Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-Small Cell Lung Cancer (NSCLC): A Real World Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection and Ethical Aspects
2.2. 18. F-FDG PET/CT Protocol
2.3. Measures of Outcome
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
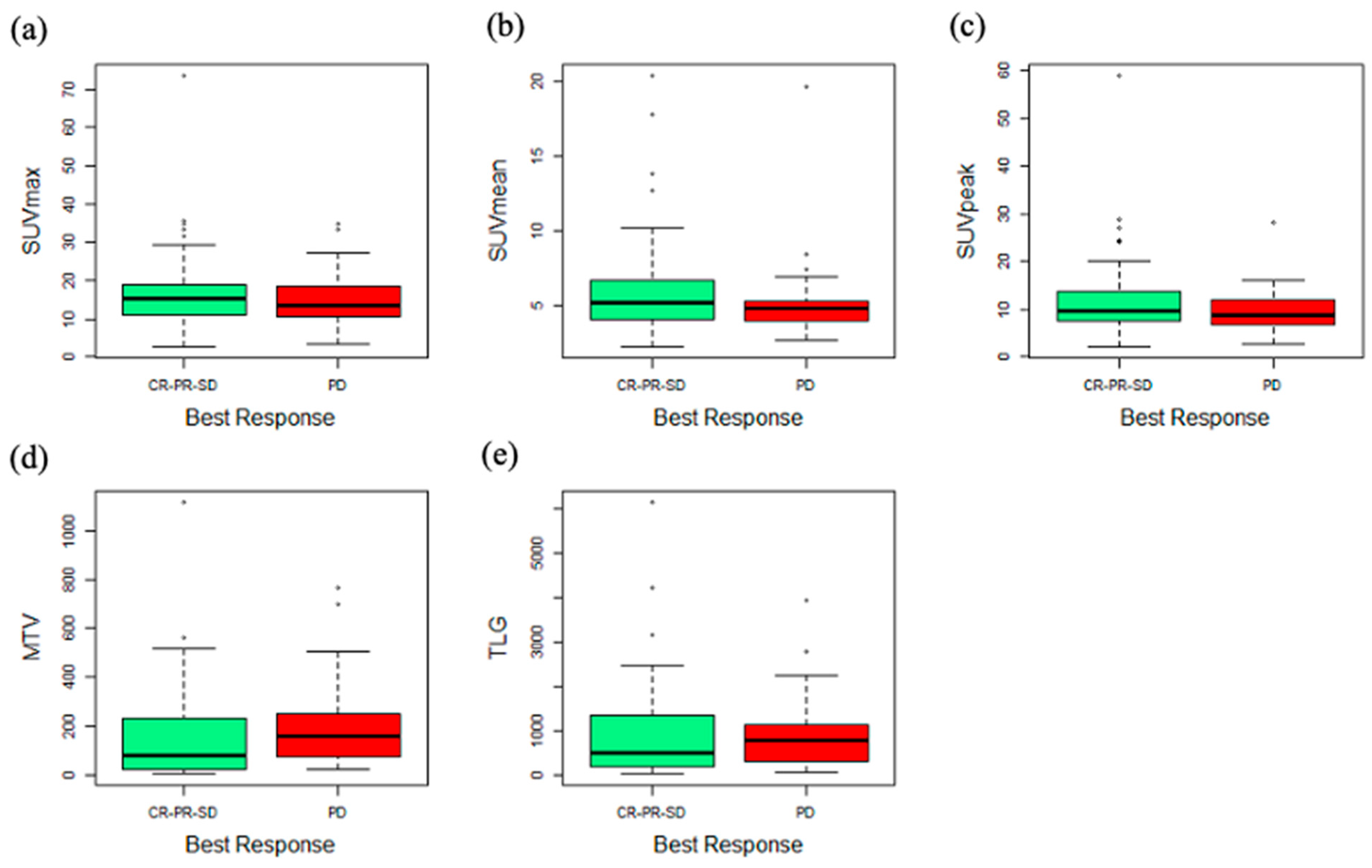
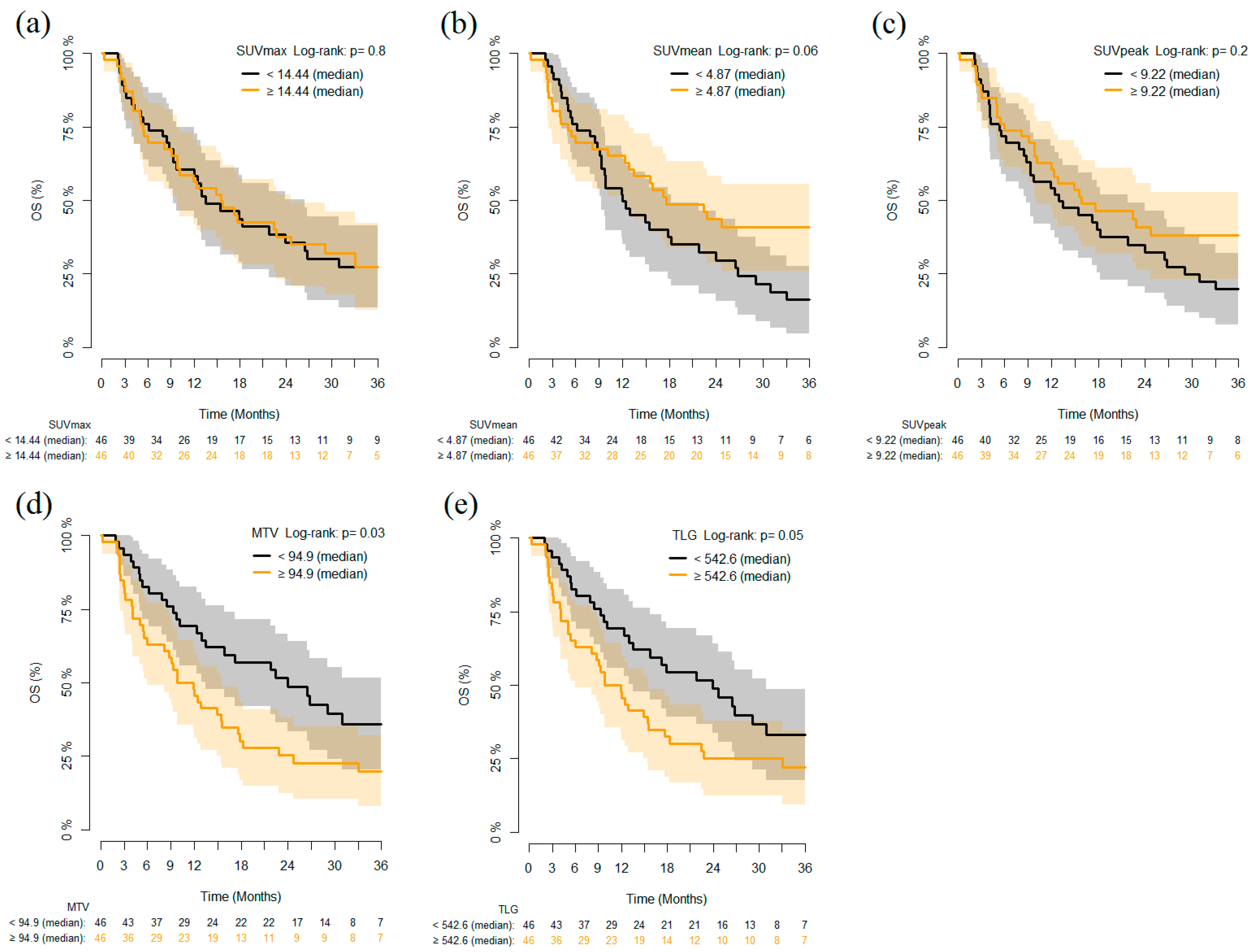
3.2. PET Parameters and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- McLaughlin, J.K.; Han, G.; Schalper, K.A.; Carvajal-Hausdorf, D.; Pelekanou, V.; Rehman, J.; Velcheti, V.; Herbst, R.S.; Lorusso, P.M.; Rimm, D.L. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non–Small-Cell Lung Cancer. JAMA Oncol. 2016, 2, 46–54. [Google Scholar] [CrossRef]
- Kandathil, A.; Kay, F.U.; Butt, Y.M.; Wachsmann, J.W.; Subramaniam, R.M. Role of FDG PET/CT in the Eighth Edition of TNM Staging of Non–Small Cell Lung Cancer. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2018, 38, 2134–2149. [Google Scholar] [CrossRef]
- Grossi, F.; Bauckneht, M.; Genova, C.; Rijavec, E.; Biello, F.; Mennella, S.; Bello, M.G.D.; Cittadini, G.; Bruzzi, P.; Piva, R.; et al. Comparison Between 18F-FDG PET–Based and CT-Based Criteria in Non–Small Cell Lung Cancer Patients Treated with Nivolumab. J. Nucl. Med. 2019, 61, 990–998. [Google Scholar] [CrossRef]
- Evangelista, L.; Cuppari, L.; Menis, J.; Bonanno, L.; Reccia, P.; Frega, S.; Pasello, G. 18F-FDG PET/CT in non-small-cell lung cancer patients: A potential predictive biomarker of response to immunotherapy. Nucl. Med. Commun. 2019, 40, 802–807. [Google Scholar] [CrossRef]
- Polverari, G.; Ceci, F.; Bertaglia, V.; Reale, M.L.; Rampado, O.; Gallio, E.; Passera, R.; Liberini, V.; Scapoli, P.; Arena, V.; et al. 18F-FDG Pet Parameters and Radiomics Features Analysis in Advanced Nsclc Treated with Immunotherapy as Predictors of Therapy Response and Survival. Cancers 2020, 12, 1163. [Google Scholar] [CrossRef]
