.; Zhao, D.; Hatanpaa, K.J.; Mickey, B.E.; Saha, D.; Boothman, D.A.; Story, M.D.; Wong, E.T.; Burma, S.; Georgescu, M.-M.; et al. RIP1 Activates PI3K-Akt via a Dual Mechanism Involving NF-κB–Mediated Inhibition of the mTOR-S6K-IRS1 Negative Feedback Loop and Down-regulation of PTEN. Cancer Res. 2009, 69, 4107–4111. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 Integrates Wnt and Energy Signals via a Coordinated Phosphorylation by AMPK and GSK3 to Regulate Cell Growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of β-Catenin by AKT Promotes β-Catenin Transcriptional Activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.; Drabsch, Y.; Gao, R.; Snaar-Jagalska, B.E.; Mickanin, C.; Huang, H.; Sheppard, K.-A.; Porter, J.A.; Lu, C.X.; et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat. Cell Biol. 2012, 14, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.; Dijke, P.T. Signaling interplay between transforming growth factor-β receptor and PI3K/AKT pathways in cancer. Trends Biochem. Sci. 2013, 38, 612–620. [Google Scholar] [CrossRef]
- Budi, E.H.; Muthusamy, B.P.; Derynck, R. The insulin response integrates increased TGF-β signaling through Akt-induced enhancement of cell surface delivery of TGF-β receptors. Sci. Signal. 2015, 8, ra96. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.-H.; Landström, M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal. 2017, 10, eaal4186. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.R. PI3K in stemness regulation: From development to cancer. Biochem. Soc. Trans. 2020, 48, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Abraham, P.; Kota, R.; Isaac, B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem. Toxicol. 2018, 118, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Arakawa, T.; Ueda, N.; Yamamoto, S. Transcriptional Roles of Nuclear Factor κB and Nuclear Factor-Interleukin-6 in the Tumor Necrosis Factor α-Dependent Induction of Cyclooxygenase-2 in MC3T3-E1 Cells. J. Biol. Chem. 1995, 270, 31315–31320. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qi, H.; Ren, J.; Cui, J.; Li, Z.; Waldum, H.L.; Cui, G. Involvement of NF-κB/IL-6 Pathway in the Processing of Colorectal Carcinogenesis in Colitis Mice. Int. J. Inflamm. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.-C.; Goode, J.; Miething, C.; Göktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. NF-κB Is a Negative Regulator of IL-1β Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKβ. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Dickerson, J.A.; Tan, L.C.; Hebert, C.A. Activation of nuclear factor-κB correlates with MCP-1 expression by human mesangial cells. Kidney Int. 1995, 48, 1263–1271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, Y.; Gaynor, R.B. IκB kinases: Key regulators of the NF-κB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef]
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-κB Pathway and Cancer Stem Cells. Cells 2016, 5, 16. [Google Scholar] [CrossRef]
- Feng, X.; Luo, Q.; Wang, H.; Zhang, H.; Chen, F. MicroRNA-22 suppresses cell proliferation, migration and invasion in oral squamous cell carcinoma by targeting NLRP3. J. Cell. Physiol. 2018, 233, 6705–6713. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, L.; Li, Y.-C.; Wu, L.; Yu, G.-T.; Zhang, W.-F.; Sun, Z.-J. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.; Sun, Y.; Liu, P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J. Pharmacol. Sci. 2019, 140, 128–136. [Google Scholar] [CrossRef]
