Change in Physical Performance Correlates with Decline in Quality of Life and Frailty Status in Head and Neck Cancer Patients Undergoing Radiation with and without Chemotherapy
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Participants
2.3. Treatments
2.4. The Short Physical Performance Battery
2.5. Health-Related Quality of Life (QoL)
2.6. Frailty
2.7. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Pre- and Post-Treatment Comparisons
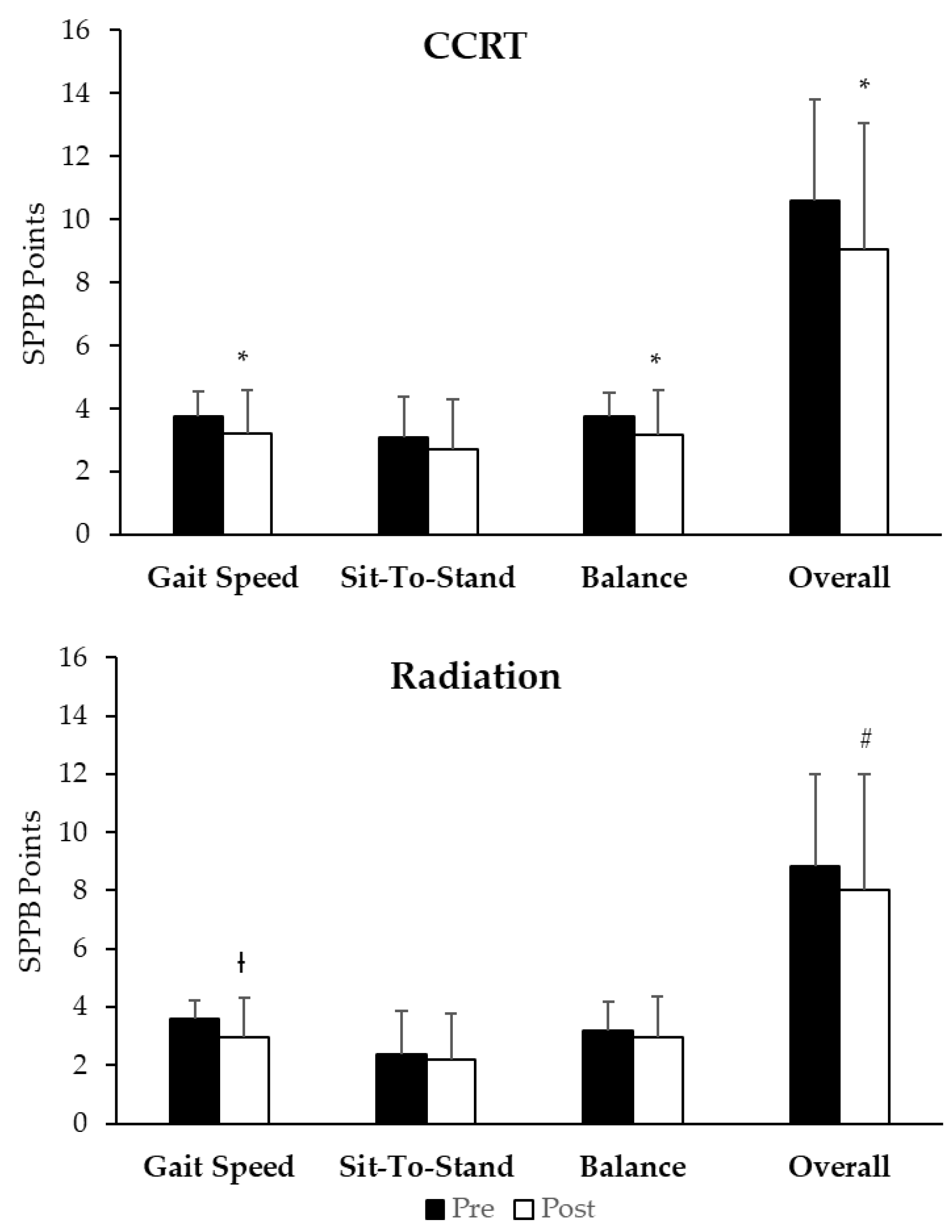
3.3. Short Physical Performance Battery
3.4. Quality of Life
3.5. SPPB and QoL
3.6. Frailty
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Head and Neck Cancer Version 2. 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 6 January 2020).
