Glioblastoma and MiRNAs
Abstract
Simple Summary
Abstract
1. Introduction
2. Glioblastoma
3. MicroRNAs
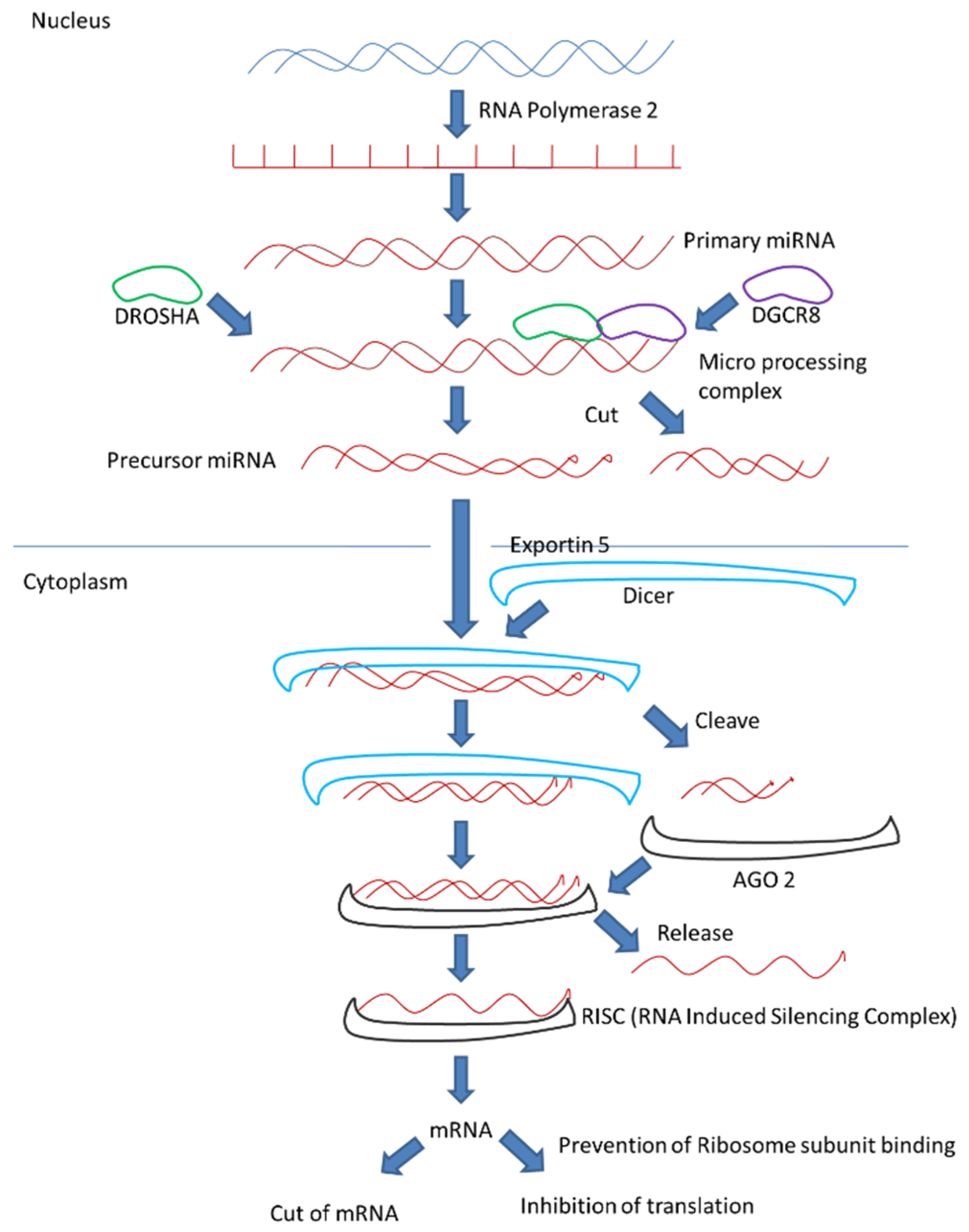
4. MicroRNA Biogenesis
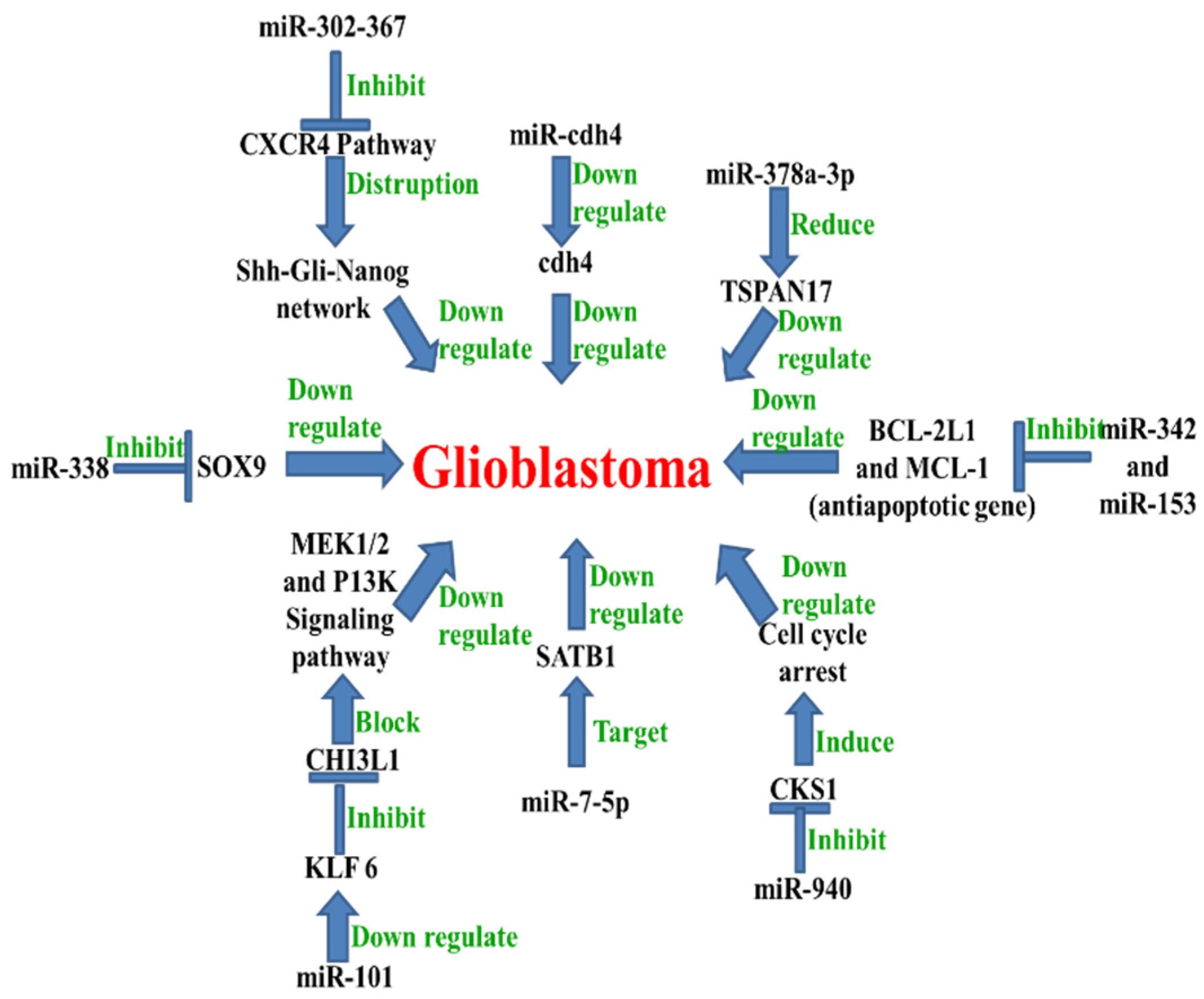
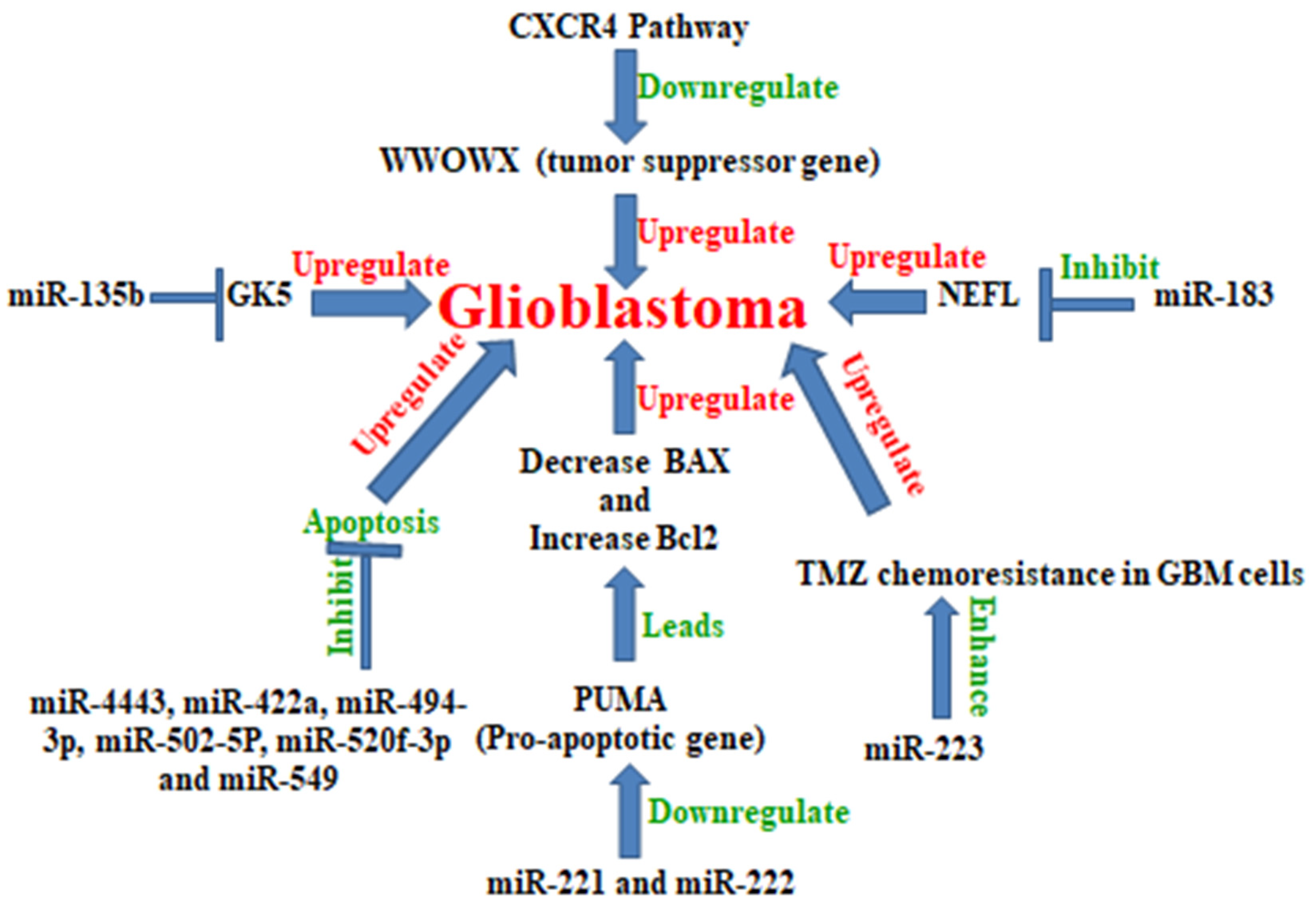
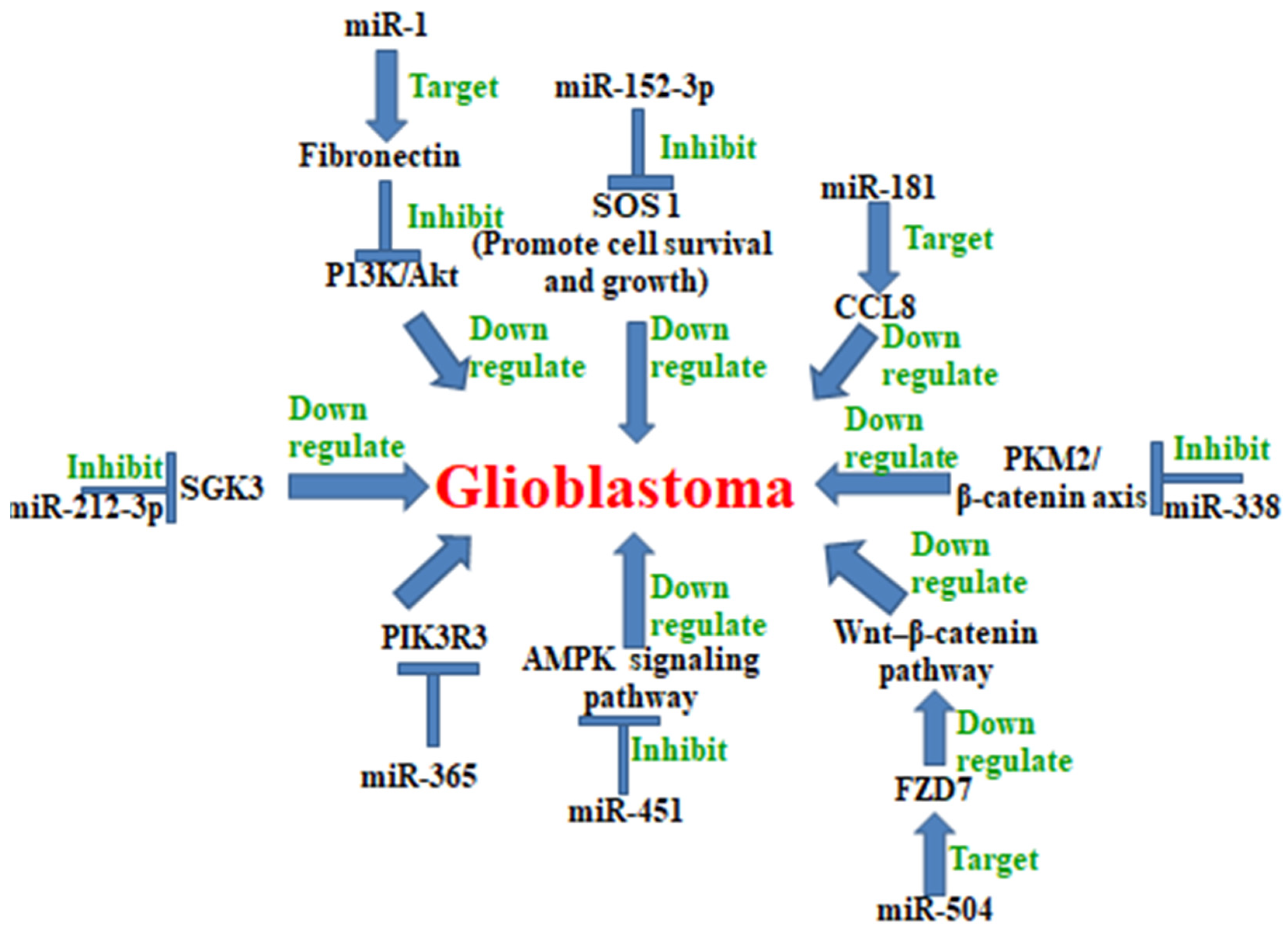
5. MicroRNA in Glioblastoma
Examples
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GBM | glioblastoma |
| miRNAs | microRNAs |
| WHO | World Health Organization |
| LGG | low-grade gliomas |
| HGG | high-grade gliomas |
| SnRNA | small non-coding RNA |
| UTR | untranslated region |
| mRNAs | messenger RNAs |
| DGCR8 | double-stranded RNA binding domain protein |
| dsDNA | double-stranded DNA |
| RISC | RNA-induced silencing complex |
| CSF | cerebrospinal fluid |
| DMEM | Dulbecco’s modified essential medium |
| FBS | fetal bovine serum |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| cDNA | complementary DNA |
| TMZ | temozolomide |
| DS | diagnostic score |
| SHH | sonic hedgehog |
| TNF | tumor necrosis factor |
| TSPAN17 | tetraspanin 17 |
| CKS1 | cyclin kinase subunit 1 |
| DMC | dimethoxy curcumin |
| KLF6 | Kruppel-like factor 6 |
| ATRA | all-trans retinoic acid |
| PRC2 | polycomb repressive complex 2 |
| UCMSCs | umbilical cord mesenchymal stem cells |
| EVs | extracellular vesicles |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-mesenchymal transition |
| MGMT | methyl-guanine-methyl-transferase |
| PAX6 | paired box 6 |
| ATM | ataxia–telangiectasia mutated |
| PLD2 | phospholipase D2 |
| CCL8 | C-C motif chemokine ligand 8 |
| FZD7 | frizzled class receptor 7 |
| FOXP1 | forkhead box P1 |
| GEF | diguanine nucleotide exchange factor |
| EGF | epidermal growth factor |
| SGK3 | serum and glucocorticoid-inducible kinase 3 |
| NEFL | neurofilament light polypeptide |
| LRIG1 | immunoglobulin-like domains protein 1 |
| GK5 | glycerol kinase 5 |
| GSK3β | glycogen synthase kinase 3β |
| CADM1 | cell adhesion molecule 1 |
| NDST1 | N-deacetyl-ase/N-sulfotransferase 1 |
| OGT | O-linked b-N-acetylglucosamine transferase |
| EGFR | epidermal growth factor receptor |
| SLIT1 | slit guidance ligand 1 |
| SFRP2 | secreted frizzled-related protein 2 |
| Six1 | sine oculis homeobox homolog 1 |
| MAPK1 | mitogen-activated protein kinase 1 |
References
- Joshi, A.; Vinay, M.; Bhaskar, P. Current management of glioblastoma multiforme. Semin. Oncol. 2004, 31, 635–644. [Google Scholar] [CrossRef]
