From Biomarkers to Models in the Changing Landscape of Chronic Lymphocytic Leukemia: Evolve or Become Extinct
Abstract
Simple Summary
Abstract
1. Introduction
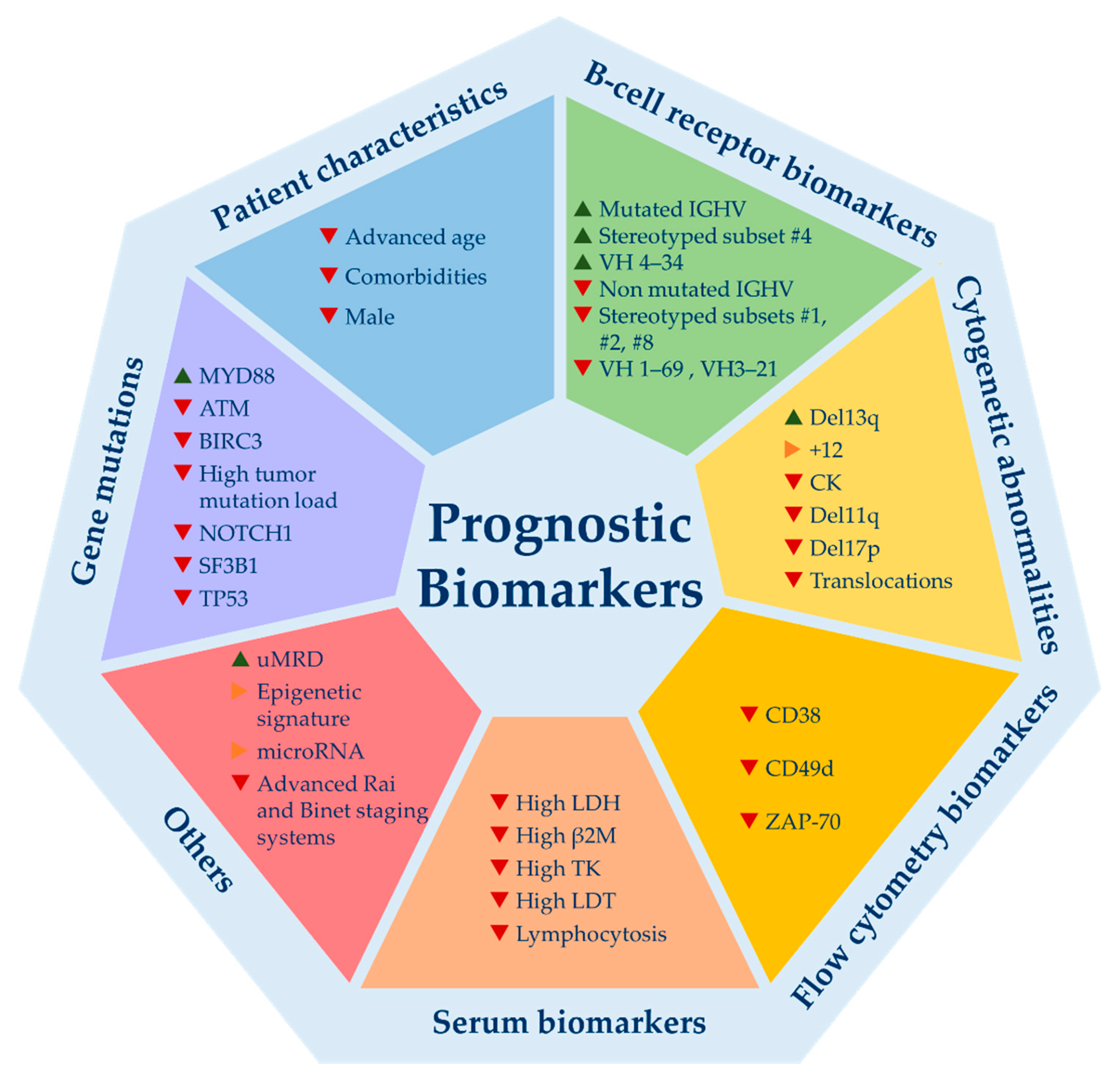
2. Prognostic Biomarkers: All That Glitters Is Not Gold
3. Predictive Biomarkers in the Targeted Therapy Era: Something Old, Something New, Something Borrowed and Something Blue
3.1. Something Old: Invalid Biomarkers for Current Treatment Algorithms That Were Important Previously and Might Reappear
11q Deletion
3.2. Something New: Novel Biomarkers for the Tas Treatments
3.2.1. Resistance Mutations to BTK Inhibitors
3.2.2. BCL-2 Mutations
3.3. Something Borrowed: Biomarkers That Retain Predictive Value on Current Treatment Algorithms
3.3.1. TP53 Abnormalities
3.3.2. IGHV Mutational Status
3.4. Something Blue: Biomarkers with Potential Predictive Value Not Fully Validated
3.4.1. Complex Karyotype
3.4.2. NOTCH1 Mutations
4. Prognostic Models. Different Models for Different Moments: Do Not Compare Apples with Oranges
4.1. Apples: Scoring Systems That Predict Time to First Treatment
4.2. Oranges: Scoring Systems That Predict OS for Patients Treated with New Targeted Agents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Version 2.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf (accessed on 9 February 2021).
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.; Sharman, J.P.; Wierda, W.; Zhao, W.; Heerema, N.A.; et al. Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study. Clin. Cancer Res. 2020, 26, 3918–3927. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Seymour, J.F.; Hillmen, P.; Eichhorst, B.; Langerak, A.W.; Owen, C.; Verdugo, M.; Wu, J.; Punnoose, E.A.; Jiang, Y.; et al. Fixed Duration of Venetoclax-Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Eradicates Minimal Residual Disease and Prolongs Survival: Post-Treatment Follow-Up of the MURANO Phase III Study. J. Clin. Oncol. 2019, 37, 269–277. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.-M.; Robrecht, S.; Samoylova, O.; Liberati, A.M.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Calabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final results of a randomized, phase iii study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef]
- Flinn, I.W.; Hillmen, P.; Montillo, M.; Nagy, Z.; Illés, Á.; Etienne, G.; Delgado, J.; Kuss, B.J.; Tam, C.S.; Gasztonyi, Z.; et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 2018, 132, 2446–2455. [Google Scholar] [CrossRef]
- Tam, C.S.; Trotman, J.; Opat, S.; Burger, J.A.; Cull, G.; Gottlieb, D.; Harrup, R.; Johnston, P.B.; Marlton, P.; Munoz, J.; et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019, 134, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.V.; Herbaux, C.; Walter, H.S.; Hillmen, P.; Rule, S.A.; Kio, E.A.; Karlin, L.; Dyer, M.J.S.; Mitra, S.S.; Yi, P.C.; et al. Phase Ib Study of Tirabrutinib in Combination with Idelalisib or Entospletinib in Previously Treated Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2020, 26, 2810–2818. [Google Scholar] [CrossRef]
- Mato, A.R.; Ghosh, N.; Schuster, S.J.; Lamanna, N.; Pagel, J.M.; Flinn, I.W.; Barrientos, J.; Rai, K.R.; Reeves, J.A.; Cheson, B.D.; et al. Phase 2 Study of the Safety and Efficacy of Umbralisib in Patients with CLL Who are Intolerant to BTK or PI3Kδ Inhibitor Therapy. Blood 2020. [Google Scholar] [CrossRef] [PubMed]
- Iskierka-Jażdżewska, E.; Robak, T. Investigational treatments for chronic lymphocytic leukemia: A focus on phase 1 and 2 clinical trials. Expert Opin. Investig. Drugs 2020, 1–14. [Google Scholar] [CrossRef]
- Rossi, D.; Gerber, B.; Stüssi, G. Predictive and prognostic biomarkers in the era of new targeted therapies for chronic lymphocytic leukemia. Leuk. Lymphoma 2017, 58, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, E.; Gale, R.P. Predicting the outcome of patients with chronic lymphocytic leukemia: Progress and uncertainty. Cancer 2019, 125, 3699–3705. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic Aberrations and Survival in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef]
- Damle, R.N.; Wasil, T.; Fais, F.; Ghiotto, F.; Valetto, A.; Allen, S.L.; Buchbinder, A.; Budman, D.; Dittmar, K.; Kolitz, J.; et al. Ig V Gene Mutation Status and CD38 Expression as Novel Prognostic Indicators in Chronic Lymphocytic Leukemia. Blood 1999, 94, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, T.J.; Davis, Z.; Gardiner, A.; Oscier, D.G.; Stevenson, F.K. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999, 94, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Bosch, F.; Villamor, N.; Bellosillo, B.; Colomer, D.; Rozman, M.; Marcé, S.; López-Guillermo, A.; Campo, E.; Montserrat, E. ZAP-70 Expression as a Surrogate for Immunoglobulin-Variable-Region Mutations in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2003, 348, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Delgado, J.; Santacruz, R.; Martínez-Trillos, A.; Rozman, M.; Aymerich, M.; López, C.; Costa, D.; Carrió, A.; Villamor, N.; et al. CD49d (ITGA4) expression is a predictor of time to first treatment in patients with chronic lymphocytic leukaemia and mutatedIGHVstatus. Br. J. Haematol. 2016, 172, 48–55. [Google Scholar] [CrossRef]
- Ghia, P.; Guida, G.; Stella, S.; Gottardi, D.; Geuna, M.; Strola, G.; Scielzo, C.; Caligaris-Cappio, F. The pattern of CD38 expression defines a distinct subset of chronic lymphocytic leukemia (CLL) patients at risk of disease progression. Blood 2003, 101, 1262–1269. [Google Scholar] [CrossRef]
- Herling, C.D.; Klaumünzer, M.; Rocha, C.K.; Altmüller, J.; Thiele, H.; Bahlo, J.; Kluth, S.; Crispatzu, G.; Herling, M.; Schiller, J.; et al. Complex karyotypes and KRAS and POT1 mutations impact outcome in CLL after chlorambucil-based chemotherapy or chemoimmunotherapy. Blood 2016, 128, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Hillmen, P.; O’Brien, S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.E.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; Barr, P.M.; et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 2018, 32, 83–91. [Google Scholar] [CrossRef]
