We read with interest the article by Lee et al. [1], which evaluated the folate enzyme ALDH1L1 as a therapeutic target in the treatment of non-small cell lung cancer. The authors hypothesized that upregulation of ALDH1L1 expression is linked to KRAS, and they performed a set of experiments to test this hypothesis in cell culture and animal models. In the initial experiment, the authors demonstrated the expression of ALDH1L1 protein in A549 lung cancer cells, a highly surprising finding. Indeed, our research group has shown in 2002 [2] that A549 cells (as in the majority of cancer cell lines) do not express this protein. In agreement with our data, the Human Protein Atlas (https://www.proteinatlas.org/ENSG00000144908-ALDH1L1/cell; accessed on 15 August 2020) also shows a lack of ALDH1L1 mRNA in A549 cells. We later established that silencing of the ALDH1L1 gene in several cancers and cancer cell lines (including A549 cells) is associated with strong methylation of the gene promoter [3].
We became curious as to why such a disagreement with earlier published data appeared in Lee’s paper. To make sure that the appearance of the band assigned as ALDH1L1 protein in Lee’s paper is not associated with abnormal properties of cells used in the study, or by specificity of a particular antibody, we obtained A549 cells from the same source, the DCTD Tumor Repository, NCI/NIH, and tested them for the presence of ALDH1L1 using an Abcam antibody (cat. #ab175198), as in Lee’s paper. As can be seen in the figure, there was no detectable ALDH1L1 protein in the A549 cells. As a control, we used RT4 cells known to express high levels of this protein. We also used our in-house ALDH1L1-specific antibody, which has been validated and used in numerous of our published studies, as well as in studies from other research groups [2,4,5,6]. This antibody did not detect any ALDH1L1 protein in the A549 cells either (Figure 1). Further, in agreement with our previous report [7], levels of ALDH1L1 mRNA evaluated by qPCR were below the detection limit in the A549 cells (Figure 1). We also re-evaluated H1299 cells for the ALDH1L1 expression, since we previously reported a lack of the protein in these cells [8]. Neither ALDH1L1 protein nor its mRNA were detected in the H1299 cells (Figure 1).
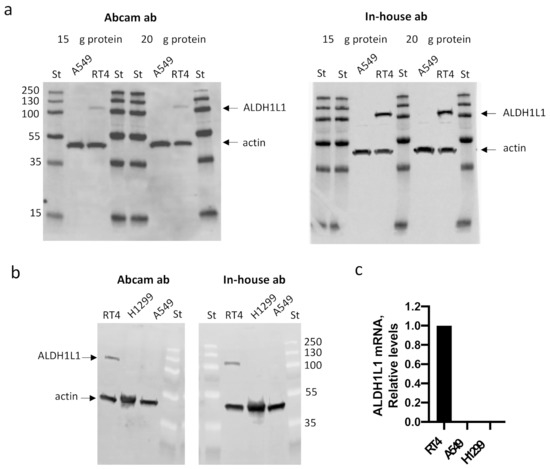
Figure 1.
Levels of ALDH1L1 protein (a,b, Western blot) and mRNA (c, qPCR) in A549, H1299, and RT4 cells. The Abcam antibody cat. #ab175198 (used in Lee’s paper [1]), as well as our in-house antibody [2], did not detect ALDH1L1 in the A549 or H1299 cells. Actin was used as loading control. Twenty µg of total protein was loaded in panel b. St, pre-stained protein ladder (ThermoFisher, cat. #26619; numbers indicate molecular masses of standards).
Surprisingly, Lee and the co-authors failed to acknowledge that there is a significant body of literature demonstrating a strong downregulation of ALDH1L1 in several cancers (recently reviewed in [9]). The only paper suggesting an upregulation of the protein in a specific cancer, lung cancer, was from the same group [10]. Curiously, the paper reported that A549 cells have the highest levels of ALDH1L1 protein among several cell lines [10]. It should be also noted that Lee’s paper [1] contains several incorrect statements regarding the published literature. In the introduction to their study, the authors cited two papers [11,12] to suggest that ALDH1L1 is upregulated in lung cancer, but neither of the references show an upregulation of ALDH1L1 in non-small cell lung cancer. In fact, these papers did not even mention ALDH1L1. Further, the domains of ALDH1L1 are not listed correctly. Curiously, throughout the paper, the authors emphasized the production of NADH by ALDH1L1. ALDH1L1, however, has exceptionally strong preference for NADP+ [13], and there is no experimental evidence that the enzyme can utilize NAD+ in vivo. Lee and the co-authors made their speculation based on the ratios of NAD+/NADH versus NADP+/NADPH, which is not a sufficient argument (we refer readers to an excellent review on the subject [14]).