- Kelly, W.J.; Gilbert, M.R. Glucocorticoids and immune checkpoint inhibitors in glioblastoma. J. Neurooncol. 2021, 151, 13–20. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Q.; Fang, M.; Huang, W.; Zhu, H. miR-152-3p Sensitizes Glioblastoma Cells Towards Cisplatin Via Regulation Of SOS1. Dovepress 2019, 12, 9513–9525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Guan, G.; Xiao, Z.; Zhao, W. Identification of a Five-Pseudogene Signature for Predicting Survival and Its ceRNA Network in Glioma. Front. Oncol. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Fareh, M.; Turchi, L.; Virolle, V.; Debruyne, D.; Almairac, F.; de-La-Forest, D.S.; Paquis, P.; Preynat-Seauve, O.; Krause, K.-H.; Chneiweiss, H.; et al. The miR 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012, 19, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and Other Malignant Gliomas: A Clinical Review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Chen, P.; Ho, K.; Shih, C. IGF-1-enhanced miR-513a-5p signaling desensitizes glioma cells to temozolomide by targeting the NEDD4L-inhibited Wnt / β—Catenin pathway. PLoS ONE 2019, 14, e0225913. [Google Scholar] [CrossRef]
- Saadatpour, L.; Fadaee, E.; Fadaei, S.; Nassiri Mansour, R.; Mohammadi, M.; Mousavi, S.M.; Goodarzi, M.; Verdi, J.; Mirzaei, H. Glioblastoma: Exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Ther. 2016, 23, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wang, Y.; Sims, M.; Cai, C.; Pfeffer, L. MicroRNA-1 suppresses glioblastoma in preclinical models by targeting fibronectin. Cancer Lett. 2019, 465, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Cheray, M.; Etcheverry, A.; Jacques, C.; Pacaud, R. Cytosine methylation of mature microRNAs inhibits their functions and is associated with poor prognosis in glioblastoma multiforme. Mol. Cancer 2020, 19, 36. [Google Scholar] [CrossRef]
- Tielking, K.; Fischer, S.; Preissner, K.T.; Vajkoczy, P.; Xu, R. Extracellular RNA in Central Nervous System Pathologies. Front. Mol. Neurosci. 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.; Arefian, E.; Rezazadeh, V.R.; Soleimani, M.; Moradimotlagh, A.; Jamshidi, A. Inhibiting the expression of anti-apoptotic genes BCL2L1 and MCL1, and apoptosis induction in glioblastoma cells by microRNA-342. Biomed. Pharmacother. 2020, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skarkova, V.; Krupova, M.; Vitovcova, B.; Skarka, A.; Kasparova, P.; Krupa, P.; Kralova, V.; Rudolf, E. The evaluation of glioblastoma cell dissociation and its influence on its behavior. Int. J. Mol. Sci. 2019, 20, 4630. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xu, R.; Chen, B.; Dong, S.; Zhou, F.; Yu, T.; Xu, G.; Zhang, J.; Wang, Y.; You, Y. MicroRNA-940 inhibits glioma cells proliferation and cell cycle progression by targeting CKS1. Am. J. Transl. Res. 2019, 11, 4851–4865. [Google Scholar] [PubMed]