- Tausch, E.; Schneider, C.; Robrecht, S.; Zhang, C.; Dolnik, A.; Bloehdorn, J.; Bahlo, J.; Al-Sawaf, O.; Ritgen, M.; Fink, A.-M.; et al. Prognostic and predictive impact of genetic markers in patients with CLL treated with obinutuzumab and venetoclax. Blood 2020. [Google Scholar] [CrossRef]
- Kater, A.P.; Wu, J.Q.; Kipps, T.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Robak, T.; de la Serna, J.; et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations from the MURANO Phase III Study. J. Clin. Oncol. 2020, 38, 4042–4054. [Google Scholar] [CrossRef]
- Binet, J.L.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F.; et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981, 48, 198–206. [Google Scholar] [CrossRef]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical staging of chronic lymphocytic leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef] [PubMed]
- International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): A meta-analysis of individual patient data. Lancet Oncol. 2016, 17, 779–790. [Google Scholar] [CrossRef]
- Gentile, M.; Shanafelt, T.D.; Mauro, F.R.; Reda, G.; Rossi, D.; Laurenti, L.; Del Principe, M.I.; Cutrona, G.; Angeletti, I.; Coscia, M.; et al. Predictive value of the CLL-IPI in CLL patients receiving chemo-immunotherapy as first-line treatment. Eur. J. Haematol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Shanafelt, T.D.; Giannarelli, D.; Gentile, M.; Mirabelli, R.; Cutrona, G.; Levato, L.; Di Renzo, N.; Di Raimondo, F.; Musolino, C.; et al. The chronic lymphocytic leukemia international prognostic index predicts time to first treatment in early CLL: Independent validation in a prospective cohort of early stage patients. Am. J. Hematol. 2016, 91, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha-Bang, C.; Christiansen, I.; Niemann, C.U. The CLL-IPI applied in a population-based cohort. Blood 2016, 128, 2181–2183. [Google Scholar] [CrossRef]
- Muñoz-Novas, C.; Poza-Santaella, M.; Marín, I.G.-G.Y., I; Hernández-Sánchez, M.; Rodríguez-Vicente, A.-E.; Infante, M.-S.; Heras, C.; Foncillas, M.-Á.; Marín, K.; Hernández-Rivas, J.-M.; et al. The International Prognostic Index for Patients with Chronic Lymphocytic Leukemia has the Higher Value in Predicting Overall Outcome Compared with the Barcelona-Brno Biomarkers Only Prognostic Model and the MD Anderson Cancer Center Prognostic Index. Biomed. Res. Int. 2018, 2018, 9506979. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Mirabelli, R.; Levato, L.; Shanafelt, T.D. Chronic lymphocytic leukemia international prognostic index (CLL-IPI) in patients receiving chemoimmuno or targeted therapy: A systematic review and meta-analysis. Ann. Hematol. 2018, 97, 2005–2008. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Ni, A.; Darif, M.; Londhe, A.; Xing, G.; Mun, Y.; Kay, N.E.; Shanafelt, T.D.; Rabe, K.G.; Byrd, J.C.; et al. Prognostic risk score for patients with relapsed or refractory chronic lymphocytic leukaemia treated with targeted therapies or chemoimmunotherapy: A retrospective, pooled cohort study with external validations. Lancet Haematol. 2019, 6, e366–e374. [Google Scholar] [CrossRef]
- Ahn, I.E.; Tian, X.; Ipe, D.; Cheng, M.; Albitar, M.; Tsao, L.C.; Zhang, L.; Ma, W.; Herman, S.E.M.; Gaglione, E.M.; et al. Prediction of Outcome in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib: Development and Validation of a Four-Factor Prognostic Model. J. Clin. Oncol. 2020, JCO2000979. [Google Scholar] [CrossRef]
- Van Dyke, D.L.; Werner, L.; Rassenti, L.Z.; Neuberg, D.; Ghia, E.; Heerema, N.A.; Dal Cin, P.; Dell Aquila, M.; Sreekantaiah, C.; Greaves, A.W.; et al. The Dohner fluorescence in situ hybridization prognostic classification of chronic lymphocytic leukaemia (CLL): The CLL Research Consortium experience. Br. J. Haematol. 2016, 173, 105–113. [Google Scholar] [CrossRef]
- Autore, F.; Strati, P.; Innocenti, I.; Corrente, F.; Trentin, L.; Cortelezzi, A.; Visco, C.; Coscia, M.; Cuneo, A.; Gozzetti, A.; et al. Elevated Lactate Dehydrogenase has Prognostic Relevance in Treatment-Naïve Patients Affected by Chronic Lymphocytic Leukemia with Trisomy 12. Cancers 2019, 11, 896. [Google Scholar] [CrossRef]
- Delgado, J.; Pratt, G.; Phillips, N.; Briones, J.; Fegan, C.; Nomdedeu, J.; Pepper, C.; Aventin, A.; Ayats, R.; Brunet, S.; et al. Beta2-microglobulin is a better predictor of treatment-free survival in patients with chronic lymphocytic leukaemia if adjusted according to glomerular filtration rate. Br. J. Haematol. 2009, 145, 801–805. [Google Scholar] [CrossRef]
- Hallek, M.; Langenmayer, I.; Nerl, C.; Knauf, W.; Dietzfelbinger, H.; Adorf, D.; Ostwald, M.; Busch, R.; Kuhn-Hallek, I.; Thiel, E.; et al. Elevated serum thymidine kinase levels identify a subgroup at high risk of disease progression in early, nonsmoldering chronic lymphocytic leukemia. Blood 1999, 93, 1732–1737. [Google Scholar]
- Montserrat, E.; Sanchez-Bisono, J.; Viñolas, N.; Rozman, C. Lymphocyte doubling time in chronic lymphocytic leukaemia: Analysis of its prognostic significance. Br. J. Haematol. 1986, 62, 567–575. [Google Scholar] [CrossRef]
- Tadmor, T.; Braester, A.; Najib, D.; Aviv, A.; Herishanu, Y.; Yuklea, M.; Shvidel, L.; Rahimi-Levene, N.; Ruchlemer, R.; Arad, A.; et al. A new risk model to predict time to first treatment in chronic lymphocytic leukemia based on heavy chain immunoparesis and summated free light chain. Eur. J. Haematol. 2019, 103, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Catovsky, D.; Fooks, J.; Richards, S. Prognostic factors in chronic lymphocytic leukaemia: The importance of age, sex and response to treatment in survival. Br. J. Haematol. 1989, 72, 141–149. [Google Scholar] [CrossRef]
- Strugov, V.; Stadnik, E.; Virts, Y.; Andreeva, T.; Zaritskey, A. Impact of age and comorbidities on the efficacy of FC and FCR regimens in chronic lymphocytic leukemia. Ann. Hematol. 2018, 97, 2153–2161. [Google Scholar] [CrossRef]