- Chardin, D.; Paquet, M.; Schiappa, R.; Darcourt, J.; Bailleux, C.; Poudenx, M.; Sciazza, A.; Ilie, M.; Benzaquen, J.; Martin, N.; et al. Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: A prospective study. J. Immunother. Cancer 2020, 8, e000645. [Google Scholar] [CrossRef]
- O, J.H.; Choi, W.H.; Han, E.J.; Choi, E.-K.; Chae, B.J.; Park, Y.-G.; Kim, S.H. The Prognostic Value of 18F-FDG PET/CT for Early Recurrence in Operable Breast Cancer: Comparison with TNM Stage. Nucl. Med. Mol. Imaging 2013, 47, 263–267. [Google Scholar] [CrossRef][Green Version]
- Takada, K.; Toyokawa, G.; Tagawa, T.; Kohashi, K.; Akamine, T.; Takamori, S.; Hirai, F.; Shoji, F.; Okamoto, T.; Oda, Y.; et al. Association Between PD-L1 Expression and Metabolic Activity on 18F-FDG PET/CT in Patients with Small-sized Lung Cancer. Anticancer Res. 2017, 37, 7073–7082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costelloe, C.M.; Macapinlac, H.A.; Madewell, J.E.; Fitzgerald, N.E.; Mawlawi, O.R.; Rohren, E.M.; Raymond, A.K.; Lewis, V.O.; Anderson, P.M.; Bassett, R.L.; et al. 18F-FDG PET/CT as an Indicator of Progression-Free and Overall Survival in Osteosarcoma. J. Nucl. Med. 2009, 50, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Choi, J.Y.; Shim, Y.M.; Kim, K.; Lee, S.J.; Cho, Y.S.; Lee, J.Y.; Lee, K.-H.; Kim, B.-T. Prognostic Value of Metabolic Tumor Volume Measured by 18F-Fluorodeoxyglucose Positron Emission Tomography in Patients with Esophageal Carcinoma. Ann. Surg. Oncol. 2009, 17, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Choi, J.Y.; Moon, S.H.; Bae, D.S.; Bin Park, S.; Choe, Y.S.; Lee, K.-H.; Kim, B.-T. Prognostic significance of volume-based metabolic parameters in uterine cervical cancer determined using 18F-fluorodeoxyglucose positron emission tomography. Int. J. Gynecol. Cancer 2012, 22, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.W.; Chiu, N.-T.; Su, W.-C.; Guo, H.-R.; Lee, B.-F. Prognostic Value of Whole-Body Total Lesion Glycolysis at Pretreatment FDG PET/CT in Non–Small Cell Lung Cancer. Radiology 2012, 264, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Ahn, H.K.; Ahn, M.