Bone Pain in Multiple Myeloma (BPMM)—A Protocol for a Prospective, Longitudinal, Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Research Methods and Analyses
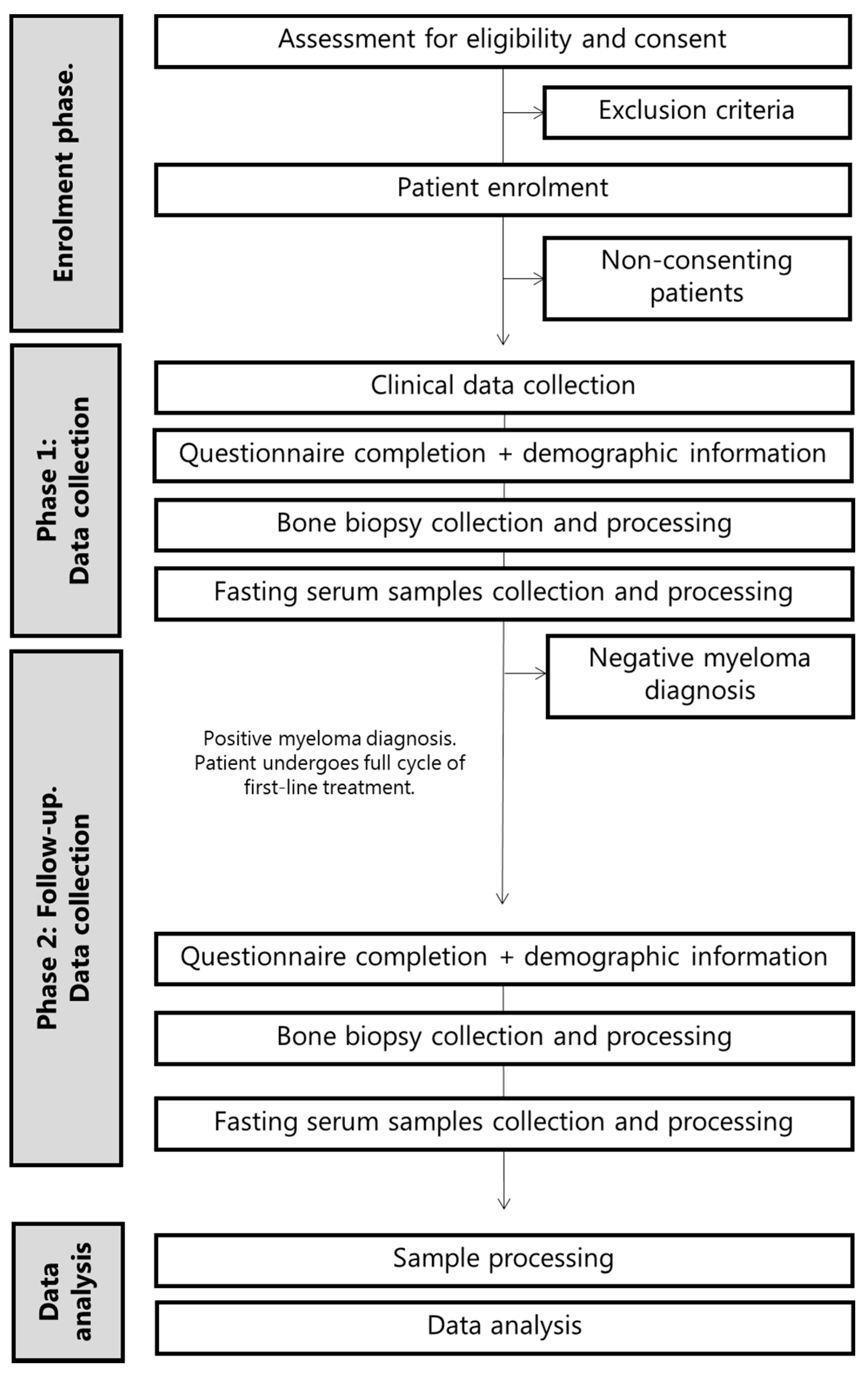
2.1. Study Design and Setting
- To provide a deep characterization of the subjective experience of pain (including pain type, location and severity) and QoL in MM patients at diagnosis, compared to patients with a negative myeloma diagnosis (control group, presumably MGUS patients).