- Bulian, P.; Shanafelt, T.D.; Fegan, C.; Zucchetto, A.; Cro, L.; Nückel, H.; Baldini, L.; Kurtova, A.V.; Ferrajoli, A.; Burger, J.A.; et al. CD49d is the Strongest Flow Cytometry-Based Predictor of Overall Survival in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2014, 32, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, L.; Elderiny, W.E.; Elhelw, L.; Ismail, M. CD49d and CD26 are independent prognostic markers for disease progression in patients with chronic lymphocytic leukemia. Blood Cells Mol. Dis. 2015, 55, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Rodríguez, A.E.; González, M.; Benito, R.; Fontanillo, C.; Sandoval, V.; Romero, M.; Martín-Núñez, G.; de Coca, A.G.; Fisac, R.; et al. A high number of losses in 13q14 chromosome band is associated with a worse outcome and biological differences in patients with B-cell chronic lymphoid leukemia. Haematologica 2009, 94, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Cavazzini, F.; Hernandez, J.A.; Gozzetti, A.; Rossi, A.R.; de Angeli, C.; Tiseo, R.; Bardi, A.; Tammiso, E.; Crupi, R.; Lenoci, M.P.; et al. Chromosome 14q32 translocations involving the immunoglobulin heavy chain locus in chronic lymphocytic leukaemia identify a disease subset with poor prognosis. Br. J. Haematol. 2008, 142, 529–537. [Google Scholar] [CrossRef]
- Hernández, J.Á.; Hernández-Sánchez, M.; Rodríguez-Vicente, A.E.; Grossmann, V.; Collado, R.; Heras, C.; Puiggros, A.; Martín, A.Á.; Puig, N.; Benito, R.; et al. A Low Frequency of Losses in 11q Chromosome is Associated with Better Outcome and Lower Rate of Genomic Mutations in Patients with Chronic Lymphocytic Leukemia. PLoS ONE 2015, 10, e0143073. [Google Scholar] [CrossRef]
- Marín, I.G.-G.Y.; Hernández-Sánchez, M.; Rodríguez-Vicente, A.-E.; Sanzo, C.; Aventín, A.; Puiggros, A.; Collado, R.; Heras, C.; Muñoz, C.; Delgado, J.; et al. A high proportion of cells carrying trisomy 12 is associated with a worse outcome in patients with chronic lymphocytic leukemia. Hematol. Oncol. 2016, 34, 84–92. [Google Scholar] [CrossRef]
- Delgado, J.; Espinet, B.; Oliveira, A.C.; Abrisqueta, P.; de la Serna, J.; Collado, R.; Loscertales, J.; Lopez, M.; Hernandez-Rivas, J.A.; Ferra, C.; et al. Chronic lymphocytic leukaemia with 17p deletion: A retrospective analysis of prognostic factors and therapy results. Br. J. Haematol. 2012, 157, 67–74. [Google Scholar] [CrossRef]
- Heerema, N.A.; Muthusamy, N.; Zhao, Q.; Ruppert, A.S.; Breidenbach, H.; Andritsos, L.A.; Grever, M.R.; Maddocks, K.J.; Woyach, J.; Awan, F.; et al. Prognostic significance of translocations in the presence of mutated IGHV and of cytogenetic complexity at diagnosis of chronic lymphocytic leukemia. Haematologica 2020. [Google Scholar] [CrossRef]
- Pérez-Carretero, C.; Hernández-Sánchez, M.; González, T.; Quijada-Álamo, M.; Martín-Izquierdo, M.; Hernández-Sánchez, J.-M.; Vidal, M.-J.; de Coca, A.G.; Aguilar, C.; Vargas-Pabón, M.; et al. Chronic lymphocytic leukemia patients with IGH translocations are characterized by a distinct genetic landscape with prognostic implications. Int. J. Cancer 2020, 147, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Baliakas, P.; Hadzidimitriou, A.; Sutton, L.-A.; Minga, E.; Agathangelidis, A.; Nichelatti, M.; Tsanousa, A.; Scarfò, L.; Davis, Z.; Yan, X.-J.; et al. Clinical effect of stereotyped B-cell receptor immunoglobulins in chronic lymphocytic leukaemia: A retrospective multicentre study. Lancet Haematol. 2014, 1, e74–e84. [Google Scholar] [CrossRef]
- Marín, I.G.-G.Y., I; Hernández, J.A.; Martín, A.; Alcoceba, M.; Sarasquete, M.E.; Rodríguez-Vicente, A.; Heras, C.; de Las Heras, N.; Fisac, R.; García de Coca, A.; et al. Mutation Status and Immunoglobulin Gene Rearrangements in Patients from Northwest and Central Region of Spain with Chronic Lymphocytic Leukemia. Biomed. Res. Int. 2014, 2014, 257517. [Google Scholar] [CrossRef]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef]
- Puente, X.S.; Pinyol, M.; Quesada, V.; Conde, L.; Ordóñez, G.R.; Villamor, N.; Escaramis, G.; Jares, P.; Beà, S.; González-Díaz, M.; et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011, 475, 101–105. [Google Scholar] [CrossRef]
- Jaramillo, S.; Agathangelidis, A.; Schneider, C.; Bahlo, J.; Robrecht, S.; Tausch, E.; Bloehdorn, J.; Hoechstetter, M.; Fischer, K.; Eichhorst, B.; et al. Prognostic impact of prevalent chronic lymphocytic leukemia stereotyped subsets: Analysis within prospective clinical trials of the German CLL Study Group (GCLLSG). Haematologica 2020, 105, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.C.; Villamor, N.; Clot, G.; Martinez-Trillos, A.; Kulis, M.; Navarro, A.; Penas, E.M.M.; Jayne, S.; Majid, A.; Richter, J.; et al. A B-cell epigenetic signature defines three biologic subgroups of chronic lymphocytic. Leukemia 2015, 29, 598–605. [Google Scholar] [CrossRef]
- Baliakas, P.; Puiggros, A.; Xochelli, A.; Sutton, L.-A.; Nguyen-Khac, F.; Gardiner, A.; Plevova, K.; Minga, E.; Hadzidimitriou, A.; Walewska, R.; et al. Additional trisomies amongst patients with chronic lymphocytic leukemia carrying trisomy 12: The accompanying chromosome makes a difference. Haematologica 2016, 101, e299–e302. [Google Scholar] [CrossRef]
- Parikh, S.A.; Strati, P.; Tsang, M.; West, C.P.; Shanafelt, T.D. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood 2016, 127, 1752–1760. [Google Scholar] [CrossRef]
- Thorsélius, M.; Kröber, A.; Murray, F.; Thunberg, U.; Tobin, G.; Bühler, A.; Kienle, D.; Albesiano, E.; Maffei, R.; Dao-Ung, L.-P.; et al. Strikingly homologous immunoglobulin gene rearrangements and poor outcome in VH3-21-using chronic lymphocytic leukemia patients independent of geographic origin and mutational status. Blood 2006, 107, 2889–2894. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Stamatopoulos, K.; Belessi, C.; Moreno, C.; Stella, S.; Guida, G.; Michel, A.; Crespo, M.; Laoutaris, N.; Montserrat, E.; et al. Geographic patterns and pathogenetic implications of IGHV gene usage in chronic lymphocytic leukemia: The lesson of the IGHV3-21 gene. Blood 2005, 105, 1678–1685. [Google Scholar] [CrossRef]