-J.; Ahn, Y.C.; Kim, J.; Shim, Y.M.; Choi, J.Y. Volume-Based Assessment With18F-FDG PET/CT Improves Outcome Prediction for Patients with Stage IIIA-N2 Non–Small Cell Lung Cancer. Am. J. Roentgenol. 2015, 205, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Ahn, H.K.; Kim, H.; Ahn, M.-J.; Park, K.; Ahn, Y.C.; Kim, J.; Shim, Y.M.; Choi, J.Y. Volume-based assessment by 18F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 41, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Son, S.H.; Kim, C.-Y.; Hong, C.M.; Oh, J.-R.; Song, B.-I.; Kim, H.W.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; et al. Prediction for Recurrence Using F-18 FDG PET/CT in Pathologic N0 Lung Adenocarcinoma After Curative Surgery. Ann. Surg. Oncol. 2013, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zaizen, Y.; Azuma, K.; Kurata, S.; Sadashima, E.; Hattori, S.; Sasada, T.; Imamura, Y.; Kaida, H.; Kawahara, A.; Kinoshita, T.; et al. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur. J. Radiol. 2012, 81, 4179–4184. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Penney, B.C.; Zhang, H.; Suzuki, K.; Pu, Y. Prognostic Value of the Quantitative Metabolic Volumetric Measurement on 18F-FDG PET/CT in Stage IV Nonsurgical Small-cell Lung Cancer. Acad. Radiol. 2012, 19, 69–77. [Google Scholar] [CrossRef]
- Dosani, M.; Yang, R.; McLay, M.; Wilson, D.; Liu, M.; Yong-Hing, C.J.; Hamm, J.; Lund, C.R.; Olson, R.; Schellenberg, D. Metabolic Tumour Volume Is Prognostic in Patients with Non-Small-Cell Lung Cancer Treated with Stereotactic Ablative Radiotherapy. Curr. Oncol. 2019, 26, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wroblewski, K.; Appelbaum, D.; Pu, Y. Independent prognostic value of whole-body metabolic tumor burden from FDG-PET in non-small cell lung cancer. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 181–191. [Google Scholar] [CrossRef]