- To evaluate disturbances in bone innervation (presence, location and density of nerve fibers innervating the bone) in MM patients at diagnosis, compared to patients with a negative myeloma diagnosis.
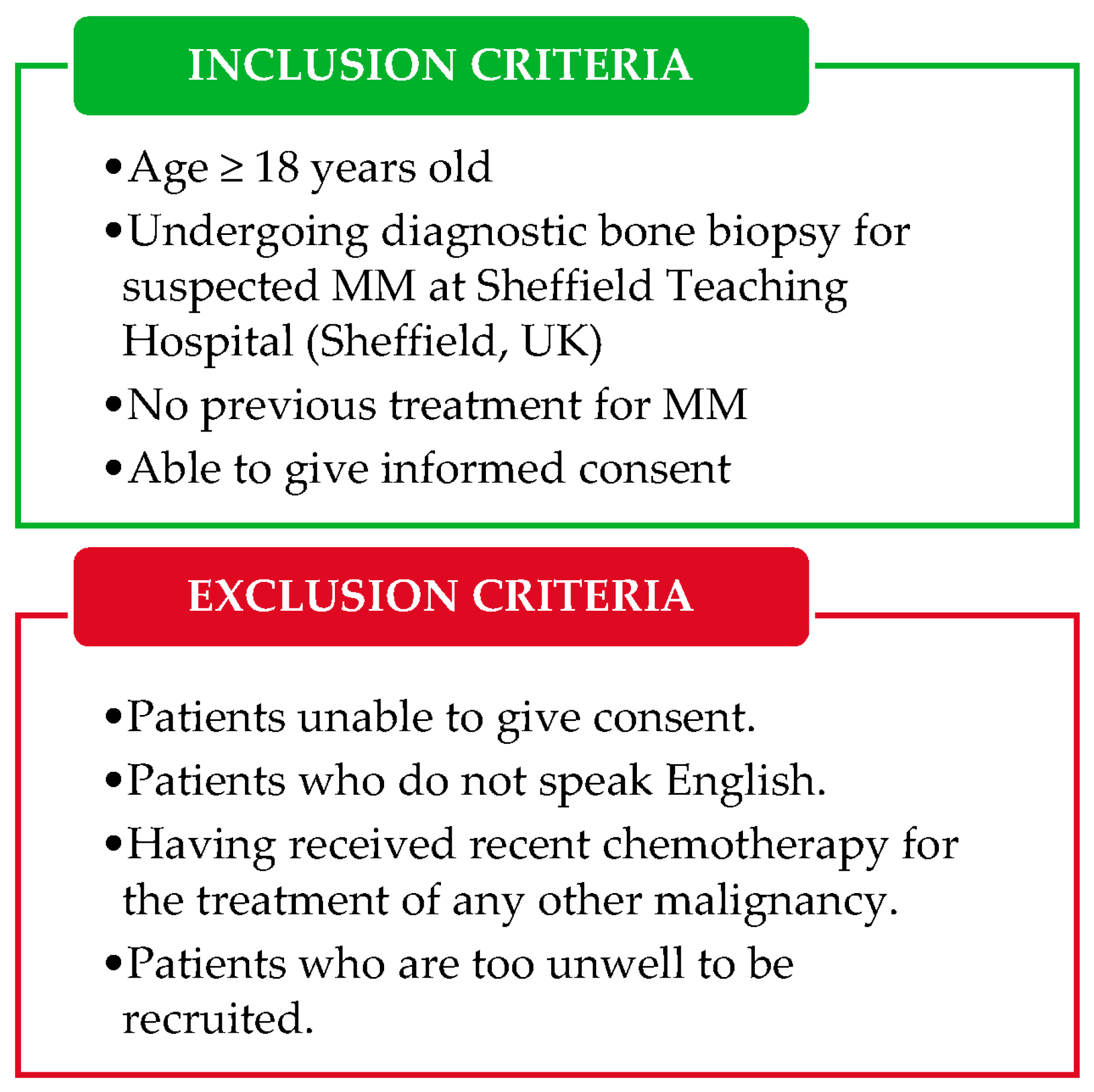
2.2. Study Population
2.3. Study Objectives
- To provide an in-depth characterization of the subjective experience of pain and QoL in MM patients through the completion of standardized questionnaires regarding general pain, bone pain, neuropathic pain, QoL and catastrophizing. Questionnaires from MM patients will be compared with those of patients receiving a negative myeloma diagnosis (control group).
