Prediction of Other-Cause Mortality in Older Patients with Breast Cancer Using Comorbidity
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Design and Patients
2.2. Definitions
2.3. Statistical Analysis
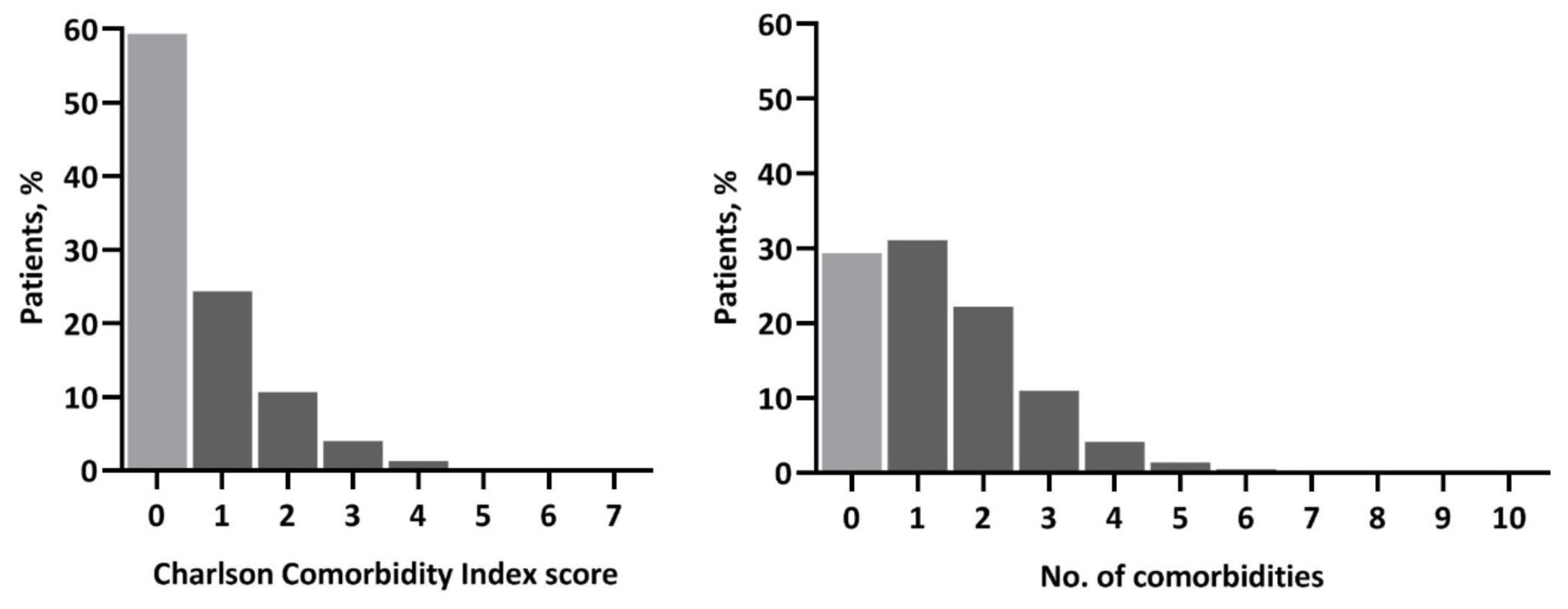
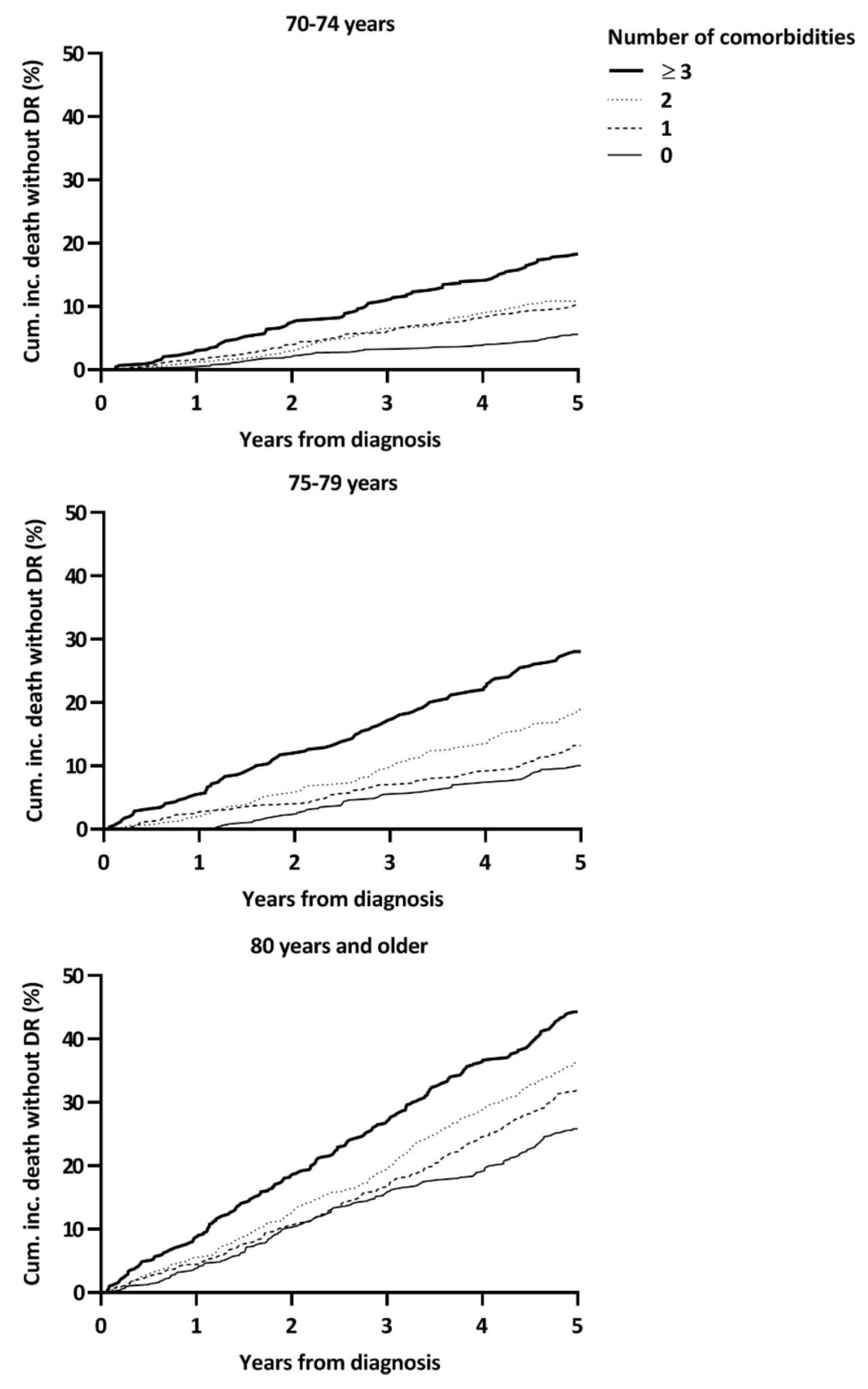
3. Results
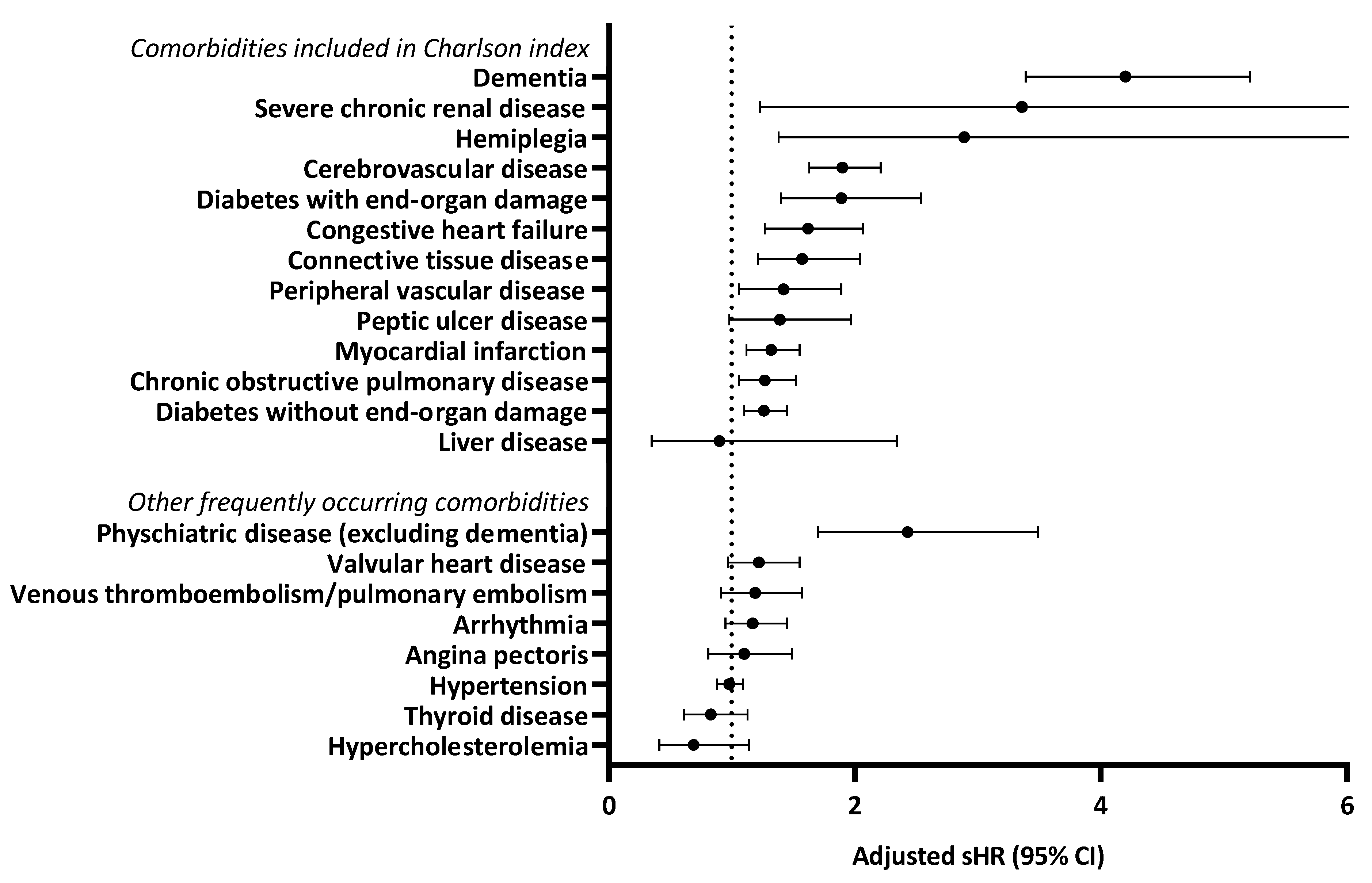
3.1. Specific Comorbidities
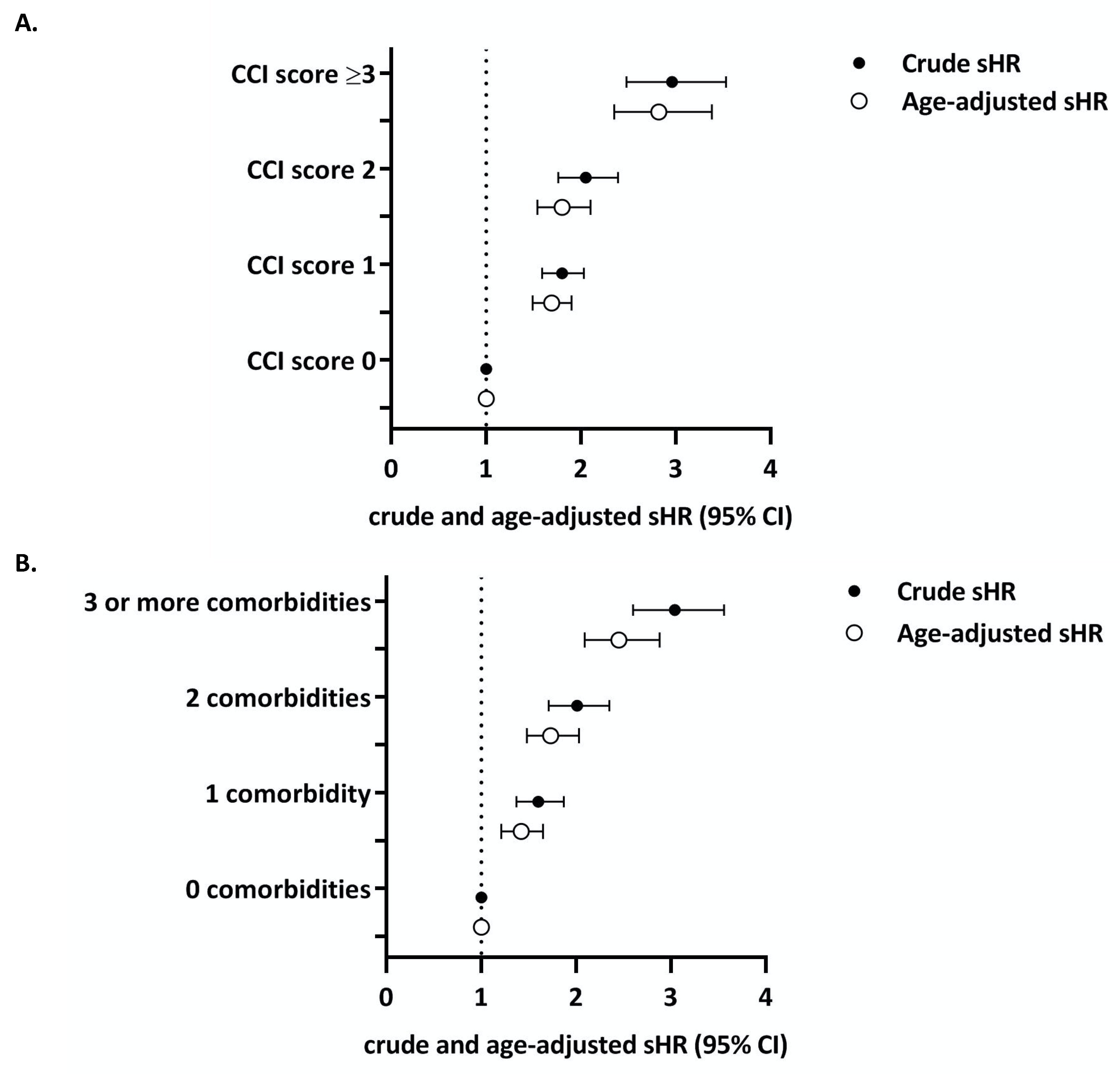
3.2. Charlson Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017. [Google Scholar] [CrossRef]
- Derks, M.G.M.; Bastiaannet, E.; van de Water, W.; de Glas, N.A.; Seynaeve, C.; Putter, H.; Nortier, J.W.R.; Rea, D.; Hasenburg, A.; Markopoulos, C.; et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur. J. Cancer 2018, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.Z.; van der Hulst, H.C.; de Glas, N.A.; Marang-van de Mheen, P.J.; Siesling, S.; de Munck, L.; de Ligt, K.M.; Portielje, J.E.A.; Bastiaannet, E.; Liefers, G.J. Impact of Older Age and Comorbidity on Locoregional and Distant Breast Cancer Recurrence: A Large Population-Based Study. Oncologist 2019. [Google Scholar] [CrossRef]
- Biganzoli, L.; Wildiers, H.; Oakman, C.; Marotti, L.; Loibl, S.; Kunkler, I.; Reed, M.; Ciatto, S.; Voogd, A.C.; Brain, E.; et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012, 13, e148–e160. [Google Scholar] [CrossRef]
- Ward, S.E.; Richards, P.D.; Morgan, J.L.; Holmes, G.R.; Broggio, J.W.; Collins, K.; Reed, M.W.R.; Wyld, L. Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer-specific survival. Br. J. Surg. 2018, 105, 1454–1463. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Land, L.H.; Dalton, S.O.; Jorgensen, T.L.; Ewertz, M. Comorbidity and survival after early breast cancer. A review. Crit. Rev. Oncol./Hematol. 2012, 81, 196–205. [Google Scholar] [CrossRef]