- Ross, J.S.; Carlson, J.A.; Brock, G. miRNA: The New Gene Silencer. Am. J. Clin. Pathol. 2007, 128, 830–836. [Google Scholar] [CrossRef]
- Bahreyni-Toossi, M.T.; Dolat, E.; Khanbabaei, H.; Zafari, N.; Azimian, H. microRNAs: Potential glioblastoma radiosensitizer by targeting radiation-related molecular pathways. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2019, 816–818, 111679. [Google Scholar] [CrossRef]
- Guo, X.B.; Zhang, X.C.; Chen, P.; Ma, L.M.; Shen, Z. MiR-378a-3p inhibits cellular proliferation and migration in glioblastoma multiforme by targeting tetraspanin 17. Oncol. Rep. 2019, 42, 1957–1971. [Google Scholar] [CrossRef]
- Qian, C.; Wang, B.; Zou, Y.; Zhang, Y.; Hu, X.; Sun, W.; Xiao, H.; Liu, H.; Shi, L. MicroRNA 145 enhances chemosensitivity of glioblastoma stem cells to demethoxycurcumin. Cancer Manag. Res. 2019, 11, 6829–6840. [Google Scholar] [CrossRef]
- Yin, C.Y.; Kong, W.E.I.; Jiang, J.; Xu, H.; Zhao, W.E. miR-7-5p inhibits cell migration and invasion in glioblastoma through targeting SATB1. Oncol. Lett. 2019, 17, 1819–1825. [Google Scholar] [CrossRef]
- Sabelström, H.; Petri, R.; Shchors, K.; Jandial, R.; Schmidt, C.; Sacheva, R.; Masic, S.; Yuan, E.; Fenster, T.; Martinez, M.; et al. Driving Neuronal Differentiation through Reversal of an ERK1/2-miR-124-SOX9 Axis Abrogates Glioblastoma Aggressiveness. Cell Rep. 2019, 28, 2064–2079.e11. [Google Scholar] [CrossRef]
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Potential therapeutic effects of exosomes packed with a miR-21-sponge construct in a rat model of glioblastoma. Front. Oncol. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Rezaei, O.; Honarmand, K.; Nateghinia, S.; Taheri, M.; Ghafouri-Fard, S. miRNA signature in glioblastoma: Potential biomarkers and therapeutic targets. Exp. Mol. Pathol. 2020, 117, 104550. [Google Scholar] [CrossRef]
- Ceresa, D.; Alessandrini, F.; Bosio, L.; Marubbi, D.; Reverberi, D.; Malatesta, P.; Appolloni, I. Cdh4 down-regulation impairs in vivo infiltration and malignancy in patients derived glioblastoma cells. Int. J. Mol. Sci. 2019, 20, 4028. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Wang, Z.; Cheng, Y.; Zhang, P.; Wang, X.; Wen, W.; Yang, H.; Liu, H.; Jin, W.; et al. MicroRNA-101 inhibits proliferation, migration and invasion of human glioblastoma by targeting SOX9. Oncotarget 2017, 8, 19244–19254. [Google Scholar] [CrossRef]