- To evaluate disturbances in the presence, location and density of sensory and sympathetic nerve fibers innervating the bone marrow of myeloma patients. Biopsies from MM patients will be compared with those of patients receiving a negative myeloma diagnosis (control group).
- To evaluate changes in the levels of serum biomarkers of bone turnover (carboxy-terminal collagen crosslinks-1, CTX-1, and pro-collagen type 1 N-terminal pro-peptide, P1NP) in MM patients. Levels of bone turnover biomarkers in the serum of MM patients will be compared with those of patients receiving a negative myeloma diagnosis (control group).
- To evaluate the levels of inflammatory serum cytokines and chemokines in MM patients. Levels of inflammatory biomarkers in the serum of MM patients will be compared with those of patients receiving a negative myeloma diagnosis (control group).
- To compare data on pain and QoL, bone innervation and serum biomarkers of bone turnover and inflammation in MM patients at diagnosis and following first-line treatment.
- To evaluate correlations between the self-reported experience of pain and disturbances in bone innervation in MM patients at diagnosis and following first-line treatment.
- To evaluate correlations between the self-reported experience of pain and serum levels of bone turnover biomarkers in MM patients at diagnosis and following first-line treatment.
- To evaluate correlations between the self-reported experience of pain and serum levels of inflammatory cytokines in MM patients at diagnosis and following first-line treatment.
2.4. Study Procedures
2.4.1. Objectives 1 and 5: In-Depth Characterization of Pain in MM Patients
- BPI (Brief Pain Inventory, short version): two-factor questionnaire assessing the severity and interference of pain [15,16]. The severity component assesses location and severity of the pain, as well the analgesic relief provided by current therapy, if any. The interference component is composed by an affective and an activity subdimension. The BPI includes a Visual Analogue Scale (VAS) for assessment of pain intensity, which will be used to establish correlations between pain and disturbances in bone innervation, serum inflammatory and serum bone turnover biomarkers. Data from this questionnaire will allow to draw comparisons between pain severity and interference between MM patients and controls.
- FACT–BP (Functional Assessment of Cancer Therapy–Bone Pain): Initially developed for the assessment of pain in bone metastases, it measures bone pain and its effect on quality of life. This will provide information on whether myeloma patients suffer from bone pain, compared to controls. Moreover, this questionnaire is sensitive to clinical changes following therapy [17], and will provide important information on whether first-line anti-myeloma treatment improves bone pain and its effect on quality of life in myeloma patients.
- PCS (Pain Catastrophizing Scale): assessment of the extent of catastrophizing, which is subdivided in three domains: rumination, magnification and helplessness [18]. Mounting evidence suggests that catastrophizing (i.e., enhancing negative, anxiety-inducing thoughts) plays an important role in the subjective experience of pain; to our knowledge, this will be the first data on the role of catastrophizing in myeloma-induced bone pain.
- EORTC QLQ-C30 (European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30): assessment of quality of life in cancer patients. This questionnaire contains a measure of global health status, functional scales (including physical, role, emotional, cognitive and social functioning) and symptoms scales (including fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties) [19]. As this questionnaire is widely used to assess the effect of cancer on QoL, it will allow to contextualize the effect of myeloma on QoL in comparison to other cancer types.
- EORTC MY20 (European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-myeloma module 20): Supplementary module to administer alongside the EORTC QLQ-C30 for the assessment of disease symptoms, side effects of treatment, future perspective and impact of disease on body image perception [20]. This questionnaire will allow comparisons to other cohorts of myeloma patients.
- EORTC CIPN (European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-chemotherapy-induced peripheral neuropathy module): assessment CIPN through a sensory, a motor and an autonomic scale [21]. Administration of this questionnaire at diagnosis will provide baseline data to compare the follow-up data to, allowing the identification of CIPN development after treatment.
- painDETECT: identification of a possible neuropathic component of pain [22]. This questionnaire, in combination with the EORTC CIPN, will allow the distinction between myeloma-induced neuropathy and chemotherapy-induced peripheral neuropathy”.