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef]
- Bossi, P.; Di Pede, P.; Guglielmo, M.; Granata, R.; Alfieri, S.; Iacovelli, N.A.; Orlandi, E.; Guzzo, M.; Bianchi, R.; Ferella, L.; et al. Prevalence of Fatigue in Head and Neck Cancer Survivors. Ann. Otol. Rhinol. Laryngol. 2019, 128, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Crary, M.A.; Madhavan, A.; Gregorio, D.C.; Im, I.; Ross, S.E.; Carnaby, G.D. Dysphagia and Oral Morbidities in Chemoradiation-Treated Head and Neck Cancer Patients. Dysphagia 2018, 33, 739–748. [Google Scholar] [CrossRef]
- van Deudekom, F.J.; Schimberg, A.S.; Kallenberg, M.H.; Slingerland, M.; van der Velden, L.A.; Mooijaart, S.P. Functional and cognitive impairment, social environment, frailty and adverse health outcomes in older patients with head and neck cancer, a systematic review. Oral Oncol. 2017, 64, 27–36. [Google Scholar] [CrossRef]
- McNeely, M.L.; Parliament, M.B.; Seikaly, H.; Jha, N.; Magee, D.J.; Haykowsky, M.J.; Courneya, K.S. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: A randomized controlled trial. Cancer 2008, 113, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mulasi, U.; Vock, D.M.; Kuchnia, A.J.; Jha, G.; Fujioka, N.; Rudrapatna, V.; Patel, M.R.; Teigen, L.; Earthman, C.P. Malnutrition Identified by the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition Consensus Criteria and Other Bedside Tools Is Highly Prevalent in a Sample of Individuals Undergoing Treatment for Head and Neck Cancer. J. Parenter. Enter. Nutr. 2016, 42, 139–147. [Google Scholar] [CrossRef]
- Inouye, S.K.; Peduzzi, P.N.; Robison, J.T.; Hughes, J.S.; Horwitz, R.I.; Concato, J. Importance of Functional Measures in Predicting Mortality Among Older Hospitalized Patients. JAMA 1998, 279, 1187–1193. [Google Scholar] [CrossRef]
- Rhoten, B.A.; Murphy, B.; Ridner, S.H. Body image in patients with head and neck cancer: A review of the literature. Oral Oncol. 2013, 49, 753–760. [Google Scholar] [CrossRef]
- Ringash, J. Quality of Life in Head and Neck Cancer: Where We Are, and Where We Are Going. Int. J. Radiat. Oncol. 2017, 97, 662–666. [Google Scholar] [CrossRef]
- Jameson, M.J.; Karnell, L.H.; Christensen, A.J.; Funk, G.F. First-Year Trends in Self-reported General Health Predict Survival in Patients with Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 958. [Google Scholar] [CrossRef][Green Version]
- Karvonen-Gutierrez, C.A.; Ronis, D.L.; Fowler, K.E.; Terrell, J.E.; Gruber, S.B.; Duffy, S.A. Quality of Life Scores Predict Survival Among Patients With Head and Neck Cancer. J. Clin. Oncol. 2008, 26, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Fortin, A.; Gélinas, M.; Nabid, A.; Brochet, F.; Têtu, B.; Bairati, I. Health-Related Quality of Life As a Survival Predictor for Patients With Localized Head and Neck Cancer Treated With Radiation Therapy. J. Clin. Oncol. 2009, 27, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Oskam, I.M.; Leeuw, I.M.V.-D.; Aaronson, N.K.; Kuik, D.J.; de Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, R.C. Quality of life as predictor of survival: A prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiother. Oncol. 2010, 97, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Østhus, A.A.; Aarstad, A.K.H.; Olofsson, J.; Aarstad, H.J. Prediction of Survival by Pretreatment Health-Related Quality-of-Life Scores in a Prospective Cohort of Patients With Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Neck Surg. 2013, 139, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Quinten, C.; Martinelli, F.; Coens, C.; Sprangers, M.A.G.; Ringash, J.; Gotay, C.; Bjordal, K.; Greimel, E.; Reeve, B.B.; Maringwa, J.; et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014, 120, 302–311. [Google Scholar] [CrossRef]