- Yao, Y.L.; Ma, J.; Wang, P.; Xue, Y.X.; Li, Z.; Zhao, L.N.; Li, Z.Q.; Feng, T.D.; Liu, Y.H. miR-101 Acts as a Tumor Suppressor by Targeting Kruppel-like Factor 6 in Glioblastoma Stem Cells. CNS Neurosci. Ther. 2015, 21, 40–51. [Google Scholar] [CrossRef]
- Han, B.; Meng, X.; Chen, H.; Chen, L.; Liu, X.; Wang, H.; Liu, D.; Gao, F.; Lin, L.; Ming, J.; et al. Epigenetic silencing of miR-338 facilitates glioblastoma progression by de-repressing the pyruvate kinase M2-β-catenin axis. Aging 2017, 9, 1885–1897. [Google Scholar] [CrossRef]
- Besse, A.; Sana, J.; Lakomy, R.; Kren, L.; Fadrus, P.; Smrcka, M.; Hermanova, M.; Jancalek, R.; Reguli, S.; Lipina, R.; et al. MiR-338-5p sensitizes glioblastoma cells to radiation through regulation of genes involved in DNA damage response. Tumor Biol. 2016, 37, 7719–7727. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Qi, Z.; Sun, T.; Zhou, Y. MiR-338-5p Promotes Glioma Cell Invasion by Regulating TSHZ3 and MMP2. Cell. Mol. Neurobiol. 2018, 38, 669–677. [Google Scholar] [CrossRef]
- Howe, J.R.; Li, E.S.; Streeter, S.E.; Rahme, G.J.; Chipumuro, E.; Russo, G.B.; Litzky, J.F.; Hills, L.B.; Rodgers, K.R.; Skelton, P.D.; et al. MIR-338-3p regulates neuronal maturation and suppresses glioblastoma proliferation. PLoS ONE 2017, 12, e177661. [Google Scholar] [CrossRef]
- Lima, L.; de Melo, T.C.T.; Marques, D.; de Araújo, J.N.G.; Leite, I.S.F.; Alves, C.X.; Genre, J.; Silbiger, V.N. Modulation of all-trans retinoic acid-induced MiRNA expression in neoplastic cell lines: A systematic review. BMC Cancer 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Xu, J.; Liao, X.; Wong, C. Downregulations of B-cell lymphoma 2 and myeloid cell leukemia sequence 1 by microRNA 153 induce apoptosis in a glioblastoma cell line DBTRG-05MG. Int. J. Cancer 2010, 126, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Shivram, H.; Le, S.V.; Iyer, V.R. PRC2 activates interferon-stimulated genes indirectly by repressing miRNAs in glioblastoma. PLoS ONE 2019, 14, e222435. [Google Scholar] [CrossRef] [PubMed]
- Ngadiono, E.; Hardiany, N.S. Advancing towards effective glioma therapy: MicroRNA derived from umbilical cord mesenchymal stem cells’ extracellular vesicles. Malays. J. Med. Sci. 2019, 26, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Kang, T.; Wang, Y.; Sun, J.; Li, C.; Liu, H.; Yang, Y.; Jiao, B. Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410. Biosci. Rep. 2019, 39, 1–11. [Google Scholar] [CrossRef]