- Tobin, G.; Rosén, A.; Rosenquist, R. What is the current evidence for antigen involvement in the development of chronic lymphocytic leukemia? Hematol. Oncol. 2006, 24, 7–13. [Google Scholar] [CrossRef]
- Xochelli, A.; Baliakas, P.; Kavakiotis, I.; Agathangelidis, A.; Sutton, L.-A.; Minga, E.; Ntoufa, S.; Tausch, E.; Yan, X.-J.; Shanafelt, T.; et al. Chronic Lymphocytic Leukemia with Mutated IGHV4-34 Receptors: Shared and Distinct Immunogenetic Features and Clinical Outcomes. Clin. Cancer Res. 2017, 23, 5292–5301. [Google Scholar] [CrossRef]
- Agathangelidis, A.; Darzentas, N.; Hadzidimitriou, A.; Brochet, X.; Murray, F.; Yan, X.-J.; Davis, Z.; van Gastel-Mol, E.J.; Tresoldi, C.; Chu, C.C.; et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: A molecular classification with implications for targeted therapies. Blood 2012, 119, 4467–4475. [Google Scholar] [CrossRef]
- Nadeu, F.; Royo, R.; Clot, G.; Duran-Ferrer, M.; Navarro, A.; Martin, S.; Lu, J.; Zenz, T.; Baumann, T.S.; Jares, P.; et al. IGLV3-21R110 identifies an aggressive biological subtype of chronic lymphocytic leukemia with intermediate epigenetics. Blood 2020. [Google Scholar] [CrossRef]
- Puente, X.S.; Beà, S.; Valdés-Mas, R.; Villamor, N.; Gutiérrez-Abril, J.; Martín-Subero, J.I.; Munar, M.; Rubio-Pérez, C.; Jares, P.; Aymerich, M.; et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 2015, 526, 519–524. [Google Scholar] [CrossRef]
- Landau, D.A.; Carter, S.L.; Stojanov, P.; McKenna, A.; Stevenson, K.; Lawrence, M.S.; Sougnez, C.; Stewart, C.; Sivachenko, A.; Wang, L.; et al. Evolution and Impact of Subclonal Mutations in Chronic Lymphocytic Leukemia. Cell 2013, 152, 714–726. [Google Scholar] [CrossRef]
- Bosch, F.; Dalla-Favera, R. Chronic lymphocytic leukaemia: From genetics to treatment. Nat. Rev. Clin. Oncol. 2019, 16, 684–701. [Google Scholar] [CrossRef]
- Yun, X.; Zhang, Y.; Wang, X. Recent progress of prognostic biomarkers and risk scoring systems in chronic lymphocytic leukemia. Biomark. Res. 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Rigolin, G.M.; Cavallari, M.; Quaglia, F.M.; Formigaro, L.; Lista, E.; Urso, A.; Guardalben, E.; Liberatore, C.; Faraci, D.; Saccenti, E.; et al. In CLL, comorbidities and the complex karyotype are associated with an inferior outcome independently of CLL-IPI. Blood 2017, 129, 3495–3498. [Google Scholar] [CrossRef]
- Kovacs, G.; Robrecht, S.; Fink, A.M.; Bahlo, J.; Cramer, P.; von Tresckow, J.; Maurer, C.; Langerbeins, P.; Fingerle-Rowson, G.; Ritgen, M.; et al. Minimal Residual Disease Assessment Improves Prediction of Outcome in Patients With Chronic Lymphocytic Leukemia (CLL) Who Achieve Partial Response: Comprehensive Analysis of Two Phase III Studies of the German CLL Study Group. J. Clin. Oncol. 2016, 34, 3758–3765. [Google Scholar] [CrossRef]
- Tam, C.S.; Siddiqi, T.; Allan, J.N.; Kipps, T.J.; Flinn, I.W.; Kuss, B.J.; Opat, S.; Barr, P.M.; Tedeschi, A.; Jacobs, R.; et al. Ibrutinib (Ibr) Plus Venetoclax (Ven) for First-Line Treatment of Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL): Results from the MRD Cohort of the Phase 2 CAPTIVATE Study. Blood 2019, 134, 35. [Google Scholar] [CrossRef]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Fowler, N.; Kadia, T.; et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N. Engl. J. Med. 2019, 380, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Lampson, B.L.; Tyekucheva, S.; Crombie, J.L.; Kim, A.I.; Merryman, R.W.; Lowney, J.; Montegaard, J.; Patterson, V.; Jacobson, C.A.; Jacobsen, E.D.; et al. Preliminary Safety and Efficacy Results from a Phase 2 Study of Acalabrutinib, Venetoclax and Obinutuzumab in Patients with Previously Untreated Chronic Lymphocytic Leukemia (CLL). Blood 2019, 134, 32. [Google Scholar] [CrossRef]
- Munir, T.; Webster, N.; Boucher, R.; Dalal, S.; Brock, K.; Yates, F.J.; Sankhalpara, C.; MacDonald, D.; Fegan, C.; McCaig, A.; et al. Continued Long Term Responses to Ibrutinib + Venetoclax Treatment for Relapsed/Refractory CLL in the Blood Cancer UK TAP Clarity Trial. Blood 2020, 136, 17–18. [Google Scholar] [CrossRef]
- Wierda, W.G.; Tam, C.; Allan, J.N.; Siddiqi, T.; Kipps, T.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, B.; et al. Ibrutinib (Ibr) Plus Venetoclax (Ven) for First-Line Treatment of Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL): 1-Year Disease-Free Survival (DFS) Results from the MRD Cohort of the Phase 2 CAPTIVATE Study. Blood 2020, 136, 16–17. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Schnaiter, A.; Paschka, P.; Zenz, T.; Rossi, M.; Döhner, K.; Bühler, A.; Böttcher, S.; Ritgen, M.; Kneba, M.; et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: Results from the CLL8 trial. Blood 2014, 123, 3247–3254. [Google Scholar] [CrossRef] [PubMed]
- Zenz, T.; Fröhling, S.; Mertens, D.; Döhner, H.; Stilgenbauer, S. Moving from prognostic to predictive factors in chronic lymphocytic leukaemia (CLL). Best Pr. Res. Clin. Haematol. 2010, 23, 71–84. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.-S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Bahlo, J.; Fink, A.M.; Goede, V.; Herling, C.D.; Cramer, P.; Langerbeins, P.; von Tresckow, J.; Engelke, A.; Maurer, C.; et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016, 127, 208–215. [Google Scholar] [CrossRef]
- Rossi, D.; Terzi-di-Bergamo, L.; De Paoli, L.; Cerri, M.; Ghilardi, G.; Chiarenza, A.; Bulian, P.; Visco, C.; Mauro, F.R.; Morabito, F.; et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood 2015, 126, 1921–1924. [Google Scholar] [CrossRef]
- Kipps, T.J.; Fraser, G.; Coutre, S.E.; Brown, J.R.; Barrientos, J.C.; Barr, P.M.; Byrd, J.C.; O’Brien, S.M.; Dilhuydy, M.-S.; Hillmen, P.; et al. Long-Term Studies Assessing Outcomes of Ibrutinib Therapy in Patients With Del(11q) Chronic Lymphocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2019, 19, 715–722.e6. [Google Scholar] [CrossRef]
