Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Data Source
2.2. Cohort Extraction
2.3. Variables and Outcomes
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Metastatic Cohorts
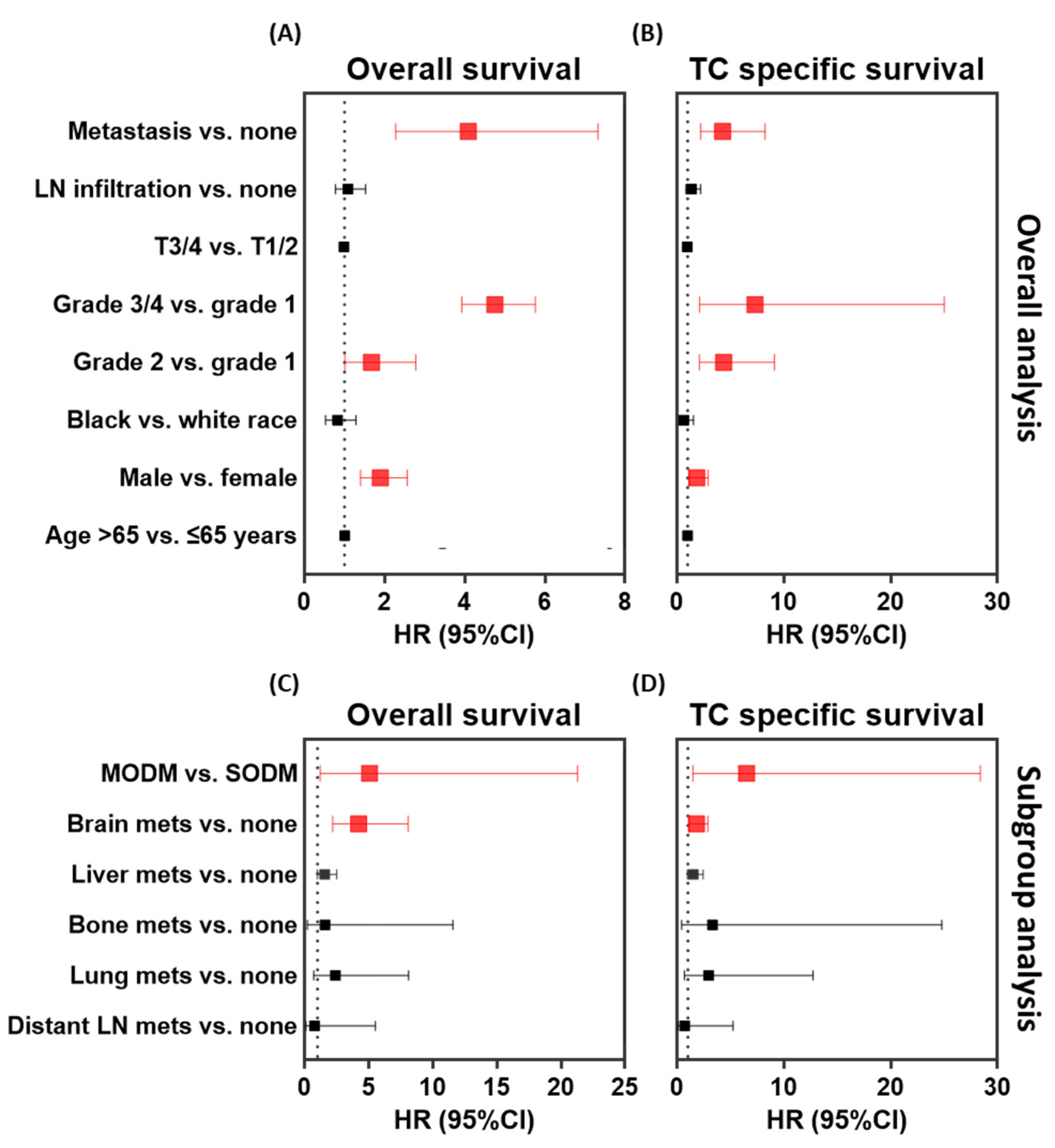
3.2. Impact of Single and Multi-Organ Distant Metastases on Prognosis
3.3. Impact of the Site of Metastasis at Presentation on Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Kilfoy, B.A.; Zheng, T.; Holford, T.R.; Han, X.; Ward, M.H.; Sjodin, A.; Zhang, Y.; Bai, Y.; Zhu, C.; Guo, G.L.; et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2008, 20, 525–531. [Google Scholar] [CrossRef]
- Ringel, M.D.; Ladenson, P.W. Controversies in the follow-up and management of well-differentiated thyroid cancer. Endocr. Relat. Cancer 2004, 11, 97–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmadi, S.; Stang, M.; Jiang, X.S.; Sosa, J.A. Hürthle cell carcinoma: Current perspectives. OncoTargets Ther. 2016, 9, 6873–6884. [Google Scholar] [CrossRef] [PubMed]
- Boone, R.T.; Fan, C.-Y.; Hanna, E.Y. Well-differentiated carcinoma of the thyroid. Otolaryngol. Clin. N. Am. 2003, 36, 73–90. [Google Scholar] [CrossRef]
- Khanafshar, E.; Lloyd, R.V. The Spectrum of Papillary Thyroid Carcinoma Variants. Adv. Anat. Pathol. 2011, 18, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Yamashita, K.; Enomoto, T.; Watanabe, M. Classification and General Considerations of Thyroid Cancer. Ann. Clin. Pathol. 2015, 3, 1045. [Google Scholar]
- Kazaure, H.S.; Roman, S.A.; Sosa, J.A. Aggressive Variants of Papillary Thyroid Cancer: Incidence, Characteristics and Predictors of Survival among 43,738 Patients. Ann. Surg. Oncol. 2011, 19, 1874–1880. [Google Scholar] [CrossRef]
- Ho, A.S.; Luu, M.; Barrios, L.; Chen, I.; Melany, M.; Ali, N.; Patio, C.; Chen, Y.; Bose, S.; Fan, X.; et al. Incidence and Mortality Risk Spectrum Across Aggressive Variants of Papillary Thyroid Carcinoma. JAMA Oncol. 2020, 6, 706–713. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Leboeuf, R.; Martorella, A.J. Papillary Thyroid Cancer: Monitoring and Therapy. Endocrinol. Metab. Clin. N. Am. 2007, 36, 753–778. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base Report on 53,856 Cases of Thyroid Carcinoma Treated in the U.S., 1985–1995. Cancer 1998, 83, 2638–2648. [Google Scholar] [CrossRef]
- Baek, S.-K.; Jung, K.-Y.; Kang, S.-M.; Kwon, S.-Y.; Woo, J.-S.; Cho, S.-H.; Chung, E.-J. Clinical Risk Factors Associated with Cervical Lymph Node Recurrence in Papillary Thyroid Carcinoma. Thyroid 2010, 20, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Palmer, F.L.; Nixon, I.J.; Thomas, D.; Patel, S.G.; Shaha, A.R.; Shah, J.P.; Tuttle, R.M.; Ganly, I. Multi-Organ Distant Metastases Confer Worse Disease-Specific Survival in Differentiated Thyroid Cancer. Thyroid 2014, 24, 1594–1599. [Google Scholar] [CrossRef]