Considering that the information presented by the authors on the expression of ALDH1L1 in A549 and H1299 cell lines contradicts the previously published expression pattern of the protein, as well as their failure to acknowledge commonly observed ALDH1L1 downregulation in cancers and their unsubstantiated statements with incorrect citations, we suggest that this paper needs to be revised in order to prevent misleading the scientific readership.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, S.H.; Jeon, Y.; Kang, J.H.; Jang, H.; Lee, H.; Kim, S.Y. The Combination of Loss of ALDH1L1 Function and Phenformin Treatment Decreases Tumor Growth in KRAS-Driven Lung Cancer. Cancers 2020, 12, 1382. [Google Scholar] [CrossRef] [PubMed]
- Krupenko, S.A.; Oleinik, N.V. 10-formyltetrahydrofolate dehydrogenase, one of the major folate enzymes, is down-regulated in tumor tissues and possesses suppressor effects on cancer cells. Cell Growth Differ. 2002, 13, 227–236. [Google Scholar] [PubMed]
- Oleinik, N.V.; Krupenko, N.I.; Krupenko, S.A. Epigenetic silencing of ALDH1L1, a metabolic regulator of cellular proliferation, in cancers. Genes Cancer 2011, 2, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.F.; Courcol, M.; Mariet, C.; Charbonnier, A.; Boitier, E.; Duchesne, M.; Parker, F.; Genet, B.; Supatto, F.; Roberts, R.; et al. Proteomic characterization of the effects of clofibrate on protein expression in rat liver. Proteomics 2006, 6, 1915–1933. [Google Scholar] [CrossRef] [PubMed]
- Cains, S.; Shepherd, A.; Nabiuni, M.; Owen-Lynch, P.J.; Miyan, J. Addressing a folate imbalance in fetal cerebrospinal fluid can decrease the incidence of congenital hydrocephalus. J. Neuropathol. Exp. Neurol. 2009, 68, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Krupenko, N.I.; Dubard, M.E.; Strickland, K.C.; Moxley, K.M.; Oleinik, N.V.; Krupenko, S.A. ALDH1L2 is the mitochondrial homolog of 10-formyltetrahydrofolate dehydrogenase. J. Biol. Chem. 2010, 285, 23056–23063. [Google Scholar] [CrossRef]
- Oleinik, N.V.; Krupenko, N.I.; Priest, D.G.; Krupenko, S.A. Cancer cells activate p53 in response to 10-formyltetrahydrofolate dehydrogenase expression. Biochem. J. 2005, 391, 503–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krupenko, S.A.; Krupenko, N.I. Loss of ALDH1L1 folate enzyme confers a selective metabolic advantage for tumor progression. Chem. Biol. Interact. 2019, 302, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, S.H.; Lee, J.S.; Nam, B.; Seong, T.W.; Son, J.; Jang, H.; Hong, K.M.; Lee, C.; Kim, S.Y. Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion. Oncotarget 2016, 7, 49397–49410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, L.; Geng, J.; Wu, C.L.; Bai, X. Aldehyde dehydrogenase 1A1 possesses stem-like properties and predicts lung cancer patient outcome. J. Thorac. Oncol. 2012, 7, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.; Wang, H.; Liu, Z.; Su, Y.; Stass, S.A.; et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Krupenko, S.A.; Wagner, C.; Cook, R.J. Expression, purification, and properties of the aldehyde dehydrogenase homologous carboxyl-terminal domain of rat 10-formyltetrahydrofolate dehydrogenase. J. Biol. Chem. 1997, 272, 10266–10272. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Calvo, S.E.; Mootha, V.K. Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J. Biol. Chem. 2018, 293, 7508–7516. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).