Three-Dimensional Radiological Assessment of Ablative Margins in Hepatocellular Carcinoma: Pilot Study of Overlay Fused CT/MRI Imaging with Automatic Registration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
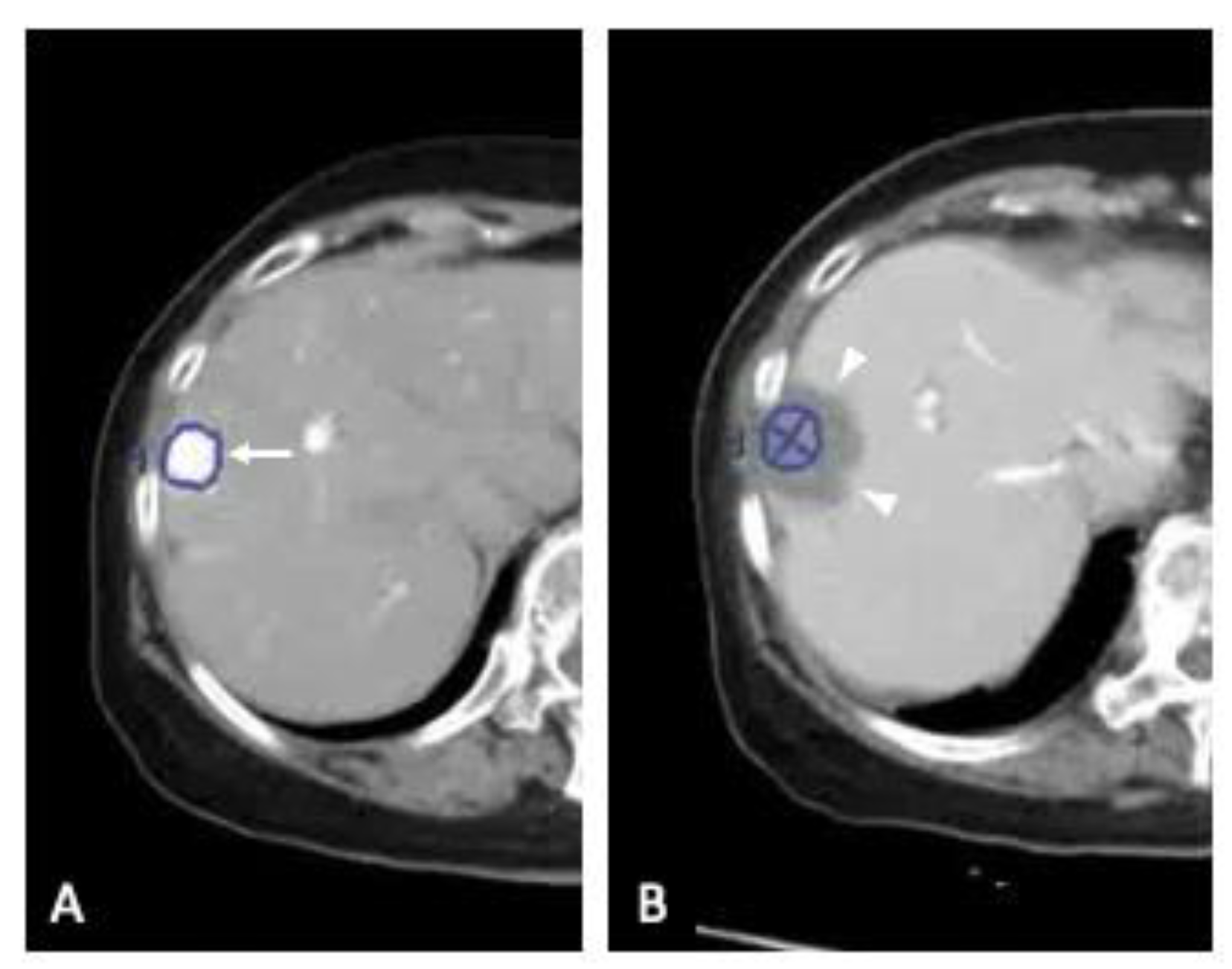
2.2. Three-Dimensional Assessment of Ablative Margins Using Overlay Fused CT/MRI Imaging
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Equipment
4.3. Assessment of Initial Technical Effectiveness
4.4. Diagnosis of LTP
4.5. Image Fusion and Visualization of the Ablative Margin