- Liao, S.; Penney, B.C.; Wroblewski, K.; Zhang, H.; Simon, C.A.; Kampalath, R.; Shih, M.-C.; Shimada, N.; Chen, S.; Salgia, R.; et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 27–38. [Google Scholar] [CrossRef]
- Ito, K.; Schöder, H.; Teng, R.; Humm, J.L.; Ni, A.; Wolchok, J.D.; Weber, W.A. Prognostic value of baseline metabolic tumor volume measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 930–939. [Google Scholar] [CrossRef]
- Marinelli, B.; Espinet-Col, C.; Ulaner, G.A.; McArthur, H.L.; Gonen, M.; Jochelson, M.; Weber, W.A. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 120–127. [Google Scholar]
- Seban, R.-D.; Assié, J.-B.; Giroux-Leprieur, E.; Massiani, M.-A.; Soussan, M.; Bonardel, G.; Chouaid, C.; Playe, M.; Goldfarb, L.; Duchemann, B.; et al. Association of the Metabolic Score Using Baseline FDG-PET/CT and dNLR with Immunotherapy Outcomes in Advanced NSCLC Patients Treated with First-Line Pembrolizumab. Cancers 2020, 12, 2234. [Google Scholar] [CrossRef]
- Seban, R.-D.; Mezquita, L.; Berenbaum, A.; Dercle, L.; Botticella, A.; Le Pechoux, C.; Caramella, C.; Deutsch, E.; Grimaldi, S.; Adam, J.; et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kaira, K.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Miura, Y.; Murayama, Y.; Kobayashi, K.; Kagamu, H.; Kuji, I. Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, N.; Wu, Z.; Pan, N.; Shen, X.; Liu, T.; Wei, F.; You, J.; Xu, W.; Ren, X. New insight on the correlation of metabolic status on 18F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 1127–1136. [Google Scholar] [CrossRef]
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. S1), 17–31. [Google Scholar] [CrossRef]
- Houdu, B.; Lasnon, C.; Licaj, I.; Thomas, G.; Do, P.; Guizard, A.-V.; Desmonts, C.; Aide, N. Why harmonization is needed when using FDG PET/CT as a prognosticator: Demonstration with EARL-compliant SUV as an independent prognostic factor in lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 421–428. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.; Pruim, J.; Price, P; European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- De Geus-Oei, L.