- Rogers, S.N.; Waylen, A.E.; Thomas, S.; Penfold, C.; Pring, M.; Waterboer, T.; Pawlita, M.; Hurley, K.; Ness, A.R. Quality of life, cognitive, physical and emotional function at diagnosis predicts head and neck cancer survival: Analysis of cases from the Head and Neck 5000 study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Verdonck-de Leeuw, I.M.; Buffart, L.M.; Heymans, M.W.; Rietveld, D.H.; Doornaert, P.; de Bree, R.; Buter, J.; Aaronson, N.K.; Slotman, B.J.; Leemans, C.R.; et al. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: A prospective cohort study. Radiother. Oncol. 2014, 110, 422–428. [Google Scholar] [CrossRef]
- de Vries, J.; Bras, L.; Sidorenkov, G.; Festen, S.; Steenbakkers, R.J.H.M.; Langendijk, J.A.; Witjes, M.J.H.; van der Laan, B.F.A.M.; de Bock, G.H.; Halmos, G.B. Frailty is associated with decline in health-related quality of life of patients treated for head and neck cancer. Oral Oncol. 2020, 111, 105020. [Google Scholar] [CrossRef]
- Fu, T.S.; Sklar, M.; Cohen, M.; De Almeida, J.R.; Sawka, A.M.; Alibhai, S.M.; Goldstein, D.P. Is Frailty Associated With Worse Outcomes After Head and Neck Surgery? A Narrative Review. Laryngoscope 2020, 130, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.P.; Sklar, M.C.; De Almeida, J.R.; Gilbert, R.; Gullane, P.; Irish, J.; Brown, D.; Higgins, K.; Enepekides, D.; Xu, W.; et al. Frailty as a predictor of outcomes in patients undergoing head and neck cancer surgery. Laryngoscope 2019, 130, E340–E345. [Google Scholar] [CrossRef]
- Maggiore, R.; Zumsteg, Z.S.; BrintzenhofeSzoc, K.; Trevino, K.M.; Gajra, A.; Korc-Grodzicki, B.; Epstein, J.B.; Bond, S.M.; Parker, I.; Kish, J.A.; et al. The Older Adult with Locoregionally Advanced Head and Neck Squamous Cell Carcinoma: Knowledge Gaps and Future Direction in Assessment and Treatment. Int. J. Radiat. Oncol. 2017, 98, 868–883. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Harhay, M.O. Physical function as a prognostic biomarker among cancer survivors. Br. J. Cancer 2014, 112, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Cerullo, F.; Zamboni, V.; Di Palma, R.; Scambia, G.; Balducci, L.; Incalzi, R.A.; Vellas, B.; Gambassi, G. Functional Status and Mortality in Older Women With Gynecological Cancer. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, L.C.; Nishimura, K.C.; McNeely, M.L.; Lau, H.; Culos-Reed, S.N. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: A systematic review. Br. J. Sports Med. 2016, 50, 325–338. [Google Scholar] [CrossRef]