- Mazzaferri, E.L. An Overview of the Management of Papillary and Follicular Thyroid Carcinoma. Thyroid 1999, 9, 421–427. [Google Scholar] [CrossRef]
- Mazzaferri, E.L. Long-Term Outcome of Patients with Differentiated Thyroid Carcinoma: Effect of Therapy. Endocr. Pract. 2000, 6, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhahri, S.F.; Al-Amro, A.S.; Al-Shakwer, W.; Terkawi, A.S. Cerebellar mass as a primary presentation of papillary thyroid carcinoma: Case report and literature review. Head Neck Oncol. 2009, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, J.C.; Dinauer, C.; Herrick-Reynolds, K.; Morotti, R.; Callender, G.G.; Christison-Lagay, E.R. Lymph node ratio predicts recurrence in pediatric papillary thyroid cancer. J. Pediatr. Surg. 2019, 54, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K. Metastatic sites and survival in lung cancer. Lung Cancer 2014, 86, 78–84. [Google Scholar] [CrossRef]
- Chen, D.; Huang, L.; Chen, S.; Huang, Y.; Hu, D.; Zeng, W.; Wang, M.; Zhou, W.; Feng, H.; Wei, W.; et al. Innovative analysis of distant metastasis in differentiated thyroid cancer. Oncol. Lett. 2020, 19, 1985–1992. [Google Scholar] [CrossRef]
- Lang, B.H.-H.; Wong, K.P.; Cheung, C.Y.; Wan, K.Y.; Lo, C.-Y. Evaluating the Prognostic Factors Associated with Cancer-specific Survival of Differentiated Thyroid Carcinoma Presenting with Distant Metastasis. Ann. Surg. Oncol. 2012, 20, 1329–1335. [Google Scholar] [CrossRef]
- Xu, B.; Tuttle, R.M.; Sabra, M.M.; Ganly, I.; Ghossein, R. Primary Thyroid Carcinoma with Low-Risk Histology and Distant Metastases: Clinicopathologic and Molecular Characteristics. Thyroid 2017, 27, 632–640. [Google Scholar] [CrossRef]
- Shaha, A.R.; Shah, J.P.; Loree, T.R. Differentiated thyroid cancer presenting initially with distant metastasis. Am. J. Surg. 1997, 174, 474–476. [Google Scholar] [CrossRef]
- Brierley, J.; Tsang, R.; Panzarella, T.; Bana, N. Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin. Endocrinol. 2005, 63, 418–427. [Google Scholar] [CrossRef]
- Haq, M.; Harmer, C. Differentiated thyroid carcinoma with distant metastases at presentation: Prognostic factors and outcome. Clin. Endocrinol. 2005, 63, 87–93. [Google Scholar] [CrossRef]