- Quijada-Álamo, M.; Hernández-Sánchez, M.; Alonso-Pérez, V.; Rodríguez-Vicente, A.E.; García-Tuñón, I.; Martín-Izquierdo, M.; Hernández-Sánchez, J.M.; Herrero, A.B.; Bastida, J.M.; San Segundo, L.; et al. CRISPR/Cas9-generated models uncover therapeutic vulnerabilities of del(11q) CLL cells to dual BCR and PARP inhibition. Leukemia 2020, 34, 1599–1612. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Guinn, D.; Lehman, A.; Blachly, J.S.; Lozanski, A.; Heerema, N.A.; Zhao, W.; Coleman, J.; Jones, D.; et al. BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2017, 35, 1437–1443. [Google Scholar] [CrossRef]
- Quinquenel, A.; Fornecker, L.-M.; Letestu, R.; Ysebaert, L.; Fleury, C.; Lazarian, G.; Dilhuydy, M.-S.; Nollet, D.; Guieze, R.; Feugier, P.; et al. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after 3 years: A FILO group study. Blood 2019, 134, 641–644. [Google Scholar] [CrossRef]
- Scarfo, L.; Bonfiglio, S.; Sutton, L.-A.; Ljungstrom, V.; Pandzic, T.; Cortese, D.; Gaidano, G.; Trentin, L.; Bonello, L.; Reda, G.; et al. BTK and PLCG2 Mutations in Patients with Chronic Lymphocytic Leukemia Relapsing on Ibrutinib: A European Research Initiative on CLL (ERIC) Study Based on Real-World Evidence. In Proceedings of the 25th Annual European Hematology Association (EHA) Congress, 11–21 June 2020. [Google Scholar]
- Woyach, J.A.; Johnson, A.J. Targeted therapies in CLL: Mechanisms of resistance and strategies for management. Blood 2015, 126, 471–477. [Google Scholar] [CrossRef]
- Gángó, A.; Alpár, D.; Galik, B.; Marosvári, D.; Kiss, R.; Fésüs, V.; Aczél, D.; Eyüpoglu, E.; Nagy, N.; Nagy, Á.; et al. Dissection of subclonal evolution by temporal mutation profiling in chronic lymphocytic leukemia patients treated with ibrutinib. Int. J. Cancer 2020, 146, 85–93. [Google Scholar] [CrossRef]
- Woyach, J.; Huang, Y.; Rogers, K.; Bhat, S.A.; Grever, M.R.; Lozanski, A.; Doong, T.-J.; Blachly, J.S.; Lozanski, G.; Jones, D.; et al. Resistance to Acalabrutinib in CLL is Mediated Primarily by BTK Mutations. Blood 2019, 134, 504. [Google Scholar] [CrossRef]
- Puła, B.; Gołos, A.; Górniak, P.; Jamroziak, K. Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia. Cancers 2019, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Chowdhury, S.M.; Hart, A.; Sircar, A.; Singh, S.K.; Nath, U.K.; Mamgain, M.; Singhal, N.K.; Sehgal, L.; Jain, N. Ibrutinib Resistance Mechanisms and Treatment Strategies for B-Cell Lymphomas. Cancers 2020, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Skånland, S.S.; Mato, A.R. Overcoming resistance to targeted therapies in chronic lymphocytic leukemia. Blood Adv. 2021, 5, 334–343. [Google Scholar] [CrossRef]
- Lama, T.G.; Kyung, D.; O’Brien, S. Mechanisms of ibrutinib resistance in chronic lymphocytic leukemia and alternative treatment strategies. Expert Rev. Hematol. 2020, 13, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Sedlarikova, L.; Petrackova, A.; Papajik, T.; Turcsanyi, P.; Kriegova, E. Resistance-Associated Mutations in Chronic Lymphocytic Leukemia Patients Treated with Novel Agents. Front. Oncol. 2020, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Guièze, R.; Liu, V.M.; Rosebrock, D.; Jourdain, A.A.; Hernández-Sánchez, M.; Martinez Zurita, A.; Sun, J.; Ten Hacken, E.; Baranowski, K.; Thompson, P.A.; et al. Mitochondrial Reprogramming Underlies Resistance to BCL-2 Inhibition in Lymphoid Malignancies. Cancer Cell 2019, 36, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Döhner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019, 104, e434–e437. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Anderson, M.A.; Gong, J.-N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Nguyen, T.; Birkinshaw, R.W.; Gong, J.-N.; Chen, X.; McBean, M.; Thijssen, R.; Conway, T.; Anderson, M.A.; et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood 2020, 135, 773–777. [Google Scholar] [CrossRef]
- Buccheri, V.; Barreto, W.G.; Fogliatto, L.M.; Capra, M.; Marchiani, M.; Rocha, V. Prognostic and therapeutic stratification in CLL: Focus on 17p deletion and p53 mutation. Ann. Hematol. 2018, 97, 2269–2278. [Google Scholar] [CrossRef]
- Ahn, I.E.; Tian, X.; Wiestner, A. Ibrutinib for Chronic Lymphocytic Leukemia with TP53 Alterations. N. Engl. J. Med. 2020, 383, 498–500. [Google Scholar] [CrossRef]
- Kater, A.; Kipps, T.J.; Eichhorst, B.F.; Hillmen, P.; D’Rozario, J.; Owen, C.; Assouline, S.; Lamanna, N.; Robak, T.; De la Serna, J.; et al. Five-Year Analysis of Murano Study Demonstrates Enduring Undetectable Minimal Residual Disease (UMRD) in a Subset of Relapsed/Refractory Chronic Lymphocytic Leukemia (R/R CLL) Patients (Pts) Following Fixed-Duration Venetoclax-Rituximab (VenR) Therapy (Tx). In Proceedings of the 62nd ASH Annual Meeting and Exposition, Atlanta, GA, USA, 11–14 December 2020. [Google Scholar]
- Condoluci, A.; Terzi di Bergamo, L.; Langerbeins, P.; Hoechstetter, M.A.; Herling, C.D.; De Paoli, L.; Delgado, J.; Rabe, K.G.; Gentile, M.; Doubek, M.; et al. International prognostic score for asymptomatic early-stage chronic lymphocytic leukemia. Blood 2020, 135, 1859–1869. [Google Scholar] [CrossRef]
- Thompson, P.A.; Tam, C.S.; O’Brien, S.M.; Wierda, W.G.; Stingo, F.; Plunkett, W.; Smith, S.C.; Kantarjian, H.M.; Freireich, E.J.; Keating, M.J. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016, 127, 303–309. [Google Scholar] [CrossRef]
- Barr, P.M.; Robak, T.; Owen, C.; Tedeschi, A.; Bairey, O.; Bartlett, N.L.; Burger, J.A.; Hillmen, P.; Coutre, S.; Devereux, S.; et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: Extended phase 3 results from RESONATE-2. Haematologica 2018, 103, 1502–1510. [Google Scholar] [CrossRef]
- Jarošová, M.; Plevová, K.; Kotašková, J.; Doubek, M.; Pospíšilová, Š. The importance of complex karyotype in prognostication and treatment of chronic lymphocytic leukemia (CLL): A comprehensive review of the literature. Leuk. Lymphoma 2019, 60, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Leeksma, A.C.; Baliakas, P.; Moysiadis, T.; Puiggros, A.; Plevova, K.; Van der Kevie-Kersemaekers, A.-M.; Posthuma, H.; Rodriguez-Vicente, A.E.; Tran, A.N.; Barbany, G.; et al. Genomic arrays identify high-risk chronic lymphocytic leukemia with genomic complexity: A multi-center study. Haematologica 2021, 106, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Baliakas, P.; Jeromin, S.; Iskas, M.; Puiggros, A.; Plevova, K.; Nguyen-Khac, F.; Davis, Z.; Rigolin, G.M.; Visentin, A.; Xochelli, A.; et al. Cytogenetic complexity in chronic lymphocytic leukemia: Definitions, associations, and clinical impact. Blood 2019, 133, 1205–1216. [Google Scholar] [CrossRef]