- Derks, M.G.M.; van de Velde, C.J.H.; Giardiello, D.; Seynaeve, C.; Putter, H.; Nortier, J.W.R.; Dirix, L.Y.; Bastiaannet, E.; Portielje, J.E.A.; Liefers, G.J. Impact of Comorbidities and Age on Cause-Specific Mortality in Postmenopausal Patients with Breast Cancer. Oncologist 2019. [Google Scholar] [CrossRef]
- De Glas, N.A.; Bastiaannet, E.; Engels, C.C.; de Craen, A.J.; Putter, H.; van de Velde, C.J.; Hurria, A.; Liefers, G.J.; Portielje, J.E. Validity of the online PREDICT tool in older patients with breast cancer: A population-based study. Br. J. Cancer 2016, 114, 395–400. [Google Scholar] [CrossRef]
- De Glas, N.A.; van de Water, W.; Engelhardt, E.G.; Bastiaannet, E.; de Craen, A.J.M.; Kroep, J.R.; Putter, H.; Stiggelbout, A.M.; Weijl, N.I.; van de Velde, C.J.H.; et al. Validity of Adjuvant! Online program in older patients with breast cancer: A population-based study. Lancet Oncol. 2014, 15, 722–729. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Bush, T.L.; Penberthy, L.T. Comorbidity measurement in elderly female breast cancer patients with administrative and medical records data. J. Clin. Epidemiol. 1997, 50, 725–733. [Google Scholar] [CrossRef]
- Frederick, L.; Greene, C.M.B.; Daniel, G.H.; Morrow, M. AJCC Cancer Staging Manual, 6th ed.; Springer Science: New York, NY, USA, 2002; ISBN 978-3-540-00595-7. [Google Scholar]