- Sampson, E.; Brierley, J.D.; Le, L.W.; Rotstein, L.; Tsang, R.W. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 2007, 110, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Borschitz, T.; Eichhorn, W.; Fottner, C.; Hansen, T.; Schad, A.; Schadmand-Fischer, S.; Weber, M.M.; Schreckenberger, M.; Lang, H.; Musholt, T.J. Diagnosis and Treatment of Pancreatic Metastases of a Papillary Thyroid Carcinoma. Thyroid 2010, 20, 93–98. [Google Scholar] [CrossRef]
- Ding, W.; Ruan, G.; Zhu, J.; Tu, C.; Li, Z. Metastatic site discriminates survival benefit of primary tumor surgery for differentiated thyroid cancer with distant metastases. Medicine 2020, 99, e23132. [Google Scholar] [CrossRef] [PubMed]
- Soylu, S.; Arikan, A.E.; Teksoz, S.; Ozcan, M.; Bukey, Y. Skin metastasis on the neck: An unusual presentation of recurrence of papillary thyroid carcinoma. Gland Surg. 2017, 6, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.M.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef]
- Graus, F.; Walker, R.W.; Allen, J.C. Brain metastases in children. J. Pediatr. 1983, 103, 558–561. [Google Scholar] [CrossRef]
- Sundermeyer, M.L.; Meropol, N.J.; Rogatko, A.; Wang, H.; Cohen, S.J. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin. Color. Cancer 2005, 5, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Doumi, N.; Thiesse, P.; Mottolese, C.; Jouvet, A.; Lacroze, M.; Carrie, C.; Frappaz, D.; Brunat-Mentigny, M. Brain metastases in children with solid tumors. Cancer 1997, 79, 403–410. [Google Scholar] [CrossRef]
- Cacho-Diaz, B.; Cuevas-Ramos, D.; Spinola-Marono, H.; Reyes-Soto, G.; Olvera-Manzanilla, E.; Monroy-Sosa, A.; Lorenzana-Mendoza, N.A.; Granados-Garcia, M.; Herrera-Gomez, A. Thyroid Cancer Brain Metastases and Thyroglobulin. J. Endocrinol. Metab. 2016, 6, 90–94. [Google Scholar] [CrossRef][Green Version]
- Lee, H.S.; Yoo, H.; Lee, S.H.; Gwak, H.S.; Shin, S.H. Clinical characteristics and follow-up of intracranial metastases from thyroid cancer. Acta Neurochir. 2015, 157, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-W.; Lin, J.-D.; Yu, M.-C.; Hsu, C.-C.; Lin, Y.-S. Outcomes and prognostic factors in thyroid cancer patients with cranial metastases: A retrospective cohort study of 4,683 patients. Int. J. Surg. 2018, 55, 182–187. [Google Scholar] [CrossRef]
- Jakola, A.S.; Gulati, S.; Nerland, U.S.; Solheim, O. Surgical resection of brain metastases: The prognostic value of the graded prognostic assessment score. J. Neuro-Oncol. 2011, 105, 573–581. [Google Scholar] [CrossRef]