- Mei, J.; Bachoo, R.; Zhang, C.-L. MicroRNA-146a Inhibits Glioma Development by Targeting Notch1. Mol. Cell. Biol. 2011, 31, 3584–3592. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Hoadley, K.; Kushwaha, D.; Ramakrishnan, V.; Li, S.; Kang, C.; You, Y.; Jiang, C.; Song, S.W.; et al. MiR-181d: Predictive glioblastoma biomarker that downregulates MGMT expression. Neuro. Oncol. 2012, 14, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ta, W.W.; Sun, P.F.; Meng, Y.F.; Zhao, C.Z. Diagnostic and prognostic significance of serum miR-145-5p expression in glioblastoma. Int. J. Clin. Exp. Pathol. 2019, 12, 2536–2543. [Google Scholar]
- Cheng, Q.; Ma, X.; Cao, H.; Chen, Z.; Wan, X.; Chen, R.; Peng, R.; Huang, J.; Jiang, B. Role of miR-223/paired box 6 signaling in temozolomide chemoresistance in glioblastoma multiforme cells. Mol. Med. Rep. 2017, 15, 597–604. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Zeng, A.; Liu, S.; Li, R.; Wang, X.; Yan, W.; Li, H.; You, Y. Genome-wide identification of epithelial-mesenchymal transition-associated microRNAs reveals novel targets for glioblastoma therapy. Oncol. Lett. 2018, 15, 7625–7630. [Google Scholar] [CrossRef]
- Xi, Y.; Formentini, A.; Chien, M.; Weir, D.B.; Russo, J.J.; Ju, J.; Kornmann, M.; Ju, J. Prognostic Values of microRNAs in Colorectal Cancer. Biomark. Insights 2006, 1, 113–121. [Google Scholar] [CrossRef]
- Guo, P.; Lan, J.; Ge, J.; Nie, Q.; Guo, L.; Qiu, Y.; Mao, Q. MiR-26a enhances the radiosensitivity of glioblastoma multiforme cells through targeting of ataxia-telangiectasia mutated. Exp. Cell Res. 2014, 320, 200–208. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, G.; Luo, H.; Zhao, S. MicroRNA-203 as a stemness inhibitor of glioblastoma stem cells. Mol. Cells 2016, 39, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, D.; Cheng, Q.; Ma, Z.; Jiang, B.; Peng, R.; Chen, R.; Cao, Y.; Wan, X. MicroRNA-203 inhibits the proliferation and invasion of U251 glioblastoma cells by directly targeting PLD2. Mol. Med. Rep. 2014, 9, 503–508. [Google Scholar] [CrossRef]
- Zhai, F.; Chen, X.; He, Q.; Zhang, H.; Hu, Y.; Wang, D.A.N.; Liu, S.; Zhang, Y.I. MicroRNA-181 inhibits glioblastoma cell growth by directly targeting CCL8. Oncol. Lett. 2019, 18, 1922–1930. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Guan, Y.; Li, Z.; Wang, Y.; Liu, Y.; Cui, R. MiR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt-β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–18. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Shen, C.; Yin, F.; Wang, K.; Liu, Y.; Zheng, B.; Zhang, W.; Hou, X.; Chen, X.; et al. MiR-212-3p Inhibits Glioblastoma Cell Proliferation by Targeting SGK3. J. Neurooncol. 2015, 122, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.; Rao, M.; Xu, S. MicroRNA-365 inhibits proliferation, migration and invasion of glioma by targeting PIK3R3. Oncol. Rep. 2017, 37, 2185–2192. [Google Scholar] [CrossRef]