- Schaffar, R.; Rapiti, E.; Rachet, B.; Woods, L. Accuracy of cause of death data routinely recorded in a population-based cancer registry: Impact on cause-specific survival and validation using the Geneva Cancer Registry. BMC Cancer 2013, 13, 609. [Google Scholar] [CrossRef]
- Ring, A.; Sestak, I.; Baum, M.; Howell, A.; Buzdar, A.; Dowsett, M.; Forbes, J.F.; Cuzick, J. Influence of comorbidities and age on risk of death without recurrence: A retrospective analysis of the Arimidex, Tamoxifen Alone or in Combination trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- De Glas, N.A.; Kiderlen, M.; Bastiaannet, E.; de Craen, A.J.; van de Water, W.; van de Velde, C.J.; Liefers, G.J. Postoperative complications and survival of elderly breast cancer patients: A FOCUS study analysis. Breast Cancer Res. Treat. 2013, 138, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Putter, H.; Fiocco, M.; Geskus, R.B. Tutorial in biostatistics: Competing risks and multi-state models. Stat. Med. 2007, 26, 2389–2430. [Google Scholar] [CrossRef]
- Wolbers, M.; Koller, M.T.; Witteman, J.C.; Steyerberg, E.W. Prognostic models with competing risks: Methods and application to coronary risk prediction. Epidemiology 2009, 20, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tammemagi, C.M.; Nerenz, D.; Neslund-Dudas, C.; Feldkamp, C.; Nathanson, D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 2005, 294, 1765–1772. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; Diguiseppi, C.; Denberg, T.D.; Dabelea, D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J. Natl. Cancer Inst. 2011, 103, 1101–1111. [Google Scholar] [CrossRef]
