Prediction of Unplanned Hospitalizations in Older Patients Treated with Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient and Tumor Characteristics
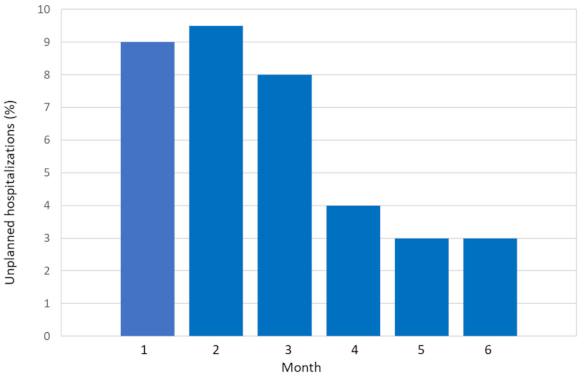
2.2. Incidence and Reasons of Unplanned Hospitalizations
2.3. Predictive Variables Associated with Unplanned Hospitalizations in the First 6 Months
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Study Schema
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haller, D.G.; O’Connell, M.J.; Cartwright, T.H.; Twelves, C.J.; McKenna, E.F.; Sun, W.; Saif, M.W.; Lee, S.; Yothers, G.; Schmoll, H.-J. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: A pooled analysis of individual patient data from four randomized, controlled trials. Ann. Oncol. 2015, 26, 715–724. [Google Scholar] [PubMed]
- Langer, C.J.; Manola, J.; Bernardo, P.; Kugler, J.W.; Bonomi, P.; Cella, D.; Johnson, D.H. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: Implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J. Natl. Cancer Inst. 2002, 94, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Feliu, J.; Heredia-Soto, V.; Gironés, R.; Jiménez-Munarriz, B.; Saldaña, J.; Guillén-Ponce, C.; Molina-Garrido, M.J. Management of the toxicity of chemotherapy and targeted therapies in elderly cancer patients. Clin. Transl. Oncol. 2020, 22, 457–467. [Google Scholar] [CrossRef]
- Creditor, M.C. Hazards of hospitalization of the elderly. Ann. Intern Med. 1993, 118, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.A.; Franke, T.; Inouye, S.K.; Landefeld, C.S.; Morgan, T.M.; Rudberg, M.A.; Sebens, H.; Winograd, C.H. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern. Med. 1996, 156, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Lamont, E.B.; Mariotto, A.; Warren, J.L.; Topor, M.; Meekins, A.; Brown, M.L. Cost of care for elderly cancer patients in the United States. J. Natl. Cancer Inst. 2008, 100, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Whitney, R.L.; Bell, J.F.; Tancredi, D.J.; Romano, P.S.; Bold, R.J.; Wun, T.; Joseph, J.G. Unplanned hospitalization among individuals with cancer in the year after diagnosis. J. Oncol. Pract. 2019, 15, e20–e29. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Kansagra, A.J.; Rao, S.R.; Weitzman, J.I.; Linden, E.A.; Jacobson, J.O. A clinical prediction model to assess risk for chemotherapy related hospitalization in patients initiating palliative chemotherapy. JAMA Oncol. 2015, 1, 441–447. [Google Scholar] [CrossRef]
- Hassett, M.J.; Rao, S.R.; Brozovic, S.; Stahl, J.E.; Schwartz, J.H.; Maloney, B.; Jacobson, J.O. Chemotherapy-related hospitalization among community cancer center patients. Oncologist 2011, 16, 378–387. [Google Scholar] [CrossRef]
- Lodewijckx, E.; Kenis, C.; Flamaing, J.; Debruyne, P.; De Groof, I.; Focan, C.; Cornélis, F.; Verschaeve, V.; Bachmann, C.; Bron, D.; et al. Unplanned hospitalizations in older patients with cancer: Occurrence and predictive factors. J. Geriatr. Oncol. 2020, 19. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Monette, J.; Girre, V.; Wolfson, C.; Monette, M.; Batist, G.; Bergman, H. Does frailty predict hospitalization, emergency department visits, and visits to the general practitioner in older newly-diagnosed cancer patients? Results of a prospective pilot study. Crit. Rev. Oncol. Hematol. 2010, 76, 142–151. [Google Scholar] [CrossRef]
- Liu, M.A.; DuMontier, C.; Murillo, A.; Hshieh, T.T.; Bean, J.F.; Soiffer, R.J.; Stone, R.M.; Abel, G.A.; Driver, J.A. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019, 134, 374–382. [Google Scholar] [CrossRef]
- Brooks, G.A.; Li, L.; Uno, H.; Hassett, M.J.; Landon, B.E.; Schrag, D. Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff. 2014, 33, 1793–1800. [Google Scholar] [CrossRef]