-F.; Van Der Heijden, H.F.; Visser, E.P.; Hermsen, R.; Van Hoorn, B.A.; Timmer-Bonte, J.N.; Willemsen, A.T.; Pruim, J.; Corstens, F.H.; Krabbe, P.F.; et al. Chemotherapy Response Evaluation with 18F-FDG PET in Patients with Non-Small Cell Lung Cancer. J. Nucl. Med. 2007, 48, 1592–1598. [Google Scholar] [CrossRef]
- Shang, J.; Ling, X.; Zhang, L.; Tang, Y.; Xiao, Z.; Cheng, Y.; Guo, B.; Gong, J.; Huang, L.; Xu, H. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1945–1953. [Google Scholar] [CrossRef]
- Grizzi, F.; Castello, A.; Qehajaj, D.; Toschi, L.; Rossi, S.; Pistillo, D.; Paleari, V.; Veronesi, G.; Novellis, P.; Monterisi, S.; et al. Independent expression of circulating and tissue levels of PD-L1: Correlation of clusters with tumor metabolism and outcome in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Toyokawa, G.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Wakasu, S.; Haro, A.; Osoegawa, A.; Tagawa, T.; et al. 18F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
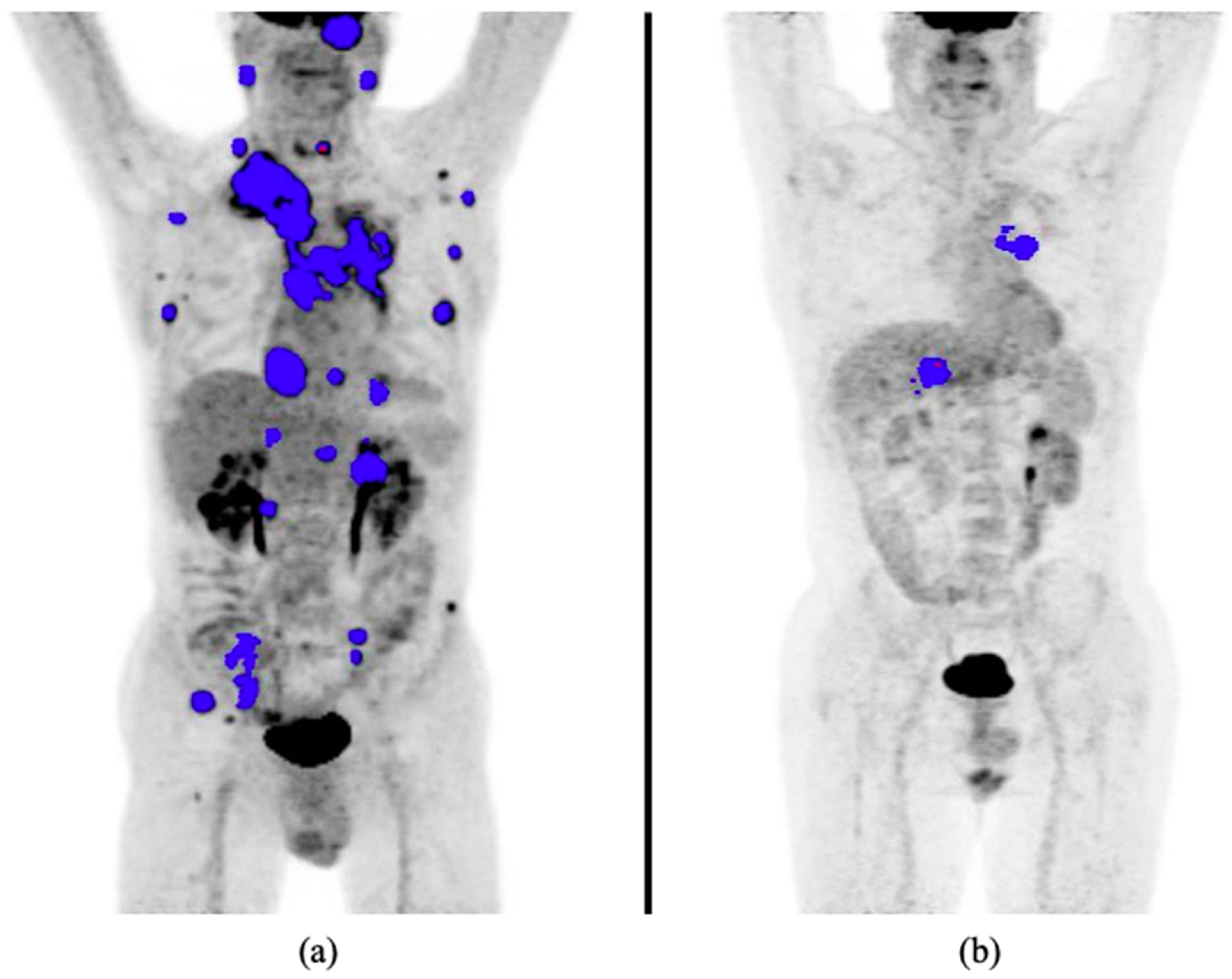
| Characteristics | Value |
|---|---|
| Age | Median 70 years old (range 61–75) |
| Sex | |
| Male | 65 (70.7%) |
| Female | 27 (29.3%) |
| Smoking status | |
| Non-smoker | 10 (10.9%) |
| Ex-smoker | 45 (48.9%) |
| Current smoker | 32 (34.8%) |
| Not known | 5 (5.4%) |
| Stage at first diagnosis | |
| IA | 2 (2.2%) |
| IB | 3 (3.3%) |
| IIA | 2 (2.2%) |
| IIB | 5 (5.4%) |
| IIIA | 10 (10.9%) |
| IIIB | 10 (10.9%) |
| IV | 56 (60.9%) |
| Not known | 4 (4.2%) |
| Histological variant | |
| Adenocarcinoma | 60 (65.2%) |
| Squamous cell carcinoma | 23 (25.3%) |
| NOS | 8 (8.8%) |
| Not known | 1 (1.1%) |
| Previous lung surgery | |
| No | 65 (70.7%) |
| Yes | 25 (27.2%) |
| Not known | 2 (2.1%) |
| Immunotherapy | |
| First line | 21 (22.8%) |
| Second line | 39 (42.4%) |
| Third line | 19 (20.7%) |
| Fourth line | 13 (14.1%) |
| Molecule | |
| Atezolizumab + Bevacizumab | 1 (1.1%) |