- Villà, S.; Weber, D.C.; Moretones, C.; Mañes, A.; Combescure, C.; Jové, J.; Puyalto, P.; Cuadras, P.; Bruna, J.; Verger, E.; et al. Validation of the new graded prognostic assessment scale for brain metastases: A multicenter prospective study. Radiat. Oncol. 2011, 6, 23. [Google Scholar] [CrossRef]
- Çeltek, N.Y.; Süren, M.; Demir, O.; Okan, I. Karnofsky Performance Scale validity and reliability of Turkish palliative cancer patients. Turk. J. Med. Sci. 2019, 49, 894–898. [Google Scholar] [CrossRef]
- De Figueiredo, B.H.; Godbert, Y.; Soubeyran, I.; Carrat, X.; Lagarde, P.; Cazeau, A.-L.; Italiano, A.; Sargos, P.; Kantor, G.; Loiseau, H.; et al. Brain Metastases from Thyroid Carcinoma: A Retrospective Study of 21 Patients. Thyroid 2014, 24, 270–276. [Google Scholar] [CrossRef]
- Misaki, T.; Iwata, M.; Kasagi, K.; Konishi, J. Brain metastasis from differentiated thyroid cancer in patients treated with radioiodine for bone and lung lesions. Ann. Nucl. Med. 2000, 14, 111–114. [Google Scholar] [CrossRef]
- Haugen, B.R.; Kane, M.A. Approach to the Thyroid Cancer Patient with Extracervical Metastases. J. Clin. Endocrinol. Metab. 2010, 95, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Soh, E.-Y. Differentiated Thyroid Carcinoma Presenting with Distant Metastasis at Initial Diagnosis. Ann. Surg. 2010, 251, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Spitzweg, C.; Bible, K.C.; Hofbauer, L.C.; Morris, J.C. Advanced radioiodine-refractory differentiated thyroid cancer: The sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2014, 2, 830–842. [Google Scholar] [CrossRef]
- Ni, W.; Chen, W.; Lu, Y. Emerging findings into molecular mechanism of brain metastasis. Cancer Med. 2018, 7, 3820–3833. [Google Scholar] [CrossRef] [PubMed]
- Janavicius, M.; Lachej, N.; Anglickiene, G.; Vincerzevskiene, I.; Brasiuniene, B. Outcomes of Treatment for Melanoma Brain Metastases. J. Ski. Cancer 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Nahed, B.V.; Alvarez-Breckenridge, C.; Brastianos, P.K.; Shih, H.; Sloan, A.; Ammirati, M.; Kuo, J.S.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Surgery in the Management of Adults with Metastatic Brain Tumors. Neurosurgery 2019, 84, E152–E155. [Google Scholar] [CrossRef]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Tvall, J.O.L.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, D. Microenvironment Determinants of Brain Metastasis. Cell Biosci. 2011, 1, 8. [Google Scholar] [CrossRef]