- Feliu, J.; Jiménez-Munárriz, B.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Antonio-Rebollo, M. Predicting Chemotherapy Toxicity in Older Patients with Cancer: A Multicenter Prospective Study. Oncologist 2020, 25, e1516–e1524. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber, F.J.; et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, J.J.; Kim, J.; Zhou, J.M.; Gomes, F.; Sehovic, M.; Extermann, M. Association of multidimensional comorbidities with survival, toxicity, and unplanned hospitalizations in older adults with metastatic colorectal cancer treated with chemotherapy. Geriatr. Oncol. 2019, 10, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Osborne, C.; Goodwin, J.S. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J. Clin. Oncol. 2002, 20, 4636–4642. [Google Scholar] [CrossRef]
- Reed, M.; Patrick, C.; Quevillon, T.; Walde, N.; Voutsadakis, I.A. Prediction of hospital admissions and grade 3-4 toxicities in cancer patients 70 years old and older receiving chemotherapy. Eur. J. Cancer Care 2019, 28, e13144. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, S.M. Chemotherapy in the elderly. Sem. Oncol. 2004, 31, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Merrill, C. Elixhauser A: Hospitalization in the United States; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005. [Google Scholar]
- Li, D.; Sun, C.L.; Kim, H.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Chien, L.; Charles, K.; Dos Santos Hughes, S.F.; et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Landrum, L.; Weinrich, S. Readmission Data for Outcomes Measurement: Identifying and Strengthening the Empirical Base. Q. Manag. Health Care 2006, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Roila, F.; Lupattelli, M.; Sassi, M.; Basurto, C.; Bracarda, S.; Picciafuoco, M.; Boschetti, E.; Milella, G.; Ballatori, E.; Tonato, M.; et al. Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann. Oncol. 1991, 2, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferruci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferruci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. NEJM 1995, 332, 556–561. [Google Scholar] [CrossRef]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficits in elderly patients. J. Am. Geriatr. Soc. 1975, 22, 433–441. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL—A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Ren, X.S.; Skinner, K.; Lee, A.; Kazis, L. Social support, social selection and self-assessed health status: Results from the veterans health study in the United States. Soc. Sci. Med. 1999, 48, 1721–1734. [Google Scholar] [CrossRef]
- Costa-Requena, G.; Salamero, M.; Gil, F. Validity of the questionnaire MOS-SSS of social support in neoplastic patients. Med. Clin. 2007, 128, 687–691. [Google Scholar]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Chen, H.; Cantor, A.B.; Corcoran, M.B.; Meyer, J.; Grendys, E.; Cavanaugh, D.; Antonek, S.; Camarata, A.; Haley, W.E.; et al. Predictors of tolerance to chemotherapy in older cancer patients: A prospective pilot study. Eur. J. Cancer 2002, 38, 1466–1473. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. (Eds.) Applied Logistic Regression; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Concato, J.; Feinstein, A.R.; Holford, T.R. The risk of determining risk with multivariable models. Ann. Intern. Med. 1993, 118, 201–210. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. (Eds.) The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]

| Characteristic | Patients, n (%) |
|---|---|
| Age | |
| 70–74 | 169 (34%) |
| 75–79 | 152 (31%) |
| ≥80 | 172 (35%) |
| Sex | |
| Male | 310 (63%) |
| Female | 183 (37%) |
| Tumor site | |
| Gastrointestinal | 256 (52) |
| Genitourinary | 64 (13) |
| Lung | 59 (12) |
| Breast | 30 (6) |
| Gynecologic | 15 (3) |
| Other | 69 (14) |
| Metastatic status | |
| M0 | 209 (42%) |
| M1 | 284 (58%) |
| ECOG PS | |
| 0 | 126 (25%) |
| 1 | 304 (62%) |
| 2 | 63 (13%) |
| IADL | |
| 8 | 217 (44%) |
| ≤7 | 276 (56%) |
| ADL | |
| 6 | 387 (79%) |
| ≤5 | 106 (21%) |
| Number of falls in the past 6 months | |
| None | 409 (83%) |
| ≥1 | 85 (17%) |
| Charlson comorbidity score | |
| 0 | 166 (34%) |
| 1 | 142 (29%) |
| ≥2 | 185 (37%) |
| VES-13 | |
| 0–2 | 171 (35%) |
| ≥3 | 322 (65%) |
| Chemotherapy | |
| Standard therapy | 215 (44%) |
| Reduced therapy or monotherapy | 278 (56%) |
| MAX2 index | |
| 0 | 151 (31%) |
| 1 | 292 (59%) |
| 2 | 50 (10%) |
| Reported Cause | n (%) |
|---|---|
| Cancer related | 53 (29) |
| Chemotherapy toxicity | 37 (19) |
| Lung infection | 30 (16) |
| Other infections | 22 (12) |
| Falls and fractures | 13 (7) |
| Cardiac events | 9 (5) |
| Thromboembolic events | 7 (4) |
| Neurological events | 5 (3) |
| Hemorrhage/bleedings | 4 (2) |
| Gastrointestinal events | 4 (2) |
| Falls | 2 (1) |
| Variable | UH (n = 184) n% | Non-UH (n = 309) n% | OR (95% CI) | p Value |
|---|---|---|---|---|
| Stage | ||||
| IV | 118 (64) | 166 (56) | 1.54 (1.06–2.24) | 0.024 |
| I–III | 66 (36) | 143 (44) | ||
| MAX2 index | ||||
| ≥1 | 138 (75) | 205 (66) | 1.52 (1.02–2.29) | 0.044 |
| 0 | 46 (25) | 104 (34) | ||
| Chemotherapy | ||||
| Standard therapy | 98 (53) | 117 (38) | 1.91 (1.32–2.76) | 0.001 |
| Reduced/monotherapy | 86 (47) | 192 (62) | ||
| Albumin g/dL | ||||
| ≤3.5 | 51 (28) | 30 (10) | 3.61 (2.21–5.92) | <0.001 |
| >3.5 | 133 (72) | 279 (90) | ||
| Creatinine Clearance mL/min | ||||
| <50 | 87 (47) | 106 (34) | 1.75 (1.20–2.54) | 0.03 |
| ≥50 | 97 (53) | 203 (66) | ||
| Unintentional weight loss % | ||||
| >5% | 80 (43) | 69 (22) | 2.67 (1.80–3.97) | <0.001 |
| ≤5% | 104 (57) | 240 (78) | ||
| ECOG PS | ||||
| 2 | 35 (19) | 28 (9) | 2.36 (1.38–4.03) | 0.02 |
| 0–1 | 149 (81) | 280 (91) | ||
| Charlson comorbidity score | ||||
| ≥2 | 84 (45) | 101 (33) | 1.73 (1.18–2.51) | 0.004 |
| 0–1 | 100 (55) | 208 (67) | ||
| ADL | ||||
| ≤5 | 52 (28) | 54 (17) | 1.87 (1.21–2.89) | 0.005 |
| 6 | 132 (72) | 255 (83) | ||
| IADL | ||||
| ≤7 | 87 (47) | 187 (60) | 1.71 (1.18–2.47) | 0.004 |
| 8 | 97 (53) | 122 (40) | ||
| Falls in the past 6 months | ||||
| ≥1 | 45 (24) | 39 (13) | 2.24 (1.39–3.60) | 0.01 |
| None | 139 (76) | 270 (87) |
| Variable | β | SE | p † | OR (95% CI) | Score |
|---|---|---|---|---|---|
| Standard therapy | 0.475 | 0.211 | 0.24 | 1.608 (1.064–2.431) | 1 |
| MAX2 index ≥ 1 | 0.558 | 0.215 | 0.02 | 1.748 (1.093–2.796) | 1 |
| Weight loss >5% | 0.659 | 0.234 | 0.005 | 1.933 (1.222–3.059) | 1 |
| Albumin ≤ 3.5 g/dL | 0.934 | 0.286 | 0.01 | 2.545 (1.453–4.456) | 2 |
| Charlson score ≥ 2 | 0.471 | 0.215 | 0.029 | 1.601 (1.050–2.440) | 1 |
| Falls in the past 6 months ≥ 1 | 0.614 | 0.268 | 0.022 | 1.847 (1.093–3.123) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliu, J.; Espinosa, E.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Losada, B.; Pinto, A.; Custodio, A.B.; Muñoz, M.d.M.; et al. Prediction of Unplanned Hospitalizations in Older Patients Treated with Chemotherapy. Cancers 2021, 13, 1437. https://doi.org/10.3390/cancers13061437
Feliu J, Espinosa E, Basterretxea L, Paredero I, Llabrés E, Jiménez-Munárriz B, Losada B, Pinto A, Custodio AB, Muñoz MdM, et al. Prediction of Unplanned Hospitalizations in Older Patients Treated with Chemotherapy. Cancers. 2021; 13(6):1437. https://doi.org/10.3390/cancers13061437
Chicago/Turabian StyleFeliu, Jaime, Enrique Espinosa, Laura Basterretxea, Irene Paredero, Elisenda Llabrés, Beatriz Jiménez-Munárriz, Beatriz Losada, Alvaro Pinto, Ana Belén Custodio, María del Mar Muñoz, and et al. 2021. "Prediction of Unplanned Hospitalizations in Older Patients Treated with Chemotherapy" Cancers 13, no. 6: 1437. https://doi.org/10.3390/cancers13061437
APA StyleFeliu, J., Espinosa, E., Basterretxea, L., Paredero, I., Llabrés, E., Jiménez-Munárriz, B., Losada, B., Pinto, A., Custodio, A. B., Muñoz, M. d. M., Gómez-Mediavilla, J., Torregrosa, M. D., Cruz, P., Higuera, O., & Molina-Garrido, M. J. (2021). Prediction of Unplanned Hospitalizations in Older Patients Treated with Chemotherapy. Cancers, 13(6), 1437. https://doi.org/10.3390/cancers13061437