- Qiu, K.; Huang, Z.; Huang, Z.; He, Z.; You, S. miR-22 regulates cell invasion, migration and proliferation in vitro through inhibiting CD147 expression in tongue squamous cell carcinoma. Arch. Oral Biol. 2016, 66, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, Y.; Wu, K.; Wang, L.; Jiang, Y.; Chen, W.; Yan, M. CD147 promotes progression of head and neck squamous cell carcinoma via NF-kappa B signaling. J. Cell. Mol. Med. 2019, 23, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Toyoma, S.; Tsuji, T.; Kawasaki, Y.; Yamada, T. CD147 mediates transforming growth factor-β1-induced epithelial-mesenchymal transition and cell invasion in squamous cell carcinoma of the tongue. Exp. Ther. Med. 2019, 17, 2855–2860. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, C.; Chen, D.; Chen, S.; Zheng, H. MicroRNA-98-HMGA2-POSTN signal pathway reverses epithelial-to-mesenchymal transition in laryngeal squamous cell carcinoma. Biomed. Pharmacother. 2019, 117, 108998. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, L.; Ren, H.; Chen, X.; Wang, Y.; Ge, J.; Wu, S.; Sun, Y.; Liu, M.; Xiao, H. MicroRNA-101 is a potential prognostic indicator of laryngeal squamous cell carcinoma and modulates CDK8. J. Transl. Med. 2015, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Bass, A.J.; Kim, S.Y.; Dunn, I.F.; Silver, S.J.; Guney, I.; Freed, E.; Ligon, A.H.; Vena, N.; Ogino, S.; et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 2008, 455, 547–551. [Google Scholar] [CrossRef] [PubMed]
- He, S.-B.; Yuan, Y.; Wang, L.; Yu, M.-J.; Zhu, Y.-B.; Zhu, X.-G. Effects of cyclin-dependent kinase 8 specific siRNA on the proliferation and apoptosis of colon cancer cells. J. Exp. Clin. Cancer Res. 2011, 30, 109. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, P.; Ma, Y.; Lu, Z.; Meng, J.; Li, H.; Wang, X.; Chen, D.; Zhang, M.; Han, Y.; et al. MicroRNA-139-5p affects cisplatin sensitivity in human nasopharyngeal carcinoma cells by regulating the epithelial-to-mesenchymal transition. Gene 2018, 652, 48–58. [Google Scholar] [CrossRef]
- Brabletz, T.; Jung, A.; Spaderna, S.; Hlubek, F.; Kirchner, T. Migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer 2005, 5, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, I.D.; Vermeulen, M. ZBTB 2 reads unmethylated CpG island promoters and regulates embryonic stem cell differentiation. EMBO Rep. 2018, 19, e44993. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; He, Z.; Xie, D.; Ni, J.; Lin, X. MicroRNA-488-3p inhibits proliferation and induces apoptosis by targeting ZBTB2 in esophageal squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 18702–18713. [Google Scholar] [CrossRef] [PubMed]
- Godar, S.; Ince, T.A.; Bell, G.W.; Feldser, D.; Donaher, J.L.; Bergh, J.; Liu, A.; Miu, K.; Watnick, R.S.; Reinhardt, F.; et al. Growth-Inhibitory and Tumor- Suppressive Functions of p53 Depend on Its Repression of CD44 Expression. Cell 2008, 134, 62–73. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, J.-W.; Liu, G.-Y.; Wang, F.-G.; Ju, Z.-S.; Zhou, N.; Wang, R.-Y. MicroRNA-372 enhances radiosensitivity while inhibiting cell invasion and metastasis in nasopharyngeal carcinoma through activating the PBK-dependent p53 signaling pathway. Cancer Med. 2019, 8, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Chao, C.-H.; Xia, W.; Yang, J.-Y.; Xiong, Y.; Li, C.-W.; Yu, W.-H.; Rehman, S.K.; Hsu, J.L.; Lee, H.-H.; et al. p53 regulates epithelial–mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 2011, 13, 317–323. [Google Scholar] [CrossRef]
- Shi, B.; Yan, W.; Liu, G.; Guo, Y. MicroRNA-488 inhibits tongue squamous carcinoma cell invasion and EMT by directly targeting ATF3. Cell. Mol. Biol. Lett. 2018, 23, 28. [Google Scholar] [CrossRef]
- Su, J.-L.; Chen, P.-S.; Johansson, G.; Kuo, M.-L. Function and Regulation of Let-7 Family microRNAs. MicroRNA 2012, 1, 34–39. [Google Scholar] [CrossRef]
- Chen, P.-S.; Su, J.-L.; Cha, S.-T.; Tarn, W.-Y.; Wang, M.-Y.; Hsu, H.-C.; Lin, M.-T.; Chu, C.-Y.; Hua, K.-T.; Chen, C.-N.; et al. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J. Clin. Investig. 2011, 121, 3442–3455. [Google Scholar] [CrossRef]