- Altuvia, Y.; Landgraf, P.; Lithwick, G.; Elefant, N.; Pfeffer, S.; Aravin, A.; Brownstein, M.J.; Tuschl, T.; Margalit, H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005, 33, 2697–2706. [Google Scholar] [CrossRef]
- De Los Reyes, V.A.A.; Jung, E.; Kim, Y. Optimal control strategies of eradicating invisible glioblastoma cells after conventional surgery. J. R. Soc. Interface 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wu, S. Mir-451: A novel biomarker and potential therapeutic target for cancer. OncoTargets Ther. 2019, 12, 11069–11082. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xiong, J.; Zhang, S.S.; Wang, J.J.; Gong, Z.J.; Dai, M.H. Up-Regulation of microRNA-183 Promotes Cell Proliferation and Invasion in Glioma By Directly Targeting NEFL. Cell. Mol. Neurobiol. 2016, 36, 1303–1310. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Z.; Wu, L.; Liu, C.; Chen, Q.; Liu, J.; Wang, X.; Zhuang, Z.; Li, W.; Xu, S.; et al. Upregulation of miR-183 expression and its clinical significance in human brain glioma. Neurol. Sci. 2016, 37, 1341–1347. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhu, S.; Jin, X.; Wang, J.; Zhu, J.; Zhou, Y. MicroRNA-590-3p enhances the radioresistance in glioblastoma cells by targeting LRIG1. Exp. Ther. Med. 2017, 14, 1818–1824. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Zhang, J.X.; Zhang, A.L.; Shi, Z.D.; Han, L.; Jia, Z.F.; Yang, W.D.; Wang, G.X.; Jiang, T.; You, Y.P.; et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 2010, 9, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.H.; Zhang, B.Z. MicroRNA-135b exerts oncogenic activity in glioblastoma via the inhibition of glycerol kinase 5 expression. Mol. Med. Rep. 2015, 12, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Lulli, V.; Buccarelli, M.; Martini, M.; Signore, M.; Biffoni, M.; Giannetti, S.; Morgante, L.; Marziali, G.; Ilari, R.; Pagliuca, A.; et al. miR-135b suppresses tumorigenesis in glioblastoma stem-like cells impairing proliferation, migration and self-renewal. Oncotarget 2015, 6, 37241–37256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, B.; Zhang, W.; Wu, J. Circular RNA CircMTO1 Inhibits Proliferation of Glioblastoma Cells via miR-92/WWOX Signaling Pathway. Med. Sci. Monit. 2019, 25, 6454–6461. [Google Scholar] [CrossRef]