- Kiderlen, M.; de Glas, N.A.; Bastiaannet, E.; van de Water, W.; de Craen, A.J.; Guicherit, O.R.; Merkus, J.W.; Extermann, M.; van de Velde, C.J.; Liefers, G.J. Impact of comorbidity on outcome of older breast cancer patients: A FOCUS cohort study. Breast Cancer Res. Treat. 2014, 145, 185–192. [Google Scholar] [CrossRef]
- Land, L.H.; Dalton, S.O.; Jensen, M.B.; Ewertz, M. Impact of comorbidity on mortality: A cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res. Treat. 2012, 131, 1013–1020. [Google Scholar] [CrossRef]
- Schonberg, M.A.; Marcantonio, E.R.; Li, D.; Silliman, R.A.; Ngo, L.; McCarthy, E.P. Breast cancer among the oldest old: Tumor characteristics, treatment choices, and survival. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.; Wigertz, A.; Adolfsson, J.; Ahlgren, J.; Fornander, T.; Warnberg, F.; Lambe, M. Impact of comorbidity on management and mortality in women diagnosed with breast cancer. Breast Cancer Res. Treat. 2012, 135, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kimmick, G.G.; Li, X.; Fleming, S.T.; Sabatino, S.A.; Wilson, J.F.; Lipscomb, J.; Cress, R.; Bergom, C.; Anderson, R.T.; Wu, X.C. Risk of cancer death by comorbidity severity and use of adjuvant chemotherapy among women with locoregional breast cancer. J. Geriatr. Oncol. 2018, 9, 214–220. [Google Scholar] [CrossRef]
- Ewertz, M.; Land, L.H.; Dalton, S.O.; Cronin-Fenton, D.; Jensen, M.B. Influence of specific comorbidities on survival after early-stage breast cancer. Acta Oncol. 2018, 57, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wasif, N.; Neville, M.; Gray, R.; Cronin, P.; Pockaj, B.A. Competing Risk of Death in Elderly Patients with Newly Diagnosed Stage I Breast Cancer. J. Am. Coll. Surg. 2019, 229, 30–36.e31. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Li, D.; Yuan, Y.; Lau, Y.M.; Hurria, A. Functional versus chronological age: Geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018, 19, e305–e316. [Google Scholar] [CrossRef]