- McCloskey, S.A.; Jaggernauth, W.; Rigual, N.R.; Hicks, W.L.; Popat, S.R.; Sullivan, M.; Mashtare, T.L.; Khan, M.K.; Loree, T.R.; Singh, A.K. Radiation Treatment Interruptions Greater Than One Week and Low Hemoglobin Levels (12 g/dL) are Predictors of Local Regional Failure After Definitive Concurrent Chemotherapy and Intensity-Modulated Radiation Therapy for Squamous Cell Carcinoma of the Head and Neck. Am. J. Clin. Oncol. 2009, 32, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Platek, M.E.; McCloskey, S.A.; Cruz, M.; Burke, M.S.; Reid, M.E.; Wilding, G.E.; Rigual, N.R.; Popat, S.R.; Loree, T.R.; Gupta, V.; et al. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck 2013, 35, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; Di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Tan, K.-Y.; Kawamura, Y.J.; Tokomitsu, A.; Tang, T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am. J. Surg. 2012, 204, 139–143. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Impact of physical frailty on disability in community-dwelling older adults: A prospective cohort study. BMJ Open 2015, 5, e008462. [Google Scholar] [CrossRef]
- Collins, J.T.; Noble, S.; Chester, J.; Davies, H.E.; Evans, W.D.; Farewell, D.; Lester, J.F.; Parry, D.; Pettit, R.; Byrne, A. The value of physical performance measurements alongside assessment of sarcopenia in predicting receipt and completion of planned treatment in non-small cell lung cancer: An observational exploratory study. Support. Care Cancer 2017, 26, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Saroul, N.; Pastourel, R.; Mulliez, A.; Farigon, N.; Dupuch, V.; Mom, T.; Boirie, Y.; Gilain, L. Which Assessment Method of Malnutrition in Head and Neck Cancer? Otolaryngol. Neck Surg. 2018, 158, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Chargi, N.; Bril, S.I.; Emmelot-Vonk, M.H.; De Bree, R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 1475–1486. [Google Scholar] [CrossRef]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, P.; Karimi, A.M.; Gairola, M.; Tandon, S.; Pal, M.; Sachdeva, N.; Sharief, M.I.; Dobriyal, K. Health-related quality of life assessment for head-and-neck cancer patients during and at 3 months after radiotherapy—A prospective, analytical questionnaire-based study. Natl. J. Maxillofac. Surg. 2019, 10, 134–140. [Google Scholar] [CrossRef]
- Klein, J.; Livergant, J.; Ringash, J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: A systematic review. Oral Oncol. 2014, 50, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Morton, R. Deterioration in quality-of-life of late (10-year) survivors of head and neck cancer. Clin. Otolaryngol. 2006, 31, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Oskam, I.M.; Leeuw, I.M.V.-D.; Aaronson, N.K.; Witte, B.I.; De Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, C.R. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013, 49, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Gupta, T.; Ghosh-Laskar, S.; Murthy, V.; Budrukkar, A.; Agarwal, J. Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): Evidence from a prospective randomized study. Oral Oncol. 2013, 49, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Hammerlid, E.; Adnan, A.; Silander, E. Population-based reference values for the European Organization for Research and Treatment of Cancer Head and Neck module. Head Neck 2017, 39, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Sandmael, J.A.; Bye, A.; Solheim, T.S.; Stene, G.B.; Thorsen, L.; Kaasa, S.; Lund, J.-Å.; Oldervoll, L.M. Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: A pilot randomized trial. Cancer 2017, 123, 4440–4448. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Robbins, K.T.; Malone, J.; Seiz, A.; Koch, L.; Rao, K.; Nagarkar, M. Physical activity and quality of life in head and neck cancer survivors. Support. Care Cancer 2006, 14, 1012–1019. [Google Scholar] [CrossRef]