- Osborne, J.R.; Kondraciuk, J.D.; Rice, S.L.; Zhou, X.; Knezevic, A.; Spratt, D.E.; Sabra, M.; Larson, S.M.; Grewal, R.K. Thyroid Cancer Brain Metastasis. Clin. Nucl. Med. 2019, 44, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Jakob, J.A.; Bassett, R.L.; Ng, C.S.; Curry, J.L.; Joseph, R.W.; Alvarado, G.C.; Apn, M.L.R.; Richard, J.; Gershenwald, J.E.; Kim, K.B.; et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012, 118, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; King, J.; Tai, E.; Buchanan, N.; Ajani, U.A.; Li, J. Cancer Incidence Rates and Trends Among Children and Adolescents in the United States, 2001–2009. Pediatrics 2014, 134, e945–e955. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z. SEER Cancer Statistics Review, 1975–2010; National Cancer Institute: Bethesda, MD, USA, 2013; Volume 21, p. 12. [Google Scholar]
- Bal, C.S.; Garg, A.; Chopra, S.; Ballal, S.; Soundararajan, R. Prognostic factors in pediatric differentiated thyroid cancer patients with pulmonary metastases. J. Pediatr. Endocrinol. Metab. 2015, 28, 745–751. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, D.; Huang, Y.; Chen, S.; Zeng, W.; Zhou, L.; Zhou, W.; Wang, M.; Feng, H.; Wei, W.; et al. Factors associated with distant metastasis in pediatric thyroid cancer: Evaluation of the SEER database. Endocr. Connect. 2019, 8, 78–85. [Google Scholar] [CrossRef]
- Zanella, A.B.; Scheffel, R.S.; Weinert, L.; Dora, J.M.; Maia, A.L. New insights into the management of differentiated thyroid carcinoma in children and adolescents (Review). Int. J. Oncol. 2021, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nies, M.; Vassilopoulou-Sellin, R.; Bassett, R.L.; Yedururi, S.; Zafereo, M.E.; Cabanillas, M.E.; Sherman, S.I.; Links, T.P.; Waguespack, S.G. Distant metastases from childhood differentiated thyroid carcinoma: Clinical course and mutational landscape. J. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef]
- Hay, I.D.; Johnson, T.R.; Kaggal, S.; Reinalda, M.S.; Iniguez-Ariza, N.M.; Grant, C.S.; Pittock, S.T.; Thompson, G.B. Papillary Thyroid Carcinoma (PTC) in Children and Adults: Comparison of Initial Presentation and Long-Term Postoperative Outcome in 4432 Patients Consecutively Treated at the Mayo Clinic During Eight Decades (1936–2015). World J. Surg. 2018, 42, 329–342. [Google Scholar] [CrossRef]
- Wada, N.; Sugino, K.; Mimura, T.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; Nakayama, H.; Hirakawa, S.; Yukawa, N.; et al. Treatment Strategy of Papillary Thyroid Carcinoma in Children and Adolescents: Clinical Significance of the Initial Nodal Manifestation. Ann. Surg. Oncol. 2009, 16, 3442–3449. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, M.S.K.; Nies, M.; Bocca, G.; Brouwers, A.H.; Burgerhof, J.G.M.; Van Dam, E.W.C.M.; Havekes, B.; Heuvel-Eibrink, M.M.V.D.; Corssmit, E.P.M.; Kremer, L.C.M.; et al. Pediatric Differentiated Thyroid Carcinoma in The Netherlands: A Nationwide Follow-Up Study. J. Clin. Endocrinol. Metab. 2016, 101, 2031–2039. [Google Scholar] [CrossRef]