- Alamdari-Palangi, V.; Amini, R.; Karami, H. MiRNA-7 enhances erlotinib sensitivity of glioblastoma cells by blocking the IRS-1 and IRS-2 expression. J. Pharm. Pharmacol. 2020, 72, 531–538. [Google Scholar] [CrossRef]
- Shi, H.; Wang, D.; Ma, L.; Zhu, H. MicroRNA-362 inhibits cell growth and metastasis in glioblastoma by targeting MAPK1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8931–8939. [Google Scholar] [PubMed]
- Kong, F.; Li, X.; Li, S.; Sheng, D.; Li, W.; Song, M. MicroRNA-15a-5p promotes the proliferation and invasion of T98G glioblastoma cells via targeting cell adhesion molecule 1. Oncol. Lett. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Xue, J.; Yang, M.; Hua, L.H.; Wang, Z.P. MiRNA-191 functions as an oncogene in primary glioblastoma by directly targeting NDST1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6242–6249. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lu, Z.; Chen, Y.; Chen, X.; Liu, N.; Chen, J.; Dong, S. MicroRNA-640 promotes cell proliferation and adhesion in glioblastoma by targeting Slit guidance ligand 1. Oncol. Lett. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, P.; Tan, Y.; Feng, Q.; Zhao, R. MicroRNA-522-3p plays an oncogenic role in glioblastoma through activating Wnt/β-catenin signaling pathway via targeting SFRP2. Neuroreport 2021, 32, 88–98. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Zhao, H. MicroRNA-155-3p promotes glioma progression and temozolomide resistance by targeting Six1. J. Cell. Mol. Med. 2020, 24, 5363–5374. [Google Scholar] [CrossRef] [PubMed]
| S. No. | MiRNA Name | Expression in Glioblastoma | Relationship between the miRNA and Its Target Gene | Refs. |
|---|---|---|---|---|
| 1. | miR-153 | Down-regulated | ↓ Bcl2 and MCL1 | [31] |
| 2. | miR-940 | Down-regulated | ↓ CSK1 | [14] |
| 3. | miR-26a | Down-regulated | ↓ PTEN and RB1 | [41] |
| 4. | miR-203 | Down-regulated | ↓ PLD2 | [43] |
| 5. | miR-338-5p | Down-regulated | ↓ TSHZ3 and MMP2 | [27,28] |
| 6. | miR-338-3p | Down-regulated | ↓ glioblastoma progression | [29] |
| 7. | miR-212 | Down-regulated | ↓ SKG3 | [46] |
| 8. | miR-7-5p | Down-regulated | ↓ SATB1 | [19] |
| 9. | miR-181a | Down-regulated | ↓ CCL8 | [44] |
| 10. | miR-128 | Down-regulated | ↓ BMI1 | [16] |
| 11. | miR-124 | Down-regulated | ↓ MEK | [16] |
| 12. | miR-378a-3p | Down-regulated | ↓ TSPAN17 | [17] |
| 13. | miR-342 | Down-regulated | ↓ BCL2L1 and MCL1 | [12] |
| 14. | miR-302-367 | Down-regulated | ↓ CXCR4 and Shh-Gli1-NANGOG | [5] |
| 15. | miR-181 | Down-regulated | ↓ BCL2 | [16] |