- González-Arriagada, W.A.; Olivero, P.; Rodríguez, B.; Lozano-Burgos, C.; De Oliveira, C.E.; Coletta, R.D. Clinicopathological significance of miR-26, miR-107, miR-125b, and miR-203 in head and neck carcinomas. Oral Dis. 2018, 24, 930–939. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Z.; Zhang, K.; Dong, Y.; Zhang, A.; Lu, C.; Liu, L. MicroRNA-107 inhibits proliferation and invasion of laryngeal squamous cell carcinoma cells by targeting CACNA2D1 in vitro. Anti-Cancer Drugs 2020, 31, 260–271. [Google Scholar] [CrossRef]
- Sui, X.; Geng, J.-H.; Li, Y.-H.; Zhu, G.-Y.; Wang, W.-H. Calcium channel α2δ1 subunit (CACNA2D1) enhances radioresistance in cancer stem-like cells in non-small cell lung cancer cell lines. Cancer Manag. Res. 2018, 10, 5009–5018. [Google Scholar] [CrossRef]
- Patel, S.; Rawal, R. Role of miRNA dynamics and cytokine profile in governing CD44v6/Nanog/PTEN axis in oral cancer: Modulating the master regulators. Tumor Biol. 2016, 37, 14565–14575. [Google Scholar] [CrossRef]
- Hersi, H.M.; Raulf, N.; Gaken, J.; Folarin, N.; Tavassoli, M. Micro RNA-9 inhibits growth and invasion of head and neck cancer cells and is a predictive biomarker of response to plerixafor, an inhibitor of its target CXCR 4. Mol. Oncol. 2018, 12, 2023–2041. [Google Scholar] [CrossRef]
- Citron, F.; Armenia, J.; Franchin, G.; Polesel, J.; Talamini, R.; D’Andrea, S.; Sulfaro, S.; Croce, C.M.; Klement, W.; Otasek, D.; et al. An Integrated Approach Identifies Mediators of Local Recurrence in Head and Neck Squamous Carcinoma. Clin. Cancer Res. 2017, 23, 3769–3780. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, Z.; Wang, J.; Wu, Y.; Chen, W.; Zheng, L.; Xi, T.; Wang, A.; Lu, Y. MicroRNA-9 and breast cancer. Biomed. Pharmacother. 2020, 122, 109687. [Google Scholar] [CrossRef]
- Kamal, N.N.S.B.N.M.; Shahidan, W.N.S. Non-Exosomal and Exosomal Circulatory Micrornas: Which Are More Valid as Biomarkers? Front. Pharmacol. 2020, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Sharma, P.; Wise, P.; Sposto, R.; Hollingshead, D.; Lamb, J.; Lang, S.; Fabbri, M.; Whiteside, T.L. mRNA and miRNA Profiles of Exosomes from Cultured Tumor Cells Reveal Biomarkers Specific for HPV16-Positive and HPV16-Negative Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 8570. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, C.-R.; Rigas, N.K.; Kim, R.H.; Kang, M.K.; Park, N.-H.; Shin, K.-H. Human papillomavirus 16 (HPV16) enhances tumor growth and cancer stemness of HPV-negative oral/oropharyngeal squamous cell carcinoma cells via miR-181 regulation. Papillomavirus Res. 2015, 1, 116–125. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, J.; Zheng, J.; Hsueh, C.; Guo, Y.; Zhou, L. Characterization of selective exosomal microRNA expression profile derived from laryngeal squamous cell carcinoma detected by next generation sequencing. Oncol. Rep. 2018, 40, 2584–2594. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Zou, L.; Zhu, Z. Role of Exosomes in Crosstalk between Cancer-Associated Fibroblasts and Cancer Cells. Front. Oncol. 2019, 9, 356. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.G.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef]
- Benjamins, J.A.; Nedelkoska, L.; Touil, H.; Stemmer, P.M.; Carruthers, N.J.; Jena, B.P.; Naik, A.R.; Bar-Or, A.; Lisak, R.P. Exosome-enriched fractions from MS B cells induce oligodendrocyte death. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e550. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, H.; He, Q.; Tian, W.; Xia, A.; Lu, X.-J. Exosomes derived from exhausted CD8+ T cells impaired the anticancer function of normal CD8+ T cells. J. Med. Genet. 2019, 56, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Int. Rev. Cytol. 2016, 74, 103–141. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Tao, Y.-W.; Gao, S.; Li, P.; Zheng, J.-M.; Zhang, S.-E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A. miRNAs in oncogenesis and tumor suppression. In International Review of Cell and Molecular Biology, MiRNAs in Differentiation and Development, 1st ed.; Galluzzi, L., Vitale, I., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 333. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).