- Zimmerman, D.; Hay, I.D.; Gough, I.R.; Goellner, J.R.; Ryan, J.J.; Grant, C.S.; McConahey, W.M. Papillary thyroid carcinoma in children and adults: Long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 1988, 104, 1157–1166. [Google Scholar]
- Alzahrani, A.S.; Alswailem, M.; Moria, Y.; Almutairi, R.; Alotaibi, M.; Murugan, A.K.; Qasem, E.; Alghamdi, B.; Al-Hindi, H.; Al-Handi, H. Lung Metastasis in Pediatric Thyroid Cancer: Radiological Pattern, Molecular Genetics, Response to Therapy, and Outcome. J. Clin. Endocrinol. Metab. 2018, 104, 103–110. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Song, H.-J.; Qiu, Z.-L.; Shen, C.-T.; Chen, X.-Y.; Sun, Z.-K.; Wei, W.-J.; Zhang, G.-Q.; Luo, Q.-Y. Pulmonary metastases in children and adolescents with papillary thyroid cancer in China: Prognostic factors and outcomes from treatment with 131I. Endocrine 2018, 62, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; De Vathaire, F.; Travagli, J.P.; Vassal, G.; Lemerle, J.; Parmentier, C.; Tubiana, M. Differentiated Thyroid Carcinoma in Childhood: Long Term Follow-Up of 72 Patients. J. Clin. Endocrinol. Metab. 1987, 65, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A. An attractive force in metastasis. Nat. Cell Biol. 2001, 410, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Molecular mechanisms of cancer metastasis: Tumor and host properties and the role of oncogenes and suppressor genes. Curr. Opin. Oncol. 1991, 3, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Dellacasagrande, J.; Schreurs, O.J.F.; Hofgaard, P.O.; Omholt, H.; Steinsvoll, S.; Schenck, K.; Bogen, B.; Dembic, Z. Liver Metastasis of Cancer Facilitated by Chemokine Receptor CCR6. Scand. J. Immunol. 2003, 57, 534–544. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Level | Total Population (n = 89,694) | No Metastasis (n = 87,875) | Metastasis (n = 1819) | p Value |
|---|---|---|---|---|---|
| Age, years | Mean ± SD | 65.18 ± 14.94 | 50.25 ± 15.64 | 65.38 ± 15.95 | <0.001 |
| <18 years | 1035 (1.2) | 1013 (1.2) | 22 (1.2) | <0.001 | |
| 18–45 years | 33,088 (36.9) | 32,926 (37.5) | 162 (8.9) | ||
| 46–65 years | 39,198 (43.7) | 38,558 (43.9) | 640 (35.2) | ||
| >65 years | 16,373 (18.3) | 15,378 (17.5) | 995 (54.7) | ||
| Gender | Female | 67,635 (75.4) | 66,647 (75.8) | 988 (54.3) | <0.001 |