- Visentin, A.; Bonaldi, L.; Rigolin, G.M.; Mauro, F.R.; Martines, A.; Frezzato, F.; Imbergamo, S.; Scomazzon, E.; Pravato, S.; Bardi, M.A.; et al. The combination of complex karyotype subtypes and IGHV mutational status identifies new prognostic and predictive groups in chronic lymphocytic leukaemia. Br. J. Cancer 2019, 121, 150–156. [Google Scholar] [CrossRef]
- Puiggros, A.; Collado, R.; Calasanz, M.J.; Ortega, M.; Ruiz-Xivillé, N.; Rivas-Delgado, A.; Luño, E.; González, T.; Navarro, B.; García-Malo, M.; et al. Patients with chronic lymphocytic leukemia and complex karyotype show an adverse outcome even in absence of TP53/ATM FISH deletions. Oncotarget 2017, 8, 54297–54303. [Google Scholar] [CrossRef]
- Baliakas, P.; Iskas, M.; Gardiner, A.; Davis, Z.; Plevova, K.; Nguyen-Khac, F.; Malcikova, J.; Anagnostopoulos, A.; Glide, S.; Mould, S.; et al. Chromosomal translocations and karyotype complexity in chronic lymphocytic leukemia: A systematic reappraisal of classic cytogenetic data. Am. J. Hematol. 2014, 89, 249–255. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Furman, R.R.; Coutre, S.; Flinn, I.W.; Burger, J.A.; Blum, K.; Sharman, J.; Wierda, W.; Jones, J.; Zhao, W.; et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: A 5-year experience. Blood 2018, 131, 1910–1919. [Google Scholar] [CrossRef]
- Thompson, P.A.; O’Brien, S.M.; Wierda, W.G.; Ferrajoli, A.; Stingo, F.; Smith, S.C.; Burger, J.A.; Estrov, Z.; Jain, N.; Kantarjian, H.M.; et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer 2015, 121, 3612–3621. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Harrington, B.; O’Brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Lilienweiss, E.; Bahlo, J.; Robrecht, S.; Fink, A.-M.; Patz, M.; Tandon, M.; Jiang, Y.; Schary, W.; Ritgen, M.; et al. High efficacy of venetoclax plus obinutuzumab in patients with complex karyotype and chronic lymphocytic leukemia. Blood 2020, 135, 866–870. [Google Scholar] [CrossRef]
- Mato, A.R.; Thompson, M.; Allan, J.N.; Brander, D.M.; Pagel, J.M.; Ujjani, C.S.; Hill, B.T.; Lamanna, N.; Lansigan, F.; Jacobs, R.; et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica 2018, 103, 1511–1517. [Google Scholar] [CrossRef]
- Anderson, M.A.; Tam, C.; Lew, T.E.; Juneja, S.; Juneja, M.; Westerman, D.; Wall, M.; Lade, S.; Gorelik, A.; Huang, D.C.S.; et al. Clinicopathological features and outcomes of progression of CLL on the BCL2 inhibitor venetoclax. Blood 2017, 129, 3362–3370. [Google Scholar] [CrossRef]
- Woyach, J.A.; Blachly, J.S.; Rogers, K.A.; Bhat, S.A.; Jianfar, M.; Lozanski, G.; Weiss, D.M.; Andersen, B.L.; Gulrajani, M.; Frigault, M.M.; et al. Acalabrutinib plus Obinutuzumab in Treatment-Naïve and Relapsed/Refractory Chronic Lymphocytic Leukemia. Cancer Discov. 2020, 10, 394–405. [Google Scholar] [CrossRef]
- Goldstein, D.; Beckwith, K.A.; Miller, C.; Huang, Y.; Abruzzo, L.V.; Bhat, S.A.; Bond, D.A.; Byrd, J.C.; Grever, M.R.; Heerema, N.A.; et al. Increasing Karyotypic Complexity Predicts Outcomes in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Blood 2020, 136, 2–3. [Google Scholar] [CrossRef]
- Kreuzer, K.-A.; Furman, R.R.; Stilgenbauer, S.; Dubowy, R.L.; Kim, Y.; Munugalavadla, V.; Lilienweiss, E.; Reinhardt, H.C.; Cramer, P.; Eichhorst, B.; et al. The impact of complex karyotype on the overall survival of patients with relapsed chronic lymphocytic leukemia treated with idelalisib plus rituximab. Leukemia 2020, 34, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Villamor, N.; Conde, L.; Martínez-Trillos, A.; Cazorla, M.; Navarro, A.; Beà, S.; López, C.; Colomer, D.; Pinyol, M.; Aymerich, M.; et al. NOTCH1 mutations identify a genetic subgroup of chronic lymphocytic leukemia patients with high risk of transformation and poor outcome. Leukemia 2013, 27, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Balatti, V.; Bottoni, A.; Palamarchuk, A.; Alder, H.; Rassenti, L.Z.; Kipps, T.J.; Pekarsky, Y.; Croce, C.M. NOTCH1 mutations in CLL associated with trisomy 12. Blood 2012, 119, 329–331. [Google Scholar] [CrossRef]
- Bo, M.D.; Del Principe, M.I.; Pozzo, F.; Ragusa, D.; Bulian, P.; Rossi, D.; Capelli, G.; Rossi, F.M.; Niscola, P.; Buccisano, F.; et al. NOTCH1 mutations identify a chronic lymphocytic leukemia patient subset with worse prognosis in the setting of a rituximab-based induction and consolidation treatment. Ann. Hematol. 2014, 93, 1765–1774. [Google Scholar] [CrossRef]
- Jeromin, S.; Weissmann, S.; Haferlach, C.; Dicker, F.; Bayer, K.; Grossmann, V.; Alpermann, T.; Roller, A.; Kohlmann, A.; Haferlach, T.; et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia 2014, 28, 108–117. [Google Scholar] [CrossRef]
- Rossi, D.; Rasi, S.; Fabbri, G.; Spina, V.; Fangazio, M.; Forconi, F.; Marasca, R.; Laurenti, L.; Bruscaggin, A.; Cerri, M.; et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood 2012, 119, 521–529. [Google Scholar] [CrossRef]
- Del Giudice, I.; Rossi, D.; Chiaretti, S.; Marinelli, M.; Tavolaro, S.; Gabrielli, S.; Laurenti, L.; Marasca, R.; Rasi, S.; Fangazio, M.; et al. NOTCH1 mutations in +12 chronic lymphocytic leukemia (CLL) confer an unfavorable prognosis, induce a distinctive transcriptional profiling and refine the intermediate prognosis of +12 CLL. Haematologica 2012, 97, 437–441. [Google Scholar] [CrossRef]
- Weissmann, S.; Roller, A.; Jeromin, S.; Hernández, M.; Abáigar, M.; Hernández-Rivas, J.M.; Grossmann, V.; Haferlach, C.; Kern, W.; Haferlach, T.; et al. Prognostic impact and landscape of NOTCH1 mutations in chronic lymphocytic leukemia (CLL): A study on 852 patients. Leukemia 2013, 27, 2393–2396. [Google Scholar] [CrossRef][Green Version]