- Eldridge, R.C.; Pugh, S.L.; Trotti, A.; Hu, K.; Spencer, S.; Yom, S.S.; Rosenthal, D.; Read, N.; Desai, A.; Gore, E.; et al. Changing functional status within 6 months posttreatment is prognostic of overall survival in patients with head and neck cancer: NRG Oncology Study. Head Neck 2019, 41, 3924–3932. [Google Scholar] [CrossRef]
- Osthus, A.A.; Aarstad, A.K.; Olofsson, J.; Aarstad, H.J. Head and neck specific Health Related Quality of Life scores predict subsequent survival in successfully treated head and neck cancer patients: A prospective cohort study. Oral Oncol. 2011, 47, 974–979. [Google Scholar] [CrossRef]
- Fang, F.-M.; Chiu, H.-C.; Kuo, W.-R.; Wang, C.-J.; Leung, S.W.; Chen, H.-C.; Sun, L.-M.; Hsu, H.-C. Health-related quality of life for nasopharyngeal carcinoma patients with cancer-free survival after treatment. Int. J. Radiat. Oncol. 2002, 53, 959–968. [Google Scholar] [CrossRef]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Kenis, C.; Decoster, L.; Bastin, J.; Bode, H.; Van Puyvelde, K.; De Grève, J.; Conings, G.; Fagard, K.; Flamaing, J.; Milisen, K.; et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J. Geriatr. Oncol. 2017, 8, 196–205. [Google Scholar] [CrossRef]
- Pitts, K.D.; Arteaga, A.A.; Stevens, B.P.; White, W.C.; Su, D.; Spankovich, C.; Jefferson, G.D.; Jackson, L.L. Frailty as a Predictor of Postoperative Outcomes among Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 2019, 160, 664–671. [Google Scholar] [CrossRef]
- Nieman, C.L.; Pitman, K.T.; Tufaro, A.P.; Eisele, D.W.; Frick, K.D.; Gourin, C.G. The effect of frailty on short-term outcomes after head and neck cancer surgery. Laryngoscope 2018, 128, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Kim, S.; Roh, J.; Lee, S.; Kim, S.; Choi, S.; Nam, S.Y.; Kim, S.Y. An Introduction to a Head and Neck Cancer-Specific Frailty Index and Its Clinical Implications in Elderly Patients: A Prospective Observational Study Focusing on Respiratory and Swallowing Functions. Oncologist 2016, 21, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- VanderWalde, N.A.; Deal, A.M.; Comitz, E.; Stravers, L.; Muss, H.; Reeve, B.B.; Basch, E.; Tepper, J.; Chera, B. Geriatric Assessment as a Predictor of Tolerance, Quality of Life, and Outcomes in Older Patients With Head and Neck Cancers and Lung Cancers Receiving Radiation Therapy. Int. J. Radiat. Oncol. 2017, 98, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.E.; Orsak, B.; Wang, C.P.; MacCarthy, D.; Kellogg, D.; Powers, B.; Conde, A.; Moris, M.; Padala, P.R.; Padala, K.P. An Individualized Low-Intensity Walking Clinic Leads to Improvement in Frailty Characteristics in Older Veterans. J. Frailty Aging 2019, 8, 205–209. [Google Scholar]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.P.; Kurian, J.R.; Lin, P.-J.; Mustian, K.M. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support. Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef]
- Paleri, V.; Wight, R.G.; Silver, C.E.; Haigentz, M.; Takes, R.P.; Bradley, P.J.; Rinaldo, A.; Sanabria, A.; Bień, S.; Ferlito, A. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol. 2010, 46, 712–719. [Google Scholar] [CrossRef]