| Male | 22,059 (24.6) | 21,228 (24.2) | 831 (45.7) | ||
| Race | White | 71,973 (80.2) | 70,635 (80.4) | 1338 (73.6) | <0.001 |
| Black | 6356 (7.1) | 6157 (7) | 199 (10.9) | ||
| Asian/Pacific Islander | 9408 (10.5) | 9143 (10.4) | 265 (14.6) | ||
| American Indian/Alaska | 609 (0.7) | 597 (0.7) | 12 (0.7) | ||
| Unknown | 1348 (1.5) | 1343 (1.5) | 5 (0.3) | ||
| Insurance status | Not insured | 2194 (2.4) | 2142 (2.4) | 52 (2.9) | <0.001 |
| Private | 77,827 (86.8) | 76,390 (86.9) | 1437 (79) | ||
| Medicaid/Medicare | 9673 (10.8) | 9343 (10.6) | 330 (18.1) | ||
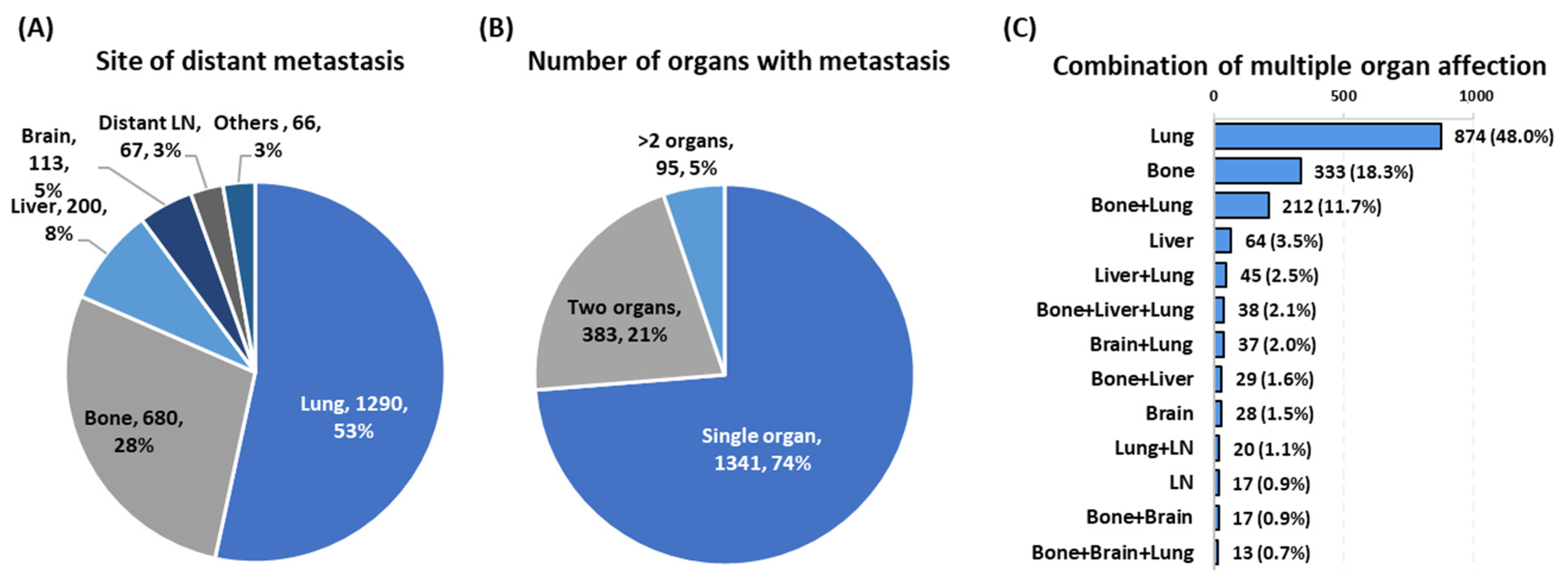
| Number of metastatic sites | 0 | 87,875 (98.0) | 87,875 (100) | --- | NA |
| 1 | 1341 (1.5) | --- | 1341 (73.7) | ||
| 2 | 383 (0.4) | --- | 383 (21.1) | ||
| 3+ | 95 (0.1) | --- | 95 (5.2) | ||
| Vital status | Alive | 84,937 (94.7) | 84,127 (95.7) | 810 (44.5) | <0.001 |
| Dead | 4757 (5.3) | 3748 (4.3) | 1009 (55.5) |
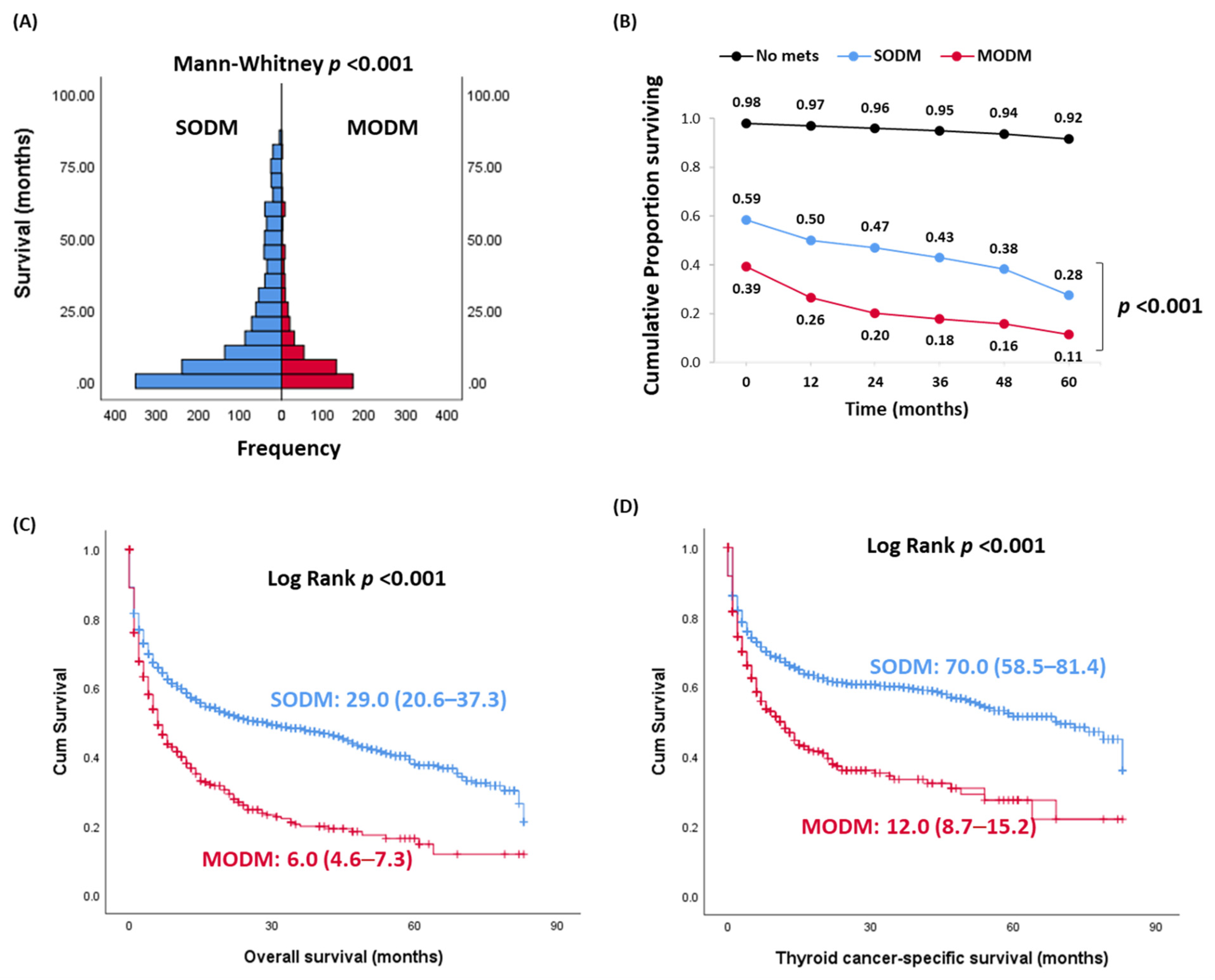
| Characteristics | SODM (n = 1341) | MODM (n = 478) | p Value | |
|---|---|---|---|---|
| Demographic Data | ||||
| Age, years | Mean ± SD | 65.37 ± 16.6 | 65.4 ± 13.9 | 0.96 |
| Sex | Female | 730 (54.4) | 258 (54) | 0.87 |
| Male | 611 (45.6) | 220 (46) | ||
| Race | White | 1002 (74.7) | 336 (70.3) | 0.28 |
| Black | 138 (10.3) | 61 (12.8) | ||
| Asian/Pacific Islander | 188 (14) | 77 (16.1) | ||
| American Indian/Alaska | 10 (0.7) | 2 (0.4) | ||
| Cancer characteristics | ||||
| Tumor size, mm | Median (Quartiles) | 13 (4–25) | 18 (7–27) | 0.42 |
| Mean ± SD | 16.7 ± 14.9 | 20.7 ± 16.8 | 0.28 | |
| Regional LN | N0 | 819 (61.1) | 325 (68) | 0.008 |
| N1 | 522 (38.9) | 153 (32) | ||
| Outcomes | ||||
| Overall survival, years | Median (Quartiles) | 6 (2–10) | 3 (1–6) | <0.001 |
| Thyroid cancer-specific death, % | Thyroid cancer | 492 (36.7) | 241 (50.4) | 0.002 |
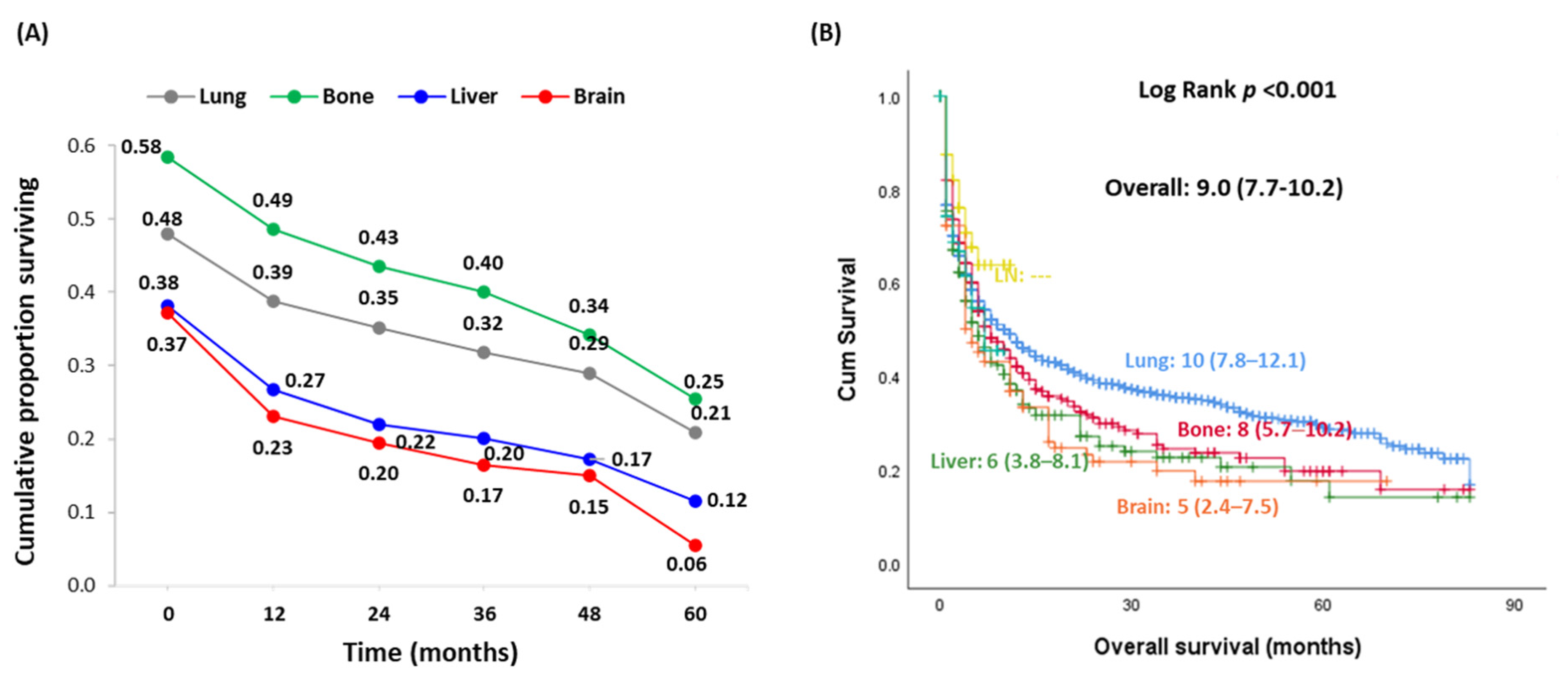
| Haracteristics | Lung (n = 1290) | Bone (n = 680) | Liver (n = 200) | Brain (n = 113) | LN (n = 67) | Others (n = 66) | p Value | |
|---|---|---|---|---|---|---|---|---|
| Demographic Data | ||||||||
| Age, years | Mean ± SD | 65.61 ± 16.45 | 65.61 ± 13.25 | 65.83 ± 15.11 | 62.87 ± 14.19 | 63.82 ± 16.66 | 65.58 ± 14.51 | 0.54 |
| Sex | Female | 695 (53.9) | 370 (54.4) | 111 (55.5) | 70 (61.9) | 36 (53.7) | 41 (62.1) | 0.51 |
| Male | 595 (46.1) | 310 (45.6) | 89 (44.5) | 43 (38.1) | 31 (46.3) | 25 (37.9) | ||
| Race | White | 960 (74.4) | 468 (68.8) | 159 (79.5) | 77 (68.1) | 52 (77.6) | 53 (80.3) | 0.001 |
| Black | 129 (10) | 105 (15.4) | 20 (10) | 8 (7.1) | 4 (6) | 6 (9.1) | ||