| 16. | miR-34 | Down-regulated | ↓ Enhance ROSs | [16] |
| 17. | miR-137 | Down-regulated | ↓ Neuronal differentiation | [20] |
| 18. | miR-504 | Down-regulated | ↓ WNT-β Catenin pathway | [45] |
| 19. | miR-101 | Down-regulated | ↓ Sox9 And MEK ½ | [25] |
| 20. | miR-152-3p | Down-regulated | ↓ SOS1 | [3] |
| 21. | miR-451 | Down-regulated | ↓ AMPK signaling | [49] |
| 22. | miR-145 | Down-regulated | ↓ BNIP3 and Notch signaling | [18] |
| 23. | miR-1 | Down-regulated | ↓ PI3K/AKT | [9] |
| 24. | miR-199a | Down-regulated | ↓ AKT-mTOR | [33] |
| 25. | miR-410 | Down-regulated | ↓ MET | [33] |
| 26. | miR-146a | Down-regulated | ↓ EGFR | [35] |
| 27. | miR-365 | Down-regulated | ↓ PIK3R3 | [47] |
| 28. | miR-7 | Down-regulated | ↓ OGT, EGFR, and Akt pathway | [58] |
| 29. | miR- 362 | Down-regulated | ↓ MAPK1 | [59] |
| 30. | miR-21 | Up-regulated | ↓ | [21,22] |
| 31. | miR-183 | Up-regulated | ↓ NEFL | [51] |
| 32. | miR-590-3p | Up-regulated | ↑ LRIG1 | [53] |
| 33. | miR-221 | Up-regulated | ↑ PUMA | [54] |
| 34. | miR-222 | Up-regulated | ↑ PUMA and Bcl2 ↓ BAX | [54] |
| 35. | miR-135b | Up-regulated | ↓ GK5 | [55] |
| 36. | miR-4443 | Up-regulated | ↓ Apoptosis | [11] |
| 37. | miR-422a | Up-regulated | ↓ Apoptosis | [11] |
| 38. | miR-494-3p | Up-regulated | ↓ Apoptosis | [11] |
| 39. | miR-502-5p | Up-regulated | ↓ Apoptosis | [11] |
| 40. | miR-520f-3p | Up-regulated | ↓ Apoptosis | [11] |
| 41. | miR-549a | Up-regulated | ↓ Apoptosis | [11] |
| 42. | miR-92 | Up-regulated | ↓ WWOX | [57] |
| 43. | miR-223 | Up-regulated | ↑ EMT | [39] |
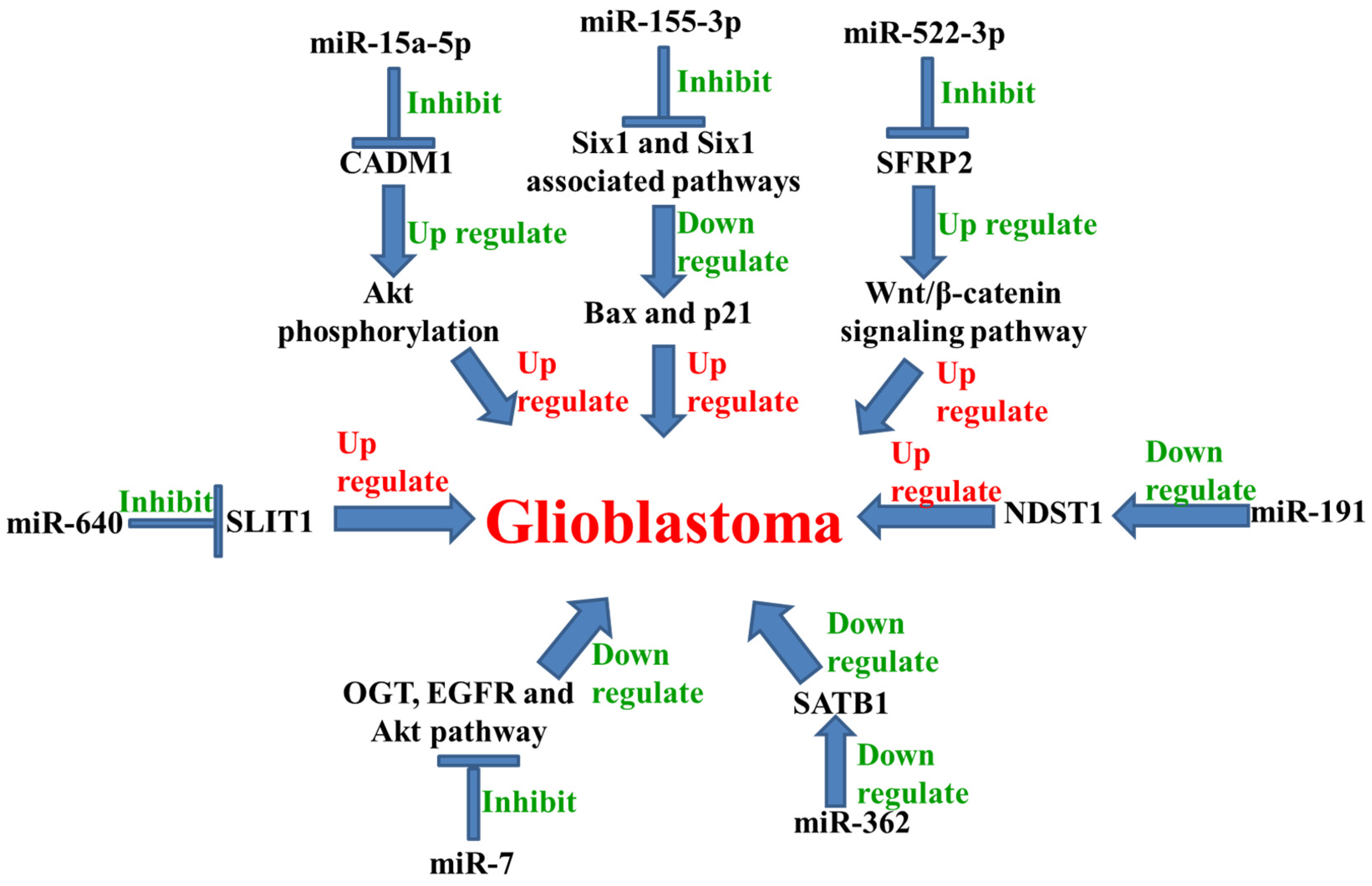
| 44. | miR-15a-5p | Up-regulated | ↓ CADM1 ↑ Akt | [60] |
| 45. | miR-191 | Up-regulated | ↓ NDST1 | [61] |
| 46. | miR-640 | Up-regulated | ↓ SLIT1 | [62] |
| 47. | miR-522-3p | Up-regulated | ↓ SFRP2 ↑ Wnt/β-catenin | [63] |
| 48. | miR-155-3p | Up-regulated | ↓ Six1, Bax, and p21 | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.P.; Castresana, J.S.; Shahi, M.H. Glioblastoma and MiRNAs. Cancers 2021, 13, 1581. https://doi.org/10.3390/cancers13071581
Ahmed SP, Castresana JS, Shahi MH. Glioblastoma and MiRNAs. Cancers. 2021; 13(7):1581. https://doi.org/10.3390/cancers13071581
Chicago/Turabian StyleAhmed, Swalih P., Javier S. Castresana, and Mehdi H. Shahi. 2021. "Glioblastoma and MiRNAs" Cancers 13, no. 7: 1581. https://doi.org/10.3390/cancers13071581
APA StyleAhmed, S. P., Castresana, J. S., & Shahi, M. H. (2021). Glioblastoma and MiRNAs. Cancers, 13(7), 1581. https://doi.org/10.3390/cancers13071581