- Akbaba, S.; Rühle, A.; Rothhaar, S.; Zamboglou, C.; Gkika, E.; Foerster, R.; Oebel, L.; Klodt, T.; Schmidberger, H.; Grosu, A.-L.; et al. Treatment outcomes of elderly salivary gland cancer patients undergoing radiotherapy—Results from a large multicenter analysis. Radiother. Oncol. 2020, 156, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Stromberger, C.; Haehl, E.; Senger, C.; David, H.; Stoian, R.; Zamboglou, C.; Knopf, A.; Budach, V.; Grosu, A.-L.; et al. Development and validation of a novel prognostic score for elderly head-and-neck cancer patients undergoing radiotherapy or chemoradiation. Radiother. Oncol. 2021, 154, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Habbous, S.; Espin-Garcia, O.; Chen, D.; Huang, S.H.; Simpson, C.; Xu, W.; Liu, F.-F.; Brown, D.H.; Gilbert, R.W.; et al. Comorbidity and performance status as independent prognostic factors in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 736–742. [Google Scholar] [CrossRef]
- Schimansky, S.; Lang, S.; Beynon, R.; Penfold, C.; Davies, A.; Waylen, A.; Thomas, S.; Pring, M.; Pawlita, M.; Waterboer, T.; et al. Association between comorbidity and survival in head and neck cancer: Results from Head and Neck 5000. Head Neck 2018, 41, 1053–1062. [Google Scholar] [CrossRef] [PubMed]

| Overall | CCRT | Radiation | p-Value | |
|---|---|---|---|---|
| # (%) | 106 (100) | 75 (70.8) | 31 (29.2) | |
| Age (Years) | 64.0 ± 10.5 | 61.5 ± 9.0 | 69.3 ± 11.5 | <0.001 |
| Male (%) | 74.5 | 76.4 | 70.6 | 0.33 |
| ECOG | <0.01 | |||
| 0 | 65.1 | 74.7 | 41.9 | |
| 1 | 34.9 | 25.3 | 58.1 | |
| KPS (%) | <0.001 | |||
| 100 | 21.7 | 25.3 | 12.9 | |
| 90 | 44.3 | 50.7 | 29 | |
| 80 | 25.5 | 18.7 | 41.9 | |
| 70 | 8.5 | 5.3 | 16.1 | |
| Caucasian (%) | 87.7 | 84.7 | 94.1 | 0.76 |
| Height (cm) | 173.0 ± 9.6 | 174.5 ± 9.8 | 170.1 ± 8.4 | <0.05 |
| Weight (Kg) | 86.7 ± 21.5 | 87.6 ± 20.7 | 84.7 ± 24.0 | 0.51 |
| BMI (kg/m2) | 29.0 ± 6.6 | 28.7 ± 6.1 | 29.5 ± 7.4 | 0.07 |
| HPV+ (%) | 51.9 | 59.7 | 35.3 | 0.09 |
| Surgery (%) | 36.8 | 26.4 | 58.8 | <0.05 |
| Smoking (%) | 0.39 | |||
| Former | 56.6 | 56.9 | 55.9 | |
| Never | 35.8 | 37.5 | 32.4 | |
| Current | 6.6 | 5.6 | 8.8 | |
| Unknown | 0.9 | 0 | 2.9 | |
| Site (%) | <0.01 | |||
| Pharynx | 61.3 | 72.2 | 38.2 | |
| Larynx | 17.9 | 13.9 | 26.5 | |
| Lip/Oral Cavity | 11.3 | 5.6 | 23.5 | |
| Other | 9.4 | 8.4 | 11.7 | |
| Stage (%) | 0.1 | |||
| I | 23.1 | 26.7 | 13.8 | |
| II | 21.1 | 16 | 34.4 | |
| III | 26 | 24 | 31 | |
| IV | 29.8 | 33.3 | 20.7 | |
| Treatment Days | 44.9 ± 4.2 | 45.7 ± 4.3 | 43.0 ± 3.4 | <0.01 |
| Dose | 68.3 ± 3.9 | 68.9 ± 3.9 | 66.8 ± 3.7 | <0.05 |
| Fraction | 34 ± 1.7 | 34.6 ± 1.1 | 33.1 ± 2.3 | <0.001 |
| CCRT | Radiation | Pre | Post | Change | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value | Pre | Post | p-Value | CCRT vs. Radiation | CCRT vs. Radiation | CCRT vs. Radiation | |
| Functional | |||||||||
| Global | 73.0 ± 24.3 | 56.5 ± 23.8 | <0.001 | 67.5 ± 20.8 | 57.5 ± 27.5 | 0.023 | 0.119 | 0.521 | 0.381 |
| Physical | 90.4 ± 18.7 | 76.1 ± 22.3 | <0.001 | 80.0 ± 19.5 | 69.5 ± 28.2 | 0.019 | 0.077 | 0.262 | 0.488 |
| Role | 85.2 ± 27.1 | 60.2 ± 30.8 | <0.001 | 76.4 ± 31.9 | 59.1 ± 34.7 | 0.001 | 0.249 | 0.579 | 0.583 |
| Emotional | 74.7 ± 22.1 | 73.5 ± 24.0 | 0.628 | 78.1 ± 17.7 | 76.3 ± 18.7 | 0.301 | 0.908 | 0.725 | 0.793 |
| Cognitive | 85.9 ± 18.2 | 79.4 ± 25.5 | 0.005 | 88.9 ± 14.7 | 83.9 ± 20.0 | 0.458 | 0.941 | 0.629 | 0.536 |
| Social | 83.1 ± 23.0 | 68.1 ± 27.3 | <0.001 | 81.1 ± 23.1 | 70.4 ± 30.9 | 0.050 | 0.851 | 0.791 | 0.689 |
| Symptoms | |||||||||
| Fatigue | 76.7 ± 23.4 | 49.6 ± 26.6 | <0.001 | 69.3 ± 19.7 | 53.1 ± 28.7 | 0.009 | 0.184 | 0.682 | 0.099 |
| Nausea | 4.2 ± 14.3 | 17.9 ± 23.6 | <0.001 | 5.0 ± 10.9 | 14.0 ± 23.6 | 0.021 | 0.417 | 0.269 | 0.139 |
| Pain | 24.9 ± 29.3 | 44.1 ± 25.4 | <0.001 | 22.7 ± 24.6 | 45.2 ± 30.0 | <0.001 | 0.935 | 0.591 | 0.655 |
| Dyspnea | 9.4 ± 17.1 | 17.9 ± 23.6 | 0.004 | 17.2 ± 25.6 | 17.2 ± 28.4 | 1.00 | 0.045 | 0.556 | 0.175 |
| Insomnia | 34.7 ± 33.1 | 39.2 ± 29.9 | 0.094 | 22.2 ± 26.7 | 32.3 ± 27.9 | 0.135 | 0.289 | 0.729 | 0.509 |
| Appetite Loss | 14.1 ± 23.7 | 48.5 ± 34.3 | <0.001 | 22.2 ± 28.1 | 51.6 ± 37.4 | 0.002 | 0.230 | 0.289 | 0.919 |
| Constipation | 9.9 ± 21.4 | 26.0 ± 27.5 | <0.001 | 16.7 ± 17.0 | 23.7 ± 37.4 | 0.205 | 0.089 | 0.554 | 0.318 |
| Diarrhea | 5.6 ± 15.9 | 13.2 ± 20.1 | 0.003 | 8.9 ± 17.4 | 15.1 ± 22.0 | 0.356 | 0.357 | 0.380 | 0.983 |
| Financial | 16.0 ± 27.0 | 16.7 ± 26.1 | 0.825 | 25.6 ± 27.2 | 19.4 ± 29.5 | 0.107 | 0.064 | 0.517 | 0.183 |
| Pre | Post | Change in QoL | |||||
|---|---|---|---|---|---|---|---|
| Beta (SE) | p-Value | Beta (SE) | p-Value | Pre–Post | Beta (SE) | p-Value | |
| Global | 0.012 (0.004) | 0.010 | 0.022 (0.004) | <0.001 | 0.095 | 0.001 (0.005) | 0.786 |
| Functional | |||||||
| Physical | 0.028 (0.005) | <0.001 | 0.032 (0.004) | <0.001 | 0.584 | 0.031 (0.007) | <0.001 |
| Role | 0.014 (0.004) | <0.001 | 0.019 (0.003) | <0.001 | 0.284 | 0.010 (0.004) | 0.017 |
| Emotional | 0.007 (0.005) | 0.178 | 0.019 (0.005) | <0.001 | 0.057 | 0.008 (0.007) | 0.241 |
| Cognitive | 0.010 (0.006) | 0.103 | 0.017 (0.004) | <0.001 | 0.305 | 0.011 (0.007) | 0.126 |
| Social | 0.009 (0.005) | 0.062 | 0.018 (0.004) | <0.001 | 0.124 | 0.010 (0.005) | 0.038 |
| Symptoms | |||||||
| Fatigue | 0.013 (0.004) | 0.006 | 0.024 (0.004) | <0.001 | 0.058 | 0.010 (0.006) | 0.080 |
| Nausea | 0.004 (0.008) | 0.617 | −0.005 (0.004) | 0.309 | 0.345 | 0.001 (0.005) | 0.870 |
| Pain | −0.006 (0.004) | 0.132 | −0.011 (0.005) | 0.012 | 0.331 | 0.001 (0.005) | 0.769 |
| Dyspnea | −0.005 (0.006) | 0.397 | −0.017 (0.005) | <0.001 | 0.113 | −0.013 (0.007) | 0.067 |
| Insomnia | −0.002 (0.003) | 0.532 | −0.010 (0.004) | 0.006 | 0.089 | 0.001 (0.004) | 0.821 |
| Appetite Loss | −0.003 (0.005) | 0.590 | −0.006 (0.003) | 0.094 | 0.626 | 0.003 (0.004) | 0.456 |
| Constipation | 0.000 (0.005) | 0.948 | 0.001 (0.004) | 0.755 | 0.881 | 0.002 (0.006) | 0.699 |
| Diarrhea | −0.013 (0.007) | 0.087 | 0.004 (0.006) | 0.526 | 0.082 | 0.005 (0.006) | 0.457 |
| Financial | −0.001 (0.004) | 0.817 | −0.013 (0.004) | 0.003 | 0.038 | −0.002 (0.006) | 0.760 |
| Pre | Post | Change in QoL | |||||
|---|---|---|---|---|---|---|---|
| Beta (SE) | p-Value | Beta (SE) | p-Value | Pre–Post | Beta (SE) | p-Value | |
| Global | −0.002 (0.009) | 0.824 | 0.011 (0.007) | 0.117 | 0.144 | 0.008 (0.010) | 0.438 |
| Functional | |||||||
| Physical | 0.005 (0.007) | 0.503 | 0.031 (0.005) | <0.001 | 0.001 | 0.020 (0.008) | 0.022 |
| Role | 0.008 (0.005) | 0.089 | 0.026 (0.004) | <0.001 | 0.003 | 0.007 (0.006) | 0.295 |
| Emotional | 0.004 (0.011) | 0.689 | −0.000 (0.010) | 0.996 | 0.712 | 0.002 (0.011) | 0.857 |
| Cognitive | 0.004 (0.012) | 0.748 | 0.017 (0.010) | 0.074 | 0.307 | 0.004 (0.012) | 0.760 |
| Social | 0.012 (0.007) | 0.122 | 0.018 (0.006) | 0.001 | 0.470 | 0.012 (0.006) | 0.070 |
| Symptoms | |||||||
| Fatigue | 0.003 (0.009) | 0.770 | 0.016 (0.006) | 0.006 | 0.171 | 0.010 (0.006) | 0.142 |
| Nausea | −0.019 (0.020) | 0.348 | −0.010 (0.008) | 0.219 | 0.614 | −0.008 (0.009) | 0.369 |
| Pain | −0.004 (0.007) | 0.550 | −0.019 (0.006) | 0.001 | 0.058 | −0.006 (0.007) | 0.455 |
| Dyspnea | 0.003 (0.007) | 0.629 | 0.002 (0.006) | 0.709 | 0.891 | −0.000 (0.006) | 0.938 |
| Insomnia | −0.004 (0.007) | 0.570 | 0.008 (0.006) | 0.219 | 0.175 | −0.004 (0.005) | 0.511 |
| Appetite Loss | −0.002 (0.006) | 0.742 | −0.008 (0.005) | 0.083 | 0.395 | −0.006 (0.004) | 0.156 |
| Constipation | −0.003 (0.010) | 0.794 | −0.004 (0.006) | 0.474 | 0.906 | −0.005 (0.005) | 0.319 |
| Diarrhea | 0.008 (0.009) | 0.356 | −0.022 (0.007) | 0.003 | 0.018 | −0.003 (0.007) | 0.654 |
| Financial | −0.006 (0.007) | 0.421 | −0.007 (0.006) | 0.231 | 0.840 | 0.002 (0.006) | 0.765 |
| Treatment | Category | Pre-Treatment | Post-Treatment | |
|---|---|---|---|---|
| Frailty | CCRT | Robust | 32 (42.7%) | 3 (4.1%) |
| Pre-frail | 35 (46.7%) | 40 (54.8%) | ||
| Frail | 8 (10.7%) | 30 (41.1%) | ||
| RT | Robust | 6 (19.4%) | 1 (3.2%) | |
| Pre-frail | 19 (61.3%) | 10 (32.3%) | ||
| Frail | 6 (19.4%) | 20 (64.5%) |
| Pre-Treatment SPPB (Beta, (SE)) | p-Value | Post-Treatment SPPB (Beta, (SE)) | p-Value | Delta SPPB and Transition to Frail (Beta, (SE)) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Frailty | CCRT | −0.151 (0.28) | <0.001 | −0.088 (0.015) | <0.001 | 0.048 (0.016) | 0.004 |
| RT | −0.043 (0.036) | 0.246 | −0.067 (0.022) | 0.004 | 0.073 (0.041) | 0.084 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrugia, M.; Erickson, K.; Wendel, E.; Platek, M.E.; Ji, W.; Attwood, K.; Ma, S.J.; Gu, F.; Singh, A.K.; Ray, A.D. Change in Physical Performance Correlates with Decline in Quality of Life and Frailty Status in Head and Neck Cancer Patients Undergoing Radiation with and without Chemotherapy. Cancers 2021, 13, 1638. https://doi.org/10.3390/cancers13071638
Farrugia M, Erickson K, Wendel E, Platek ME, Ji W, Attwood K, Ma SJ, Gu F, Singh AK, Ray AD. Change in Physical Performance Correlates with Decline in Quality of Life and Frailty Status in Head and Neck Cancer Patients Undergoing Radiation with and without Chemotherapy. Cancers. 2021; 13(7):1638. https://doi.org/10.3390/cancers13071638
Chicago/Turabian StyleFarrugia, Mark, Kayleigh Erickson, Elizabeth Wendel, Mary E. Platek, Wenyan Ji, Kristopher Attwood, Sung Jun Ma, Fangyi Gu, Anurag K. Singh, and Andrew D. Ray. 2021. "Change in Physical Performance Correlates with Decline in Quality of Life and Frailty Status in Head and Neck Cancer Patients Undergoing Radiation with and without Chemotherapy" Cancers 13, no. 7: 1638. https://doi.org/10.3390/cancers13071638
APA StyleFarrugia, M., Erickson, K., Wendel, E., Platek, M. E., Ji, W., Attwood, K., Ma, S. J., Gu, F., Singh, A. K., & Ray, A. D. (2021). Change in Physical Performance Correlates with Decline in Quality of Life and Frailty Status in Head and Neck Cancer Patients Undergoing Radiation with and without Chemotherapy. Cancers, 13(7), 1638. https://doi.org/10.3390/cancers13071638