| Asian/Pacific Islander | 185 (14.3) | 103 (15.1) | 20 (10) | 28 (24.8) | 10 (14.9) | 7 (10.6) | ||
| Cancer characteristics | ||||||||
| Tumor size, mm | Mean ± SD | 17.48 ± 14.48 | 13.25 ± 11.25 | 17.91 ± 10.49 | 28.00 ± 25.96 | 22.37 ± 21.31 | 16.64 ± 10.10 | 0.94 |
| Regional LN | N0 | 776 (60.2) | 324 (47.6) | 125 (62.5) | 76 (67.3) | 39 (58.2) | 42 (63.6) | <0.001 |
| N1 | 514 (39.8) | 356 (52.4) | 75 (37.5) | 37 (32.7) | 28 (41.8) | 24 (36.4) | ||
| Number of metastatic organs | One | 874 (67.8) | 333 (49.0) | 64 (32) | 28 (24.8) | 17 (25.4) | 25 (37.9) | <0.001 |
| Two | 324 (25.1) | 264 (38.8) | 76 (38) | 58 (51.3) | 26 (38.8) | 18 (27.3) | ||
| ≥Three | 92 (7.1) | 83 (12.2) | 60 (30) | 27 (23.9) | 24 (35.8) | 23 (34.8) | ||
| Survival | ||||||||
| Vital status | Alive | 500 (38.8) | 324 (47.6) | 64 (32) | 31 (27.4) | 49 (73.1) | 40 (60.6) | <0.001 |
| Dead | 790 (61.2) | 356 (52.4) | 136 (68) | 82 (72.6) | 18 (26.9) | 26 (39.4) | ||
| OS, years | Mean ± SD | 4.10 ± 3.28 | 4.88 ± 2.53 | 3.64 ± 3.29 | 3.50 ± 3.86 | 4.63 ± 3.74 | 4.09 ± 2.88 | <0.001 |
| Cause of death, % | TC | 587 (74.3) | 246 (69.1) | 105 (77.2) | 61 (74.4) | 11 (61.1) | 20 (76.9) | 0.40 |
| Other cancers | 53 (6.7) | 33 (9.3) | 7 (5.1) | 2 (2.4) | 2 (11.1) | 2 (7.7) | ||
| Non-cancer | 150 (19) | 77 (21.6) | 24 (17.6) | 19 (23.2) | 5 (27.8) | 4 (15.4) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toraih, E.A.; Hussein, M.H.; Zerfaoui, M.; Attia, A.S.; Marzouk Ellythy, A.; Mostafa, A.; Ruiz, E.M.L.; Shama, M.A.; Russell, J.O.; Randolph, G.W.; et al. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers 2021, 13, 1625. https://doi.org/10.3390/cancers13071625
Toraih EA, Hussein MH, Zerfaoui M, Attia AS, Marzouk Ellythy A, Mostafa A, Ruiz EML, Shama MA, Russell JO, Randolph GW, et al. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers. 2021; 13(7):1625. https://doi.org/10.3390/cancers13071625
Chicago/Turabian StyleToraih, Eman A., Mohammad H. Hussein, Mourad Zerfaoui, Abdallah S. Attia, Assem Marzouk Ellythy, Arwa Mostafa, Emmanuelle M. L. Ruiz, Mohamed Ahmed Shama, Jonathon O. Russell, Gregory W. Randolph, and et al. 2021. "Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease" Cancers 13, no. 7: 1625. https://doi.org/10.3390/cancers13071625
APA StyleToraih, E. A., Hussein, M. H., Zerfaoui, M., Attia, A. S., Marzouk Ellythy, A., Mostafa, A., Ruiz, E. M. L., Shama, M. A., Russell, J. O., Randolph, G. W., & Kandil, E. (2021). Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers, 13(7), 1625. https://doi.org/10.3390/cancers13071625