- Nadeu, F.; Delgado, J.; Royo, C.; Baumann, T.; Stankovic, T.; Pinyol, M.; Jares, P.; Navarro, A.; Martín-García, D.; Beà, S.; et al. Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood 2016, 127, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Estenfelder, S.; Tausch, E.; Robrecht, S.; Bahlo, J.; Goede, V.; Ritgen, M.; van Dongen, J.J.; Langerak, A.W.; Fingerle-Rowson, G.; Kneba, M.; et al. Gene Mutations and Treatment Outcome in the Context of Chlorambucil (Clb) without or with the Addition of Rituximab (R) or Obinutuzumab (GA-101, G)—Results of an Extensive Analysis of the Phase III Study CLL11 of the German CLL Study Group. Blood 2016, 128, 3227. [Google Scholar] [CrossRef]
- Lee, J.; Wang, Y.L. Prognostic and Predictive Molecular Biomarkers in Chronic Lymphocytic Leukemia. J. Mol. Diagn. 2020, 22, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Molica, S. Chronic lymphocytic leukemia prognostic models in real life: Still a long way off. Expert Rev. Hematol. 2021, 1–5. [Google Scholar] [CrossRef]
- Kreuzberger, N.; Damen, J.A.; Trivella, M.; Estcourt, L.J.; Aldin, A.; Umlauff, L.; Vazquez-Montes, M.D.; Wolff, R.; Moons, K.G.; Monsef, I.; et al. Prognostic models for newly-diagnosed chronic lymphocytic leukaemia in adults: A systematic review and meta-analysis. Cochrane Database Syst. Rev. 2020, 7, CD012022. [Google Scholar] [CrossRef]
- Baliakas, P.; Mattsson, M.; Stamatopoulos, K.; Rosenquist, R. Prognostic indices in chronic lymphocytic leukaemia: Where do we stand how do we proceed? J. Intern. Med. 2016, 279, 347–357. [Google Scholar] [CrossRef]
- Delgado, J.; Doubek, M.; Baumann, T.; Kotaskova, J.; Molica, S.; Mozas, P.; Rivas-Delgado, A.; Morabito, F.; Pospisilova, S.; Montserrat, E. Chronic lymphocytic leukemia: A prognostic model comprising only two biomarkers (IGHV mutational status and FISH cytogenetics) separates patients with different outcome and simplifies the CLL-IPI. Am. J. Hematol. 2017, 92, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Wierda, W.G.; O’Brien, S.; Wang, X.; Faderl, S.; Ferrajoli, A.; Do, K.-A.; Garcia-Manero, G.; Cortes, J.; Thomas, D.; Koller, C.A.; et al. Multivariable Model for Time to First Treatment in Patients with Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2011, 29, 4088–4095. [Google Scholar] [CrossRef]
- Gentile, M.; Shanafelt, T.D.; Cutrona, G.; Molica, S.; Tripepi, G.; Alvarez, I.; Mauro, F.R.; Di Renzo, N.; Di Raimondo, F.; Vincelli, I.; et al. A progression-risk score to predict treatment-free survival for early stage chronic lymphocytic leukemia patients. Leukemia 2016, 30, 1440–1443. [Google Scholar] [CrossRef]
- Baliakas, P.; Moysiadis, T.; Hadzidimitriou, A.; Xochelli, A.; Jeromin, S.; Agathangelidis, A.; Mattsson, M.; Sutton, L.-A.; Minga, E.; Scarfò, L.; et al. Tailored approaches grounded on immunogenetic features for refined prognostication in chronic lymphocytic leukemia. Haematologica 2019, 104, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Hoechstetter, M.A.; Busch, R.; Eichhorst, B.; Bühler, A.; Winkler, D.; Bahlo, J.; Robrecht, S.; Eckart, M.J.; Vehling-Kaiser, U.; Jacobs, G.; et al. Prognostic model for newly diagnosed CLL patients in Binet stage A: Results of the multicenter, prospective CLL1 trial of the German CLL study group. Leukemia 2020, 34, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Levato, L.; Mirabelli, R.; Gentile, M.; Morabito, F. Assessing time to first treatment in early chronic lymphocytic leukemia (CLL): A comparative performance analysis of five prognostic models with inclusion of CLL-international prognostic index (CLL-IPI). Leuk. Lymphoma 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- González-Gascón-Y-Marín, I.; Muñoz-Novas, C.; Figueroa, I.; Hernández-Sánchez, M.; Rodríguez-Vicente, A.-E.; Quijada-Álamo, M.; Pérez-Carretero, C.; Moreno, C.; Collado, R.; Espinet, B.; et al. Prognosis Assessment of Early-Stage Chronic Lymphocytic Leukemia: Are We Ready to Predict Clinical Evolution without a Crystal Ball? Clin. Lymphoma Myeloma Leuk. 2020, 20, 548–555.e4. [Google Scholar] [CrossRef] [PubMed]
- Brander, D.M.; Rhodes, J.; Pagel, J.M.; Nabhan, C.; Tam, C.S.; Jacobs, R.; Hill, B.T.; Lamanna, N.; Lansigan, F.; Shadman, M.; et al. Applicability of the Chronic Lymphocytic Leukemia (CLL)-IPI on Patients Treated with Front-Line Ibrutinib in the Real World: The Case for New Prognostic Models. Blood 2017, 130, 1719. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Ni, A.; Xing, G.; Huang, J.; Furman, R.R.; Jones, J.; Sharman, J.P.; Hallek, M.; Adewoye, A.H.; Dubowy, R.; et al. Evaluation of the CLL-IPI in relapsed and refractory chronic lymphocytic leukemia in idelalisib phase-3 trials. Leuk. Lymphoma 2019, 60, 1438–1446. [Google Scholar] [CrossRef]
- Molica, S.; Baumann, T.S.; Lentini, M.; Levato, L.; Delgado, J.; Montserrat, E. The BALL prognostic score identifies relapsed/refractory CLL patients who benefit the most from single-agent ibrutinib therapy. Leuk. Res. 2020, 95, 106401. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Sitlinger, A.; Salous, T.; Alqahtani, H.; Churnetski, M.; Rivera, X.; Wisniewski, P.; Cohen, J.; Patel, K.; Shadman, M.; et al. A simplified prognostic index for chronic lymphocytic leukemia treated with ibrutinib: Results from a multicenter retrospective cohort study. Leuk. Res. 2020, 89, 106302. [Google Scholar] [CrossRef]
- Gentile, M.; Morabito, F.; Del Poeta, G.; Mauro, F.R.; Reda, G.; Sportoletti, P.; Laurenti, L.; Coscia, M.; Herishanu, Y.; Recchia, A.G.; et al. Survival risk score for real-life relapsed/refractory chronic lymphocytic leukemia patients receiving ibrutinib. A campus CLL study. Leukemia 2021, 35, 235–238. [Google Scholar] [CrossRef]
- Molica, S.; Baumann, T.; Giannarelli, D. Prognostic models in chronic lymphocytic leukemia patients receiving ibrutinib therapy: Results of a comparative performance analysis. Eur. J. Haematol. 2021, 106, 425–427. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Esfahani, M.S.; Scherer, F.; Soo, J.; Jin, M.C.; Liu, C.L.; Newman, A.M.; Dührsen, U.; Hüttmann, A.; Casasnovas, O.; et al. Dynamic Risk Profiling Using Serial Tumor Biomarkers for Personalized Outcome Prediction. Cell 2019, 178, 699–713.e19. [Google Scholar] [CrossRef]
