Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
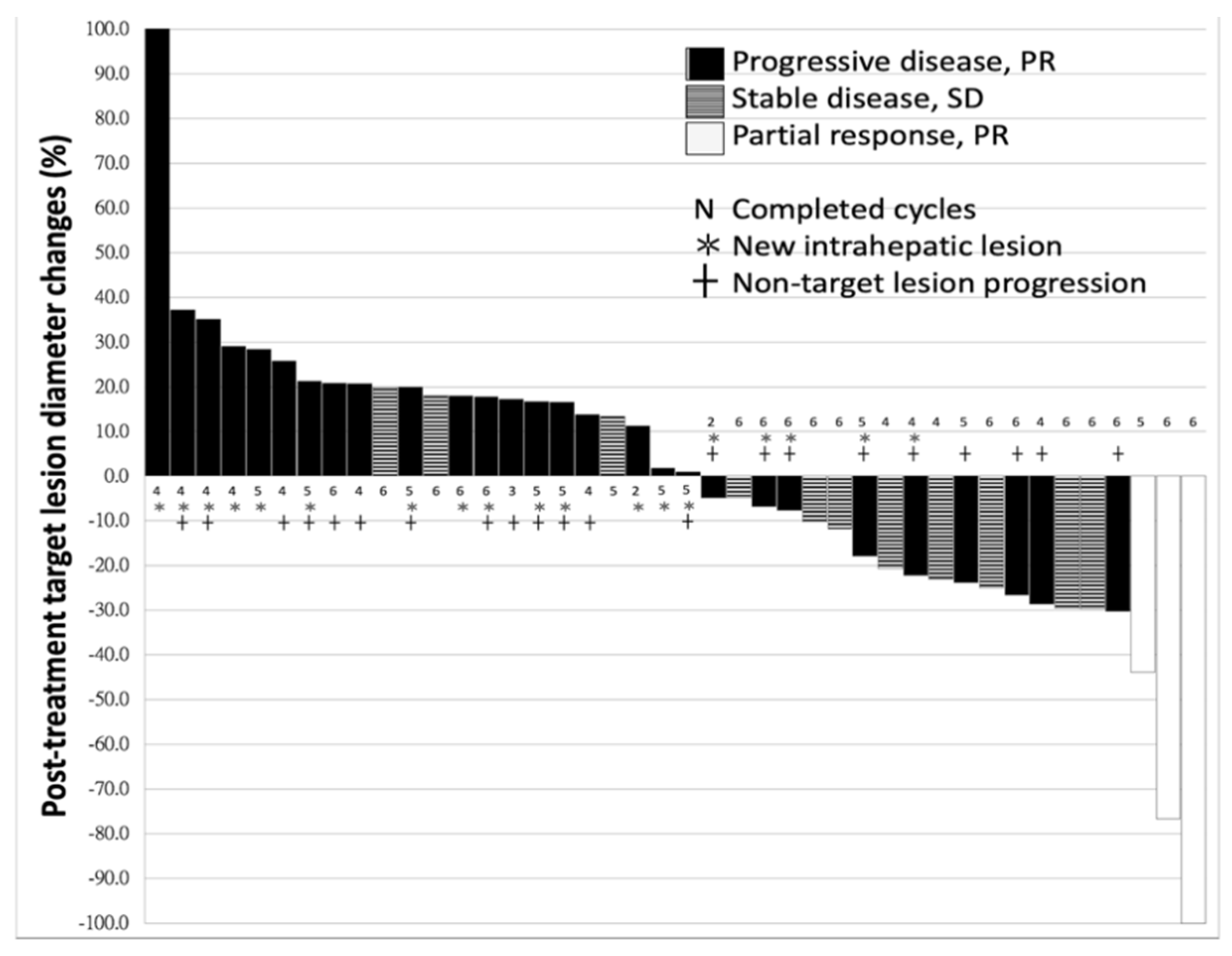
2.1. Characteristics of Patients and Response to Nivolumab
2.2. Difference between the Patients With or Without Disease-Control
2.3. Univariate and Multivariate Logistic Regression
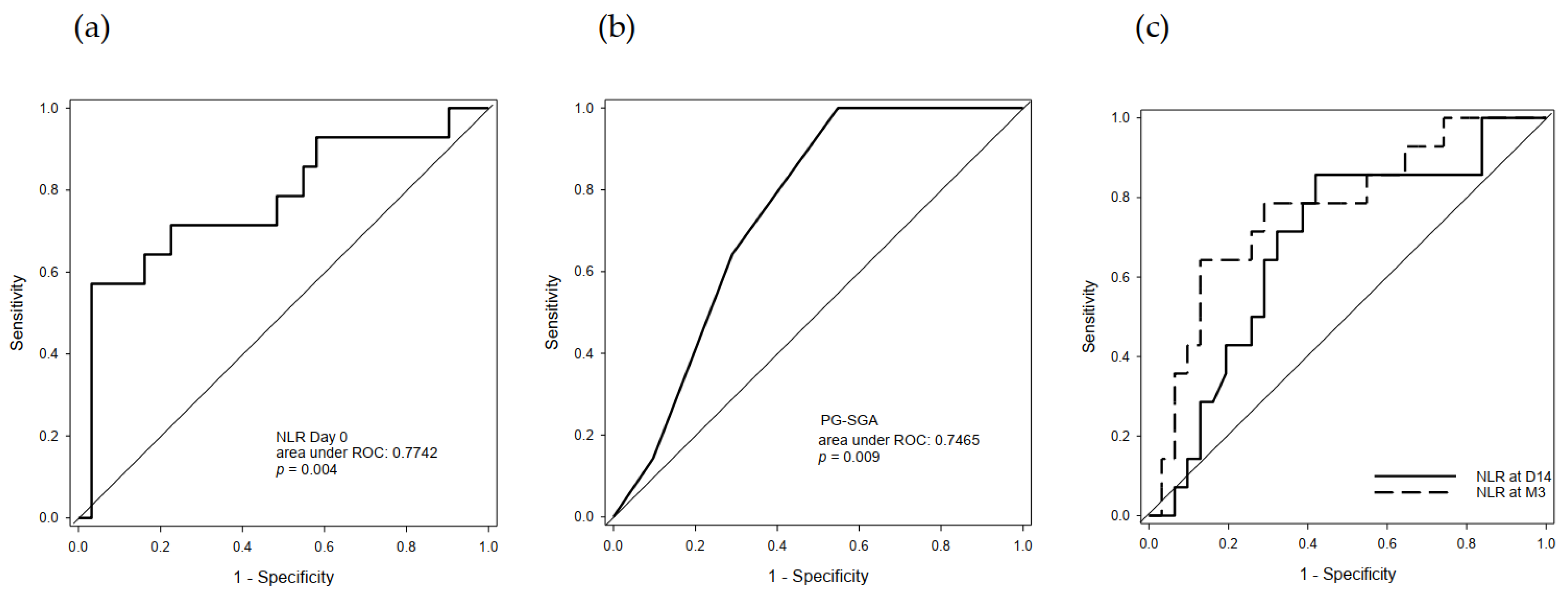
2.4. Predictive Value of Serum NLR and PG-SGA
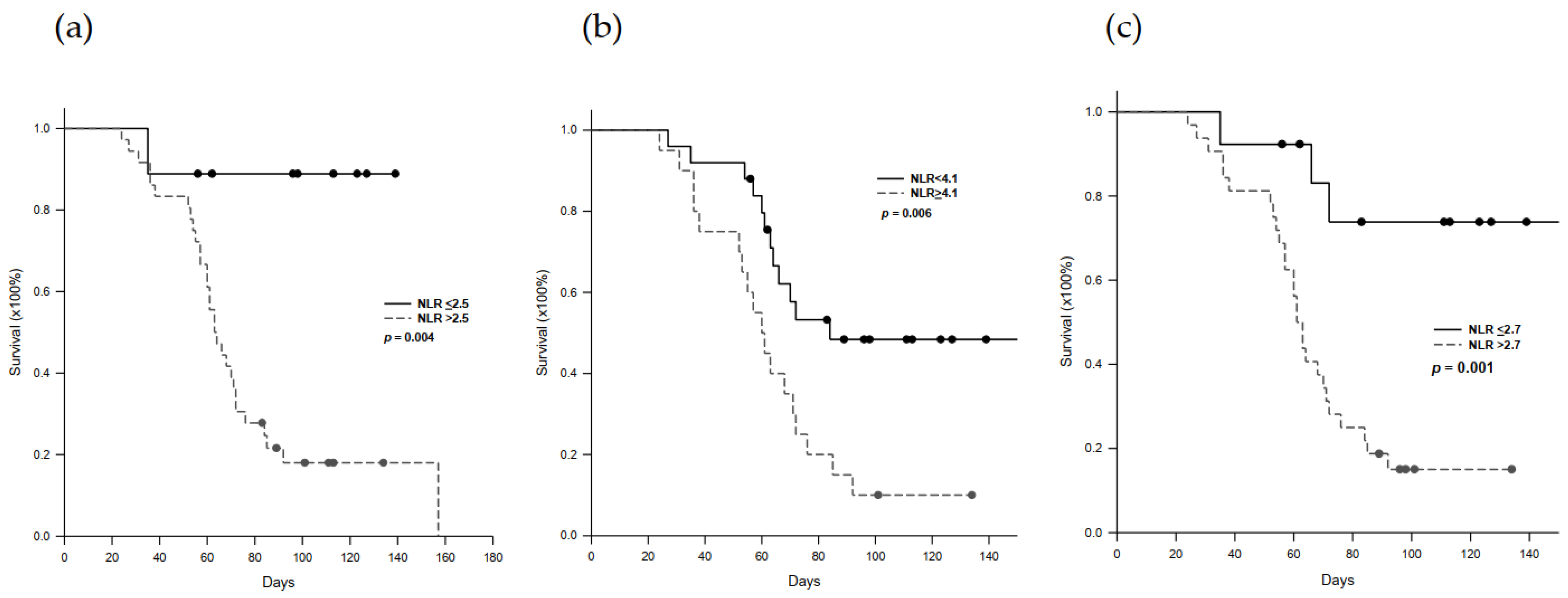
2.5. Progression-Free Survival in Nivolumab-Treated Patients
2.6. Immune-Related Adverse Effect (irAE) Profiles
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Nivolumab Administration
4.3. Assessment of Responses to Treatment
4.4. Clinical Profiles
4.5. Neutrophil-to-Lymphocyte Ratio
4.6. Clinical Benefits of Treatment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tinkle, C.L.; Haas-Kagan, D. Hepatocellular carcinoma: Natural history, current management, and emerging tool. Biol. Target Ther. 2012, 6, 207–219. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; YKang, O.-K.; Chen, Z. Effi cacy and safety of sorafenib in patients in the Asia-Pacifi c region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.-H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2017, 32, 317–326. [Google Scholar] [CrossRef]
- McDermott, D.; Haanen, J.; Chen, T.-T.; Lorigan, P.; O’Day, S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann. Oncol. 2013, 24, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, P.A. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Kang, Y.-K.; Hou, M.-M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 2019, 71, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Lavoie, J.-M.; Black, P.C.; Eigl, B.J. Predictive Biomarkers for Checkpoint Blockade in Urothelial Cancer: A Systematic Review. J. Urol. 2019, 202, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.E.; Banerji, S. Biomarkers of Immune Checkpoint Inhibitor Efficacy in Cancer. Curr. Oncol. 2020, 27, 106–114. [Google Scholar] [CrossRef]
- Bustamante-Alvarez, J.G.; Owen, D.H. Biomarkers for Immunotherapy. Thorac. Surg. Clin. 2020, 30, 207–214. [Google Scholar] [CrossRef]
- Fumet, J.-D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bellesoeur, A.; Torossian, N.; Amigorena, S.; Romano, E. Advances in theranostic biomarkers for tumor immunotherapy. Curr. Opin. Chem. Biol. 2020, 56, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, H.; Sun, B.; Karin, M. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. J. Hepatol. 2020, 72, 167–182. [Google Scholar] [CrossRef]
- Jung, M.R.; Park, Y.K.; Jeong, O.; Seon, J.W.; Ryu, S.Y.; Kim, D.Y.; Kim, Y.J. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J. Surg. Oncol. 2011, 104, 504–510. [Google Scholar] [CrossRef]
- Van Berckelaer, C.; Van Geyt, M.; Linders, S. A high neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are associated with a worse outcome in inflammatory breast cancer. Breast 2020, 53, 212–220. [Google Scholar] [CrossRef]
- Mazaki, J.; Katsumata, K.; Kasahara, K.; Tago, T.; Wada, T.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y.; Tsuchida, A. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: A propensity score analysis. BMC Cancer 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Wu, J.N.; Lv, X.D. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS ONE 2020, 15, e0237947. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Lee, J.-C.; Cheng, C.-H.; Wu, T.-H.; Wang, Y.-C.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.-K.; Chen, D.; Li, S.-Q.; Fu, S.-J.; Peng, B.-G.; Liang, L.-J. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer 2014, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-D.; Shi, X.-J.; Chen, Y.-G.; Wang, C.-L.; Ma, Q.; Lv, G.-Y. Elevated Preoperative Neutrophil-Lymphocyte Ratio Is Associated with Poor Prognosis in Hepatocellular Carcinoma Patients Treated with Liver Transplantation: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Jiang, T.; Bai, Y.; Zhou, F.; Li, W.; Gao, G.; Su, C.; Ren, S.; Chen, X.; Zhou, C. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer 2019, 130, 76–83. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef]
- Suzuki, K.; Terakawa, T.; Furukawa, J.; Harada, K.; Hinata, N.; Nakano, Y.; Fujisawa, M. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int. J. Clin. Oncol. 2020, 25, 135–144. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Wakasaki, T.; Hashimoto, K.; Nakashima, K.; Manako, T.; Taura, M.; Matsuo, M.; Nakagawa, T. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck 2019, 41, 2610–2618. [Google Scholar] [CrossRef]
- Jeyakumar, G.; Kim, S.; Bumma, N.; Landry, C.; Silski, C.; Suisham, S.; Dickow, B.; Heath, E.; Fontana, J.; Vaishampayan, U. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J. Immunother. Cancer 2017, 5, 82. [Google Scholar] [CrossRef]
- Kirsch, R.; Matthews, K.; Williams, V. Using Global Criteria to Detect Malnutrition: Application in Disease States. Nutr. Clin. Pract. 2019, 35, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Chao, Y.; Chen, M.-H.; Lan, K.-H.; Lee, C.-J.; Lee, I.-C.; Chen, S.-C.; Hou, M.-C.; Huang, Y.-H. Predictors of Response and Survival in Immune Checkpoint Inhibitor-Treated Unresectable Hepatocellular Carcinoma. Cancers 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Dharmapuri, S.; Özbek, U.; Lin, J.; Sung, M.; Schwartz, M.; Branch, A.D.; Ang, C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti–PD-1 therapy. Cancer Med. 2020, 9, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Chen, Q.; Zhang, B.; Liu, J.; Liu, S.; Mo, X.; Li, M.; Chen, Z.; Chen, L.; et al. Predicting hyperprogressive disease in patients with advanced hepatocellular carcinoma treated with anti-programmed cell death 1 therapy. EClinicalMedicine 2020, 31, 100673. [Google Scholar] [CrossRef] [PubMed]
- Kwee, S.A.; Tiirikainen, M. Beta-catenin activation and immunotherapy resistance in hepatocellular carcinoma: Mechanisms and biomarkers. Hepatoma Res. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Li, Y.; Ren, H.P. 2010 guideline for the management of hepatocellular carcinoma recommended by the American Association for the Study of Liver Diseases. Zhonghua Gan Zang Bing Za Zhi 2011, 19, 249–250. [Google Scholar]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharm. 1989, 24, 148–154. [Google Scholar] [CrossRef]
| Factors | n = 45 (100%) |
|---|---|
| Baseline conditions | |
| Age (years) | 61.8 ± 9.6 |
| Gender (Male) | 41 (91.1%) |
| CTP score | 5.3 ± 0.6 |
| ALBI score | −2.49 ± 0.39 |
| Thrombocytopenia | 11 (24.4%) |
| EV | 7 (15.6%) |
| Ascites | 10 (22.2%) |
| Cirrhosis | 41 (91.1%) |
| ECOG-PS (0/1/2) | 35/9/1 |
| Viral hepatitis | 38 (84.4%) |
| Alcohol consumption | 8 (17.8%) |
| PG-SGA score | 3.8 ± 2.9 |
| Tumor-associated factors | |
| Maximum tumor diameter (cm) | 7.2 ± 4.2 |
| Total tumor volume (cm3) | 619.0 ± 831.1 |
| Alpha-fetoprotein (ng/mL) | 82,584.2 ± 499,364.2 |
| T stage (2/3/4) | 13/12/20 (28.9/26.7/44.4%) |
| N stage (1) | 10 (22.2%) |
| M stage (1) | 22 (48.9%) |
| PVT | 19 (42.2%) |
| Serum NLR | 4.0 ± 2.2 |
| Medical treatment | |
| Anti-viral agent | 22 (48.9%) |
| Previous history of hepatectomy | 19 (42.2%) |
| Previous Sorafenib | 45 (100.0%) |
| Period between diagnosis and ICI (months) | 37.0 ± 32.5 |
| Early drop-out of ICI treatment | 11 (24.4%) |
| Response to ICI (PR/SD/PD) | 3/11/31 (6.7/24.4/68.9%) |
| Factors | PR + SD (n = 14) | PD (n = 31) | p-Value |
|---|---|---|---|
| Age (years) | 65.2 ± 10.2 | 60.3 ± 9.1 | 0.117 |
| Gender (Male) | 14 (100.0%) | 27 (87.1%) | 0.159 |
| WBC (×109/L) | 6.1 ± 2.0 | 5.7 ± 2.2 | 0.571 |
| Platelet(×103/μL) | 168.6 ± 76.3 | 193.4 ± 149.3 | 0.558 |
| INR | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.163 |
| NLR | 2.9 ± 1.3 | 4.4 ± 2.3 | 0.028 |
| PLR | 123.7 ± 65.4 | 190.7 ± 118.6 | 0.185 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 0.9 ± 0.6 | 0.734 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.9 ± 0.5 | 0.184 |
| AST (U/L) | 62.3 ± 30.5 | 82.7 ± 54.7 | 0.200 |
| ALT (U/L) | 48.1 ± 29.2 | 63.5 ± 49.9 | 0.291 |
| Albumin (g/dL) | 4.0 ± 0.4 | 3.7 ± 0.4 | 0.059 |
| CTP class (A/B) | 14/0 (100.0/0.0%) | 29/2 (93.5/6.5%) | 0.331 |
| ALBI score | −2.7 ± 0.3 | −2.4 ± 0.4 | 0.042 |
| EV | 3 (21.4%) | 4 (12.9%) | 0.465 |
| Ascites | 2 (14.3%) | 8 (25.8%) | 0.389 |
| Cirrhosis | 11 (78.6%) | 30 (96.8%) | 0.047 |
| ECOG-PS (0/1/2) | 12/2/0 (85.7/14.3/0.0%) | 23/7/1 (74.2/22.6/0.3%) | 0.623 |
| Viral hepatitis (Yes) | 12 (85.7%) | 26 (83%) | 0.874 |
| Alcohol use (Yes) | 3 (21.4%) | 5 (16.1%) | 0.667 |
| PG-SGA score | 2.3 ± 0.7 | 4.7 ± 3.2 | 0.003 |
| AFP (ng/mL) | 3540.9 ± 5481.5 | 118281.2 ± 601217.5 | 0.482 |
| Max. tumor diameter (cm) | 5.6 ± 3.7 | 8.0 ± 4.3 | 0.091 |
| Total tumor volume (cm3) | 397.2 ± 659.3 | 719.1 ± 889.6 | 0.233 |
| T (2/3/4) | 6/5/3 (42.9/35.7/21.4%) | 7/7/17 (22.6/22.6/54.8%) | 0.110 |
| N (1) | 2 (11.1%) | 8 (27.3%) | 0.389 |
| M (1) | 7 (50.0%) | 15 (48.4%) | 0.920 |
| PVT | 4 (28.6%) | 15 (48.4%) | 0.213 |
| Anti-viral agent | 8 (57.1%) | 14 (45.2%) | 0.457 |
| Period between diagnosis and IC (months) | 35.4 ± 43.2 | 37.8 ± 27.1 | 0.822 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Serum NLR | 2.07 | 1.10–3.90 | 0.025 | 2.04 | 1.10–3.80 | 0.025 |
| Max. tumor size | 1.17 | 0.97–1.40 | 0.099 | |||
| ALBI score | 6.11 | 1.01–37.23 | 0.050 | |||
| Cirrhosis | 8.18 | 0.77–87.20 | 0.082 | |||
| PG-SGA score | 2.02 | 1.08–3.80 | 0.029 | 2.30 | 1.04–5.09 | 0.039 |
| Category | Total (n = 45) | Patients, N (%) a Grade 1–2 | Grade 3–4 c | Grade 5 b | Weeks to Onset Median (Range) |
|---|---|---|---|---|---|
| Any | 29 (64.4) | 17 (37.7) | 11 (24.4) | 1 (2.2) | |
| Skin | 13 (28.9) | 13 (28.9) | 0 (0.0) | 0 (0.0) | 3.1 (0.6–7.6) |
| Rash | 6 (13.3) | 6 (13.3) | 0 (0.0) | 0 (0.0) | |
| Pruritus | 9 (20.0) | 9 (20.0) | 0 (0.0) | 0 (0.0) | |
| Pneumonitis | 4 (8.9) | 1 (2.2) | 3 (6.7) | 0 (0.0) | 8.3 (2.0–12.0) |
| Endocrine | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 6.0 (NA) |
| Thyroditis/hypothyroidism | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | |
| Hypophysitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Gastrointestinal | 7 (15.6) | 6 (13.3) | 1 (2.2) | 0 (0.0) | 7.6 (0.6–8.9) |
| Mucositis | 2 (4.4) | 2(4.4) | 0 (0.0) | 0 (0.0) | |
| Esophagitis | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | |
| Diarrhea/colitis | 5 (11.1) | 4 (8.9) | 1 (2.2) | 0 (0.0) | |
| Hepatobiliary | 11 (24.4) | 2(4.4) | 9 (20.0) | 0 (0.0) | 4.6 (1.0–14.6) |
| Hepatitis | 9 (20.0) | 2(4.4) | 6 (13.3) | 1 (2.2) | |
| Cholangitis | 4 (8.9) | 0 (0.0) | 4 (8.9) | 0 (0.0) | |
| Others | 12 (26.7) | 15 (33.3) | 0 (0.0) | 0 (0.0) | 4.3 (1.0–10.3) |
| Fatigue | 7 (15.6) | 6 (13.3) | 1 (2.2) | 0 (0.0) | |
| Anorexia | 7 (15.6) | 7 (15.6) | 0 (0.0) | 0 (0.0) | |
| Polyarthritis | 1(2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers 2021, 13, 1607. https://doi.org/10.3390/cancers13071607
Hung H-C, Lee J-C, Wang Y-C, Cheng C-H, Wu T-H, Lee C-F, Wu T-J, Chou H-S, Chan K-M, Lee W-C. Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers. 2021; 13(7):1607. https://doi.org/10.3390/cancers13071607
Chicago/Turabian StyleHung, Hao-Chien, Jin-Chiao Lee, Yu-Chao Wang, Chih-Hsien Cheng, Tsung-Han Wu, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, Kun-Ming Chan, and Wei-Chen Lee. 2021. "Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma" Cancers 13, no. 7: 1607. https://doi.org/10.3390/cancers13071607
APA StyleHung, H.-C., Lee, J.-C., Wang, Y.-C., Cheng, C.-H., Wu, T.-H., Lee, C.-F., Wu, T.-J., Chou, H.-S., Chan, K.-M., & Lee, W.-C. (2021). Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers, 13(7), 1607. https://doi.org/10.3390/cancers13071607