2.4.2. Objectives 2 and 5: Immunohistological Characterization of Nerve Fibers Innervating the Bone Marrow in MM
2.4.3. Objectives 3, 4 and 5: Quantification of Serum Biomarkers of Bone Turnover and Inflammation
2.4.4. Objectives 6, 7 and 8: Correlation Analyses
2.4.5. Sample Size Calculation
3. Discussion
Limitations of the Study
4. Ethics and Dissemination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Walker, R.E.; Lawson, M.A.; Buckle, C.H.; Snowden, J.A.; Chantry, A.D. Myeloma bone disease: Pathogenesis, current treatments and future targets. Br. Med. Bull. 2014, 111, 117–138. [Google Scholar] [CrossRef][Green Version]
- Therneau, T.M.; Kyle, R.A.; Melton, L.J., 3rd; Larson, D.R.; Benson, J.T.; Colby, C.L.; Dispenzieri, A.; Kumar, S.; Katzmann, J.A.; Cerhan, J.R.; et al. Incidence of monoclonal gammopathy of undetermined significance and estimation of duration before first clinical recognition. Mayo Clin. Proc. 2012, 87, 1071–1079. [Google Scholar] [CrossRef]
- Jordan, K.; Proskorovsky, I.; Lewis, P.; Ishak, J.; Payne, K.; Lordan, N.; Kyriakou, C.; Williams, C.D.; Peters, S.; Davies, F.E. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: Results of a European, multicenter cohort study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2014, 22, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.T.; Tholstrup, D.; Petersen, M.A.; Pedersen, L.; Groenvold, M. Health related quality of life in a nationally representative sample of haematological patients. Eur. J. Haematol. 2009, 83, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Niscola, P.; Tendas, A.; Scaramucci, L.; Giovannini, M.; De Sanctis, V. Pain in blood cancers. Indian J. Palliat. Care 2011, 17, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ramsenthaler, C.; Osborne, T.R.; Gao, W.; Siegert, R.J.; Edmonds, P.M.; Schey, S.A.; Higginson, I.J. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: A multi-centre study. BMC Cancer 2016, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- LeMay, K.; Wilson, K.G.; Buenger, U.; Jarvis, V.; Fitzgibbon, E.; Bhimji, K.; Dobkin, P.L. Fear of Pain in Patients With Advanced Cancer or in Patients with Chronic Noncancer Pain. Clin. J. Pain 2011, 27, 116–124. [Google Scholar] [CrossRef]
- Coleman, E.A.; Goodwin, J.A.; Coon, S.K.; Richards, K.; Enderlin, C.; Kennedy, R.; Stewart, M.C.; McNatt, M.P.; Lockhart, M.K.; Anaissie, E.J.; et al. Fatigue, Sleep, Pain, Mood and Performance Status in Patients with Multiple Myeloma. Cancer Nurs. 2011, 34, 219–227. [Google Scholar] [CrossRef]
- Holen, J.C.; Lydersen, S.; Klepstad, P.; Loge, J.H.; Kaasa, S. The Brief Pain Inventory: Pain’s interference with functions is different in cancer pain compared with noncancer chronic pain. Clin. J. Pain 2008, 24, 219–225. [Google Scholar] [CrossRef]
- Mateos, M.V.; Landgren, O. MGUS and Smoldering Multiple Myeloma: Diagnosis and Epidemiology. Cancer Treat Res. 2016, 169, 3–12. [Google Scholar] [CrossRef]
- Wedding, U.; Pientka, L.; Hoffken, K. Quality-of-life in elderly patients with cancer: A short review. Eur. J. Cancer 2007, 43, 2203–2210. [Google Scholar] [CrossRef]
- Diaz-delCastillo, M.; Kamstrup, D.; Olsen, R.; Hansen, R.; Pembridge, T.; Simanskaite, B.; Jimenez-Andrade, J.M.; Lawson, M.A.; Heegaard, A.M. Differential pain-related behaviours and bone disease in immunocompetent mouse models of myeloma. JBMR Plus 2019. [Google Scholar] [CrossRef]
- Hiasa, M.; Okui, T.; Allette, Y.M.; Ripsch, M.S.; Sun-Wada, G.H.; Wakabayashi, H.; Roodman, G.D.; White, F.A.; Yoneda, T. Bone pain induced by multiple myeloma is reduced by targeting V-ATPase and ASIC3. Cancer Res. 2017, 77, 1283–1295. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 1994, 23, 129–138. [Google Scholar] [PubMed]