- Agius, R.; Brieghel, C.; Andersen, M.A.; Pearson, A.T.; Ledergerber, B.; Cozzi-Lepri, A.; Louzoun, Y.; Andersen, C.L.; Bergstedt, J.; Von Stemann, J.H.; et al. Machine learning can identify newly diagnosed patients with CLL at high risk of infection. Nat. Commun. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
| Drug | Impact of CK | Study Type | Study | N (% CK) | Population | Prognostic Impact of CK | Others | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ibrutinib | CK does not impact outcome | Phase 3 CT | RESONATE | 39/153 (25%) | R/R | No impact on PFS (40 vs. 44 months, NS) | [6] | |
| Phase 3 CT | ALLIANCE A041202 | 99/333 (29%) | TN | No impact on PFS | [7] | |||
| Pooled analysis | PCYC-1102 PCYC1103 | 41/132 (31%) | TN & R/R | No impact on PFS and OS in MA | [118] | |||
| Pooled analysis | RESONATE RESONATE 2 HELIOS | 41/338 (12.1%) | TN & R/R | No impact on PFS or OS | Excluded del(17p) | [89] | ||
| CK aggravates outcome | Retrospective | Cohort | 21/56 (37.5%) | R/R | Independently associated with shorter PFS and OS | 17/21 del(17p) | [119] | |
| Pooled analysis | PCYC-1102 PCYC-1109 OSU11 RESONATE | 172/295 (58%) | R/R (8 TN) | Associated with disease progression or transformation | [91] | |||
| Acalabrutinib | CK does not impact outcome | Phase 3 CT | ASCEND | 50/154 (32%) | R/R | PFS benefit for acala arm on subgroup analysis | 16 m median follow up | [11] |
| Phase 3 CT | ELEVATE TN | 60/358 (16.7%) | TN | PFS benefit for acala arms on subgroup analysis | 28 m median follow up | [12] | ||
| CK aggravates outcome | Phase 1/2 CT | 20/57 (35%) | R/R | Shorter PFS (33m vs. NR) | [120] | |||
| Venetoclax | CK does not impact outcome | Phase 3 CT | CLL-14 | 30/200 (17%) | TN | No impact on PFS or OS (not reached in CK and non-CK) | [121] | |
| Retrospective | Cohort | 52/130 (26.8%) | R/R (2 TN) | No impact on PFS | 7 m median follow up | [122] | ||
| CK aggravates outcome | Pooled analysis | M12-175 M13-365 M13-982 | 16/38 (46%) | R/R | CK independently associated with PFS | 23 m median follow up | [123] | |
| Phase 3 CT | MURANO | 94/288 (36.3%) | R/R | Shorter PFS and uMRD for CK | 4 year follow up | [31] |
| Model | Population | Stage | Biomarkers | Risk Groups | Validation |
|---|---|---|---|---|---|
| MDAC 2011 [141] | Retrospective single-center cohort | All | IGHV ms Diameter of largest palpable LN FISH (11q/17p Vs none) N involved LN sites LDH IGHV-LDH interaction | Nomogram | 1 external |
| O-CLL1 2016 [142] | Prospective multicenter cohort | Binet A | IGHV ms Rai stage ALC B2M | 3 | 3 external |
| CLL-IPI 2016 [145] | 8 Ph3 multicenter clinical trials | All | IGHV ms TP53 status B2M Clinical stage Age | 3 | 9 external |
| Barcelona-Brno 2017 [140] | Retrospective single-center cohort | All (83% Binet A) | IGHV ms del(17p)/del(11q) | 3 | 7 external |
| Tailored approach 2019 [143] | Retrospective multicenter cohort | Binet A | M-CLL: TP53 abn; +12; subset #2 | 2 | 2 external |
| U-CLL: TP53 abn; del(11q); gender | 3 | ||||
| IPS-E 2020 [109] | Multicenter retrospective cohort | Binet A | IGHV ms ALC > 15 × 109/L Palpable LN | 3 | 9 external |
| CLL-1 PM 2020 [144] | Ph 3 clinical trial | Binet A | IGHV ms del(11q) del(17p) B2M LDT < 12 m Age | 4 | No |
| Characteristics | BALL [40] | NIH (Ahn et al.) [41] | Simplified PI [150] | SRSI [151] |
|---|---|---|---|---|
| N | 2475 | 720 | 346 | 541 |
| Study | Retrospective multicenter pool cohort from randomized trials | Retrospective pooled cohort from randomized trials | Retrospective multicenter cohort from academic medical centers | Retrospective multicenter working group, real life patients |
| Treatment | Ibrutinib Idelalisib Venetoclax CIT | Ibrutinib | Ibrutinib | Ibrutinib |
| Population | R/R | TN and R/R | TN and R/R | R/R |
| Validation cohorts | 1 internal 4 external | 1 internal 1 external | No | 1 internal 1 external |
| Scores | B2M ≥ 5 → 1p LDH> ULN → 1p Hb < 11F/12M → 1p TILT < 24 m → 1p | B2M ≥ 5 → 1p LDH > 250U/L → 1p TP53ab → 1p Prior treatment → 1p | Age ≥ 70 → 1p R/R → 1p ECOG ≥ 1 → 1p | B2M ≥ 5 → 1p LDH > ULT → 1p Hb < 11F/12M → 2p |
| Groups | Low (0–1) | Low (0–1) | Low (0–1) | Low (0) |
| Intermediate (2–3) | Intermediate (2) | Intermediate (2) | Intermediate (1–3) | |
| High (4) | High (3–4) | High (3) | High (4–5) | |
| Prediction | OS | PFS and OS | PFS and OS | OS |
| Accuracy | CS = 0.79 (OS) | CS = 0.69 (PFS) | AUC = 0.6 (PFS) AUC = 0.66 (OS) | CS = 0.71 (OS) |
| Other | BTK and PLG2 mutations detected more frequently in the high risk group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Gascón-y-Marín, I.; Muñoz-Novas, C.; Rodríguez-Vicente, A.-E.; Quijada-Álamo, M.; Hernández-Sánchez, M.; Pérez-Carretero, C.; Ramos-Ascanio, V.; Hernández-Rivas, J.-Á. From Biomarkers to Models in the Changing Landscape of Chronic Lymphocytic Leukemia: Evolve or Become Extinct. Cancers 2021, 13, 1782. https://doi.org/10.3390/cancers13081782
González-Gascón-y-Marín I, Muñoz-Novas C, Rodríguez-Vicente A-E, Quijada-Álamo M, Hernández-Sánchez M, Pérez-Carretero C, Ramos-Ascanio V, Hernández-Rivas J-Á. From Biomarkers to Models in the Changing Landscape of Chronic Lymphocytic Leukemia: Evolve or Become Extinct. Cancers. 2021; 13(8):1782. https://doi.org/10.3390/cancers13081782
Chicago/Turabian StyleGonzález-Gascón-y-Marín, Isabel, Carolina Muñoz-Novas, Ana-Eugenia Rodríguez-Vicente, Miguel Quijada-Álamo, María Hernández-Sánchez, Claudia Pérez-Carretero, Victoria Ramos-Ascanio, and José-Ángel Hernández-Rivas. 2021. "From Biomarkers to Models in the Changing Landscape of Chronic Lymphocytic Leukemia: Evolve or Become Extinct" Cancers 13, no. 8: 1782. https://doi.org/10.3390/cancers13081782
APA StyleGonzález-Gascón-y-Marín, I., Muñoz-Novas, C., Rodríguez-Vicente, A.-E., Quijada-Álamo, M., Hernández-Sánchez, M., Pérez-Carretero, C., Ramos-Ascanio, V., & Hernández-Rivas, J.-Á. (2021). From Biomarkers to Models in the Changing Landscape of Chronic Lymphocytic Leukemia: Evolve or Become Extinct. Cancers, 13(8), 1782. https://doi.org/10.3390/cancers13081782