| Nivolumab | 69 (75.0%) |
| Pembrolizumab | 22 (23.9%) |
| Variables | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| SUVmean, per 10 units | 0.423 (0.164–1.088) | 0.074 | 0.337 (0.103–1.103) | 0.072 |
| MTV, per 100 units | 1.139 (0.989–1.311) | 0.07 | 1.221 (1.063–1.402) | 0.005 |
| Gender M vs. F | 0.837 (0.506–1.384) | 0.488 | 0.924 (0.533–1.600) | 0.777 |
| Age, per 10 years | 1.287 (0.947–1.749) | 0.108 | 1.523 (1.078–2.124) | 0.017 |
| First line vs. not first line | 0.763 (0.390–1.496) | 0.432 | 0.640 (0.278–1.873) | 0.294 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, L.; Gemelli, M.; Gotuzzo, I.; Bauckneht, M.; Crivellaro, C.; Genova, C.; Cortinovis, D.; Zullo, L.; Ammoni, L.C.; Bernasconi, D.P.; et al. Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-Small Cell Lung Cancer (NSCLC): A Real World Experience. Cancers 2021, 13, 1634. https://doi.org/10.3390/cancers13071634
Monaco L, Gemelli M, Gotuzzo I, Bauckneht M, Crivellaro C, Genova C, Cortinovis D, Zullo L, Ammoni LC, Bernasconi DP, et al. Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-Small Cell Lung Cancer (NSCLC): A Real World Experience. Cancers. 2021; 13(7):1634. https://doi.org/10.3390/cancers13071634
Chicago/Turabian StyleMonaco, Lavinia, Maria Gemelli, Irene Gotuzzo, Matteo Bauckneht, Cinzia Crivellaro, Carlo Genova, Diego Cortinovis, Lodovica Zullo, Luca Carlofrancesco Ammoni, Davide Paolo Bernasconi, and et al. 2021. "Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-Small Cell Lung Cancer (NSCLC): A Real World Experience" Cancers 13, no. 7: 1634. https://doi.org/10.3390/cancers13071634
APA StyleMonaco, L., Gemelli, M., Gotuzzo, I., Bauckneht, M., Crivellaro, C., Genova, C., Cortinovis, D., Zullo, L., Ammoni, L. C., Bernasconi, D. P., Rossi, G., Morbelli, S., & Guerra, L. (2021). Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-Small Cell Lung Cancer (NSCLC): A Real World Experience. Cancers, 13(7), 1634. https://doi.org/10.3390/cancers13071634