- Cleeland, C.S. The measurement of pain from metastatic bone disease: Capturing the patient’s experience. Clin. Cancer Res. 2006, 12 (Pt 2), 6236s–6242s. [Google Scholar] [CrossRef]
- Broom, R.; Du, H.; Clemons, M.; Eton, D.; Dranitsaris, G.; Simmons, C.; Ooi, W.; Cella, D. Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: Measuring impact using the Functional Assessment of Cancer Therapy-Bone Pain. J. Pain Symptom. Manag. 2009, 38, 244–257. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Stead, M.L.; Brown, J.M.; Velikova, G.; Kaasa, S.; Wisløff, F.; Child, J.A.; Hippe, E.; Hjorth, M.; Sezer, O.; Selby, P. Development of an EORTC questionnaire module to be used in health-related quality-of-life assessment for patients with multiple myeloma. European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Br. J. Haematol. 1999, 104, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lantéri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Sayilekshmy, M.; Hansen, R.B.; Delaisse, J.M.; Rolighed, L.; Andersen, T.L.; Heegaard, A.M. Innervation is higher above Bone Remodeling Surfaces and in Cortical Pores in Human Bone: Lessons from patients with primary hyperparathyroidism. Sci. Rep. 2019, 9, 5361. [Google Scholar] [CrossRef] [PubMed]
- Poulos, A.R.; Gertz, M.A.; Pankratz, V.S.; Post-White, J. Pain, mood disturbance, and quality of life in patients with multiple myeloma. Oncol. Nurs. Forum. 2001, 28, 1163–1171. [Google Scholar] [PubMed]
- National Cancer Institute NIH. Common Terminology Criteria for Adverse Events (CTCAE) US Department of Health and Human Services. 2018. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 21 September 2020).
- Atkinson, T.M.; Ryan, S.J.; Bennett, A.V.; Stover, A.M.; Saracino, R.M.; Rogak, L.J.; Jewell, S.T.; Matsoukas, K.; Li, Y.; Basch, E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): A systematic review. Support. Care Cancer 2016, 24, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.K.; Klausen, T.W.; Jarden, M.; Frederiksen, H.; Vangsted, A.J.; Do, T.; Kristensen, I.B.; Frølund, U.C.; Andersen, C.L.; Abildgaard, N.; et al. Clarithromycin added to bortezomib-cyclophosphamide-dexamethasone impairs health-related quality of life in multiple myeloma patients. Eur. J. Haematol. 2019, 102, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Breivik, E.K.; Bjornsson, G.A.; Skovlund, E. A comparison of pain rating scales by sampling from clinical trial data. Clin. J. Pain 2000, 16, 22–28. [Google Scholar] [CrossRef]
- Ficko, S.L.; Pejsa, V.; Zadnik, V. Health-related quality of life in Croatian general population and multiple myeloma patients assessed by the EORTC QLQ-C30 and EORTC QLQ-MY20 questionnaires. Radiol. Oncol. 2019, 53, 337–347. [Google Scholar] [CrossRef]
- Proskorovsky, I.; Lewis, P.; Williams, C.D.; Jordan, K.; Kyriakou, C.; Ishak, J.; Davies, F.E. Mapping EORTC QLQ-C30 and QLQ-MY20 to EQ-5D in patients with multiple myeloma. Health Qual. Life Outcomes 2014, 12, 35. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jarden, M.; Andersen, C.L.; Frederiksen, H.; Abildgaard, N. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. Eur. J. Haematol. 2017, 99, 3–17. [Google Scholar] [CrossRef]
- Bell, M.L.; Fairclough, D.L. Practical and statistical issues in missing data for longitudinal patient-reported outcomes. Stat. Methods Med. Res. 2014, 23, 440–459. [Google Scholar] [CrossRef]
- Nielsen, L.K.; King, M.; Möller, S.; Jarden, M.; Andersen, C.L.; Frederiksen, H.; Gregersen, H.; Klostergaard, A.; Steffensen, M.S.; Pedersen, P.T.; et al. Strategies to improve patient-reported outcome completion rates in longitudinal studies. Qual. Life Res. 2019. [Google Scholar] [CrossRef]
- Andersen, T.L.; Søe, K.; Sondergaard, T.E.; Plesner, T.; Delaisse, J.M. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br. J. Haematol. 2010, 148, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst. Rev. 2017, 12, Cd003188. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.; Eiser, C.; Ezaydi, Y.; Greenfield, D.M.; Ahmedzai, S.H.; Snowden, J.A. Living with advanced but stable multiple myeloma: A study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J. Pain Symptom. Manag. 2013, 46, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Li, Y.; Xu, Y.S.; Ma, D.; Fu, P.; Gao, H.Q.; Gao, F.T.; Yang, H.S.; Yang, Z.J. Serum concentrations of IL-2 and TNF-alpha in patients with painful bone metastases: Correlation with responses to 89SrCl2 therapy. J. Nucl. Med. 2006, 47, 242–246. [Google Scholar] [PubMed]
- Fazzari, J.; Sidhu, J.; Motkur, S.; Inman, M.; Buckley, N.; Clemons, M.; Vandermeer, L.; Singh, G. Applying Serum Cytokine Levels to Predict Pain Severity in Cancer Patients. J. Pain Res. 2020, 13, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335. [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef]
| Full Questionnaire Name | Abbreviated Name | Copyright | Objective | Number of Items | |
|---|---|---|---|---|---|
| PAIN | Short Brief Pain Inventory [15,16] | BPI | Dr. Charles S. Cleeland, MD Anderson Centre | Pain severity and interference (affective and activity subdimensions) | 9 |
| Functional Assessment of Cancer Therapy-Bone Pain [17] | FACT-BP | Dr. David Cella, FACIT system | Quality of Life in patients with bone pain | 16 | |
| Pain Catastrophizing Scale [18] | PCS | Dr. Michael JL Sullivan | Measure of the extent to which a patient catastrophizes pain | 13 | |
| QUALITY OF LIFE | European Organization for the Research and Treatment of Cancer: Quality of Life Questionnaire-Core 30 [19] | EORTC QLQ-C30 | European Organization for the Research and Treatment of Cancer | Quality of Life in cancer patients | 30 |
| European Organization for the Research and Treatment of Cancer: Quality of Life Questionnaire-myeloma module 20 [20] | EORTC QLQ-MY20 | European Organization for the Research and Treatment of Cancer | Quality of Life in MM patients | 20 | |
| NEUROPATHY | European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-chemotherapy-induced peripheral neuropathy module 20 [21] | EORTC QLQ-CIPN20 | European Organization for the Research and Treatment of Cancer | Characterization of chemotherapy-induced peripheral neuropathy | 20 |
| painDETECT [22] | painDETECT | Pfizer Pharma GmbH, Germany | Identification of a neuropathic pain component | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-delCastillo, M.; Andrews, R.E.; Mandal, A.; Andersen, T.L.; Chantry, A.D.; Heegaard, A.-M. Bone Pain in Multiple Myeloma (BPMM)—A Protocol for a Prospective, Longitudinal, Observational Study. Cancers 2021, 13, 1596. https://doi.org/10.3390/cancers13071596
Diaz-delCastillo M, Andrews RE, Mandal A, Andersen TL, Chantry AD, Heegaard A-M. Bone Pain in Multiple Myeloma (BPMM)—A Protocol for a Prospective, Longitudinal, Observational Study. Cancers. 2021; 13(7):1596. https://doi.org/10.3390/cancers13071596
Chicago/Turabian StyleDiaz-delCastillo, Marta, Rebecca E. Andrews, Aritri Mandal, Thomas L. Andersen, Andrew D. Chantry, and Anne-Marie Heegaard. 2021. "Bone Pain in Multiple Myeloma (BPMM)—A Protocol for a Prospective, Longitudinal, Observational Study" Cancers 13, no. 7: 1596. https://doi.org/10.3390/cancers13071596
APA StyleDiaz-delCastillo, M., Andrews, R. E., Mandal, A., Andersen, T. L., Chantry, A. D., & Heegaard, A.-M. (2021). Bone Pain in Multiple Myeloma (BPMM)—A Protocol for a Prospective, Longitudinal, Observational Study. Cancers, 13(7), 1596. https://doi.org/10.3390/cancers13071596