- Clough-Gorr, K.M.; Thwin, S.S.; Stuck, A.E.; Silliman, R.A. Examining five- and ten-year survival in older women with breast cancer using cancer-specific geriatric assessment. Eur. J. Cancer 2012, 48, 805–812. [Google Scholar] [CrossRef]
- DuMontier, C.; Clough-Gorr, K.M.; Silliman, R.A.; Stuck, A.E.; Moser, A. Health-Related Quality of Life in a Predictive Model for Mortality in Older Breast Cancer Survivors. J. Am. Geriatr. Soc. 2018, 66, 1115–1122. [Google Scholar] [CrossRef]
- Epplein, M.; Zheng, Y.; Zheng, W.; Chen, Z.; Gu, K.; Penson, D.; Lu, W.; Shu, X.O. Quality of life after breast cancer diagnosis and survival. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 406–412. [Google Scholar] [CrossRef]
| Patients | No. (%) |
|---|---|
| Total | 7511 |
| Year of diagnosis | |
| 2003 | 309 (4.1) |
| 2004 | 452 (6.0) |
| 2005 | 548 (7.3) |
| 2006 | 564 (7.5) |
| 2007 | 1552 (20.7) |
| 2008 | 1615 (21.5) |
| 2009 | 2471 (32.9) |
| Age category | |
| 70–74 years | 3292 (43.8) |
| 75–79 years | 1778 (23.7) |
| ≥80 years | 2441 (32.5) |
| TNM stage | |
| I | 3297 (43.9) |
| II | 3259 (43.4) |
| III | 944 (12.6) |
| Unknown | 11 (0.2) |
| Tumor grade | |
| 1 | 1847 (24.6) |
| 2 | 3387 (45.1) |
| 3 | 1803 (24.0) |
| Unknown | 474 (6.3) |
| Hormone receptor status | |
| ER- and/or PR-positive | 6382 (85.0) |
| ER- and PR-negative | 968 (12.7) |
| Unknown | 180 (2.3) |
| Her2 status | |
| Positive | 610 (8.0) |
| Negative | 5667 (75.1) |
| Unknown | 1269 (16.8) |
| Type of surgery | |
| Mastectomy | 4346 (57.9) |
| Breast-conserving surgery | 3165 (42.1) |
| Endocrine treatment * | |
| Yes | 3584 (56.2) |
| No | 2798 (43.8) |
| Chemotherapy | |
| Yes | 194 (2.6) |
| No | 7317 (97.4) |
| Comorbidity | No. (%) |
|---|---|
| Comorbidities included in Charlson index | |
| Myocardial infarction | 671 (8.9) |
| Congestive heart failure | 216 (2.9) |
| Peripheral vascular disease | 216 (2.9) |
| Cerebrovascular disease | 545 (7.3) |
| Dementia | 164 (2.2) |
| Chronic obstructive pulmonary disease | 620 (8.3) |
| Connective tissue disease | 212 (2.8) |
| Peptic ulcer disease | 128 (1.7) |
| Liver disease | 31 (0.4) |
| Diabetes without end-organ damage | 1219 (16.2) |
| Diabetes with end-organ damage | 162 (2.2) |
| Hemiplegia | 16 (0.2) |
| Severe chronic renal disease | 12 (0.2) |
| Other frequently occurring comorbidities * | |
| Hypertension | 2971 (39.6) |
| Arrhythmia | 342 (4.6) |
| Valvular heart disease | 294 (3.9) |
| Thyroid disease | 293 (3.9) |
| Venous thromboembolism/pulmonary embolism | 213 (2.8) |
| Angina pectoris | 166 (2.2) |
| Tuberculosis | 100 (1.3) |
| Hypercholesterolemia | 94 (1.3) |
| Psychiatric disease (excluding dementia) | 90 (1.2) |
| Comorbidity Category | Charlson Index | Comorbidity Count | ||
|---|---|---|---|---|
| Crude sHR (95% CI) | Age-Adjusted sHR (95% CI) | Crude sHR (95% CI) | Age-Adjusted sHR (95% CI) | |
| 0 | Referent | Referent | Referent | Referent |
| 1 | 1.80 (1.59 to 2.03) | 1.69 (1.49 to 1.90) | 1.60 (1.37 to 1.87) | 1.42 (1.21 to 1.65) |
| 2 | 2.05 (1.76 to 2.39) | 1.80 (1.54 to 2.10) | 2.01 (1.71 to 2.35) | 1.73 (1.48 to 2.03) |
| ≥3 | 2.96 (2.49 to 3.53) | 2.82 (2.35 to 3.38) | 3.04 (2.60 to 3.56) | 2.45 (2.09 to 2.88) |
| Model c-statistic (95% CI) * | 0.58 (0.57 to 0.59) | 0.65 (0.64 to 0.66) | 0.58 (0.58 to 0.59) | 0.64 (0.64 to 0.65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Boer, A.Z.; Bastiaannet, E.; Putter, H.; Marang-van de Mheen, P.J.; Siesling, S.; de Munck, L.; de Ligt, K.M.; Portielje, J.E.A.; Liefers, G.J.; de Glas, N.A. Prediction of Other-Cause Mortality in Older Patients with Breast Cancer Using Comorbidity. Cancers 2021, 13, 1627. https://doi.org/10.3390/cancers13071627
de Boer AZ, Bastiaannet E, Putter H, Marang-van de Mheen PJ, Siesling S, de Munck L, de Ligt KM, Portielje JEA, Liefers GJ, de Glas NA. Prediction of Other-Cause Mortality in Older Patients with Breast Cancer Using Comorbidity. Cancers. 2021; 13(7):1627. https://doi.org/10.3390/cancers13071627
Chicago/Turabian Stylede Boer, Anna Z., Esther Bastiaannet, Hein Putter, Perla J. Marang-van de Mheen, Sabine Siesling, Linda de Munck, Kelly M. de Ligt, Johanneke E. A. Portielje, Gerrit Jan Liefers, and Nienke A. de Glas. 2021. "Prediction of Other-Cause Mortality in Older Patients with Breast Cancer Using Comorbidity" Cancers 13, no. 7: 1627. https://doi.org/10.3390/cancers13071627
APA Stylede Boer, A. Z., Bastiaannet, E., Putter, H., Marang-van de Mheen, P. J., Siesling, S., de Munck, L., de Ligt, K. M., Portielje, J. E. A., Liefers, G. J., & de Glas, N. A. (2021). Prediction of Other-Cause Mortality in Older Patients with Breast Cancer Using Comorbidity. Cancers, 13(7), 1627. https://doi.org/10.3390/cancers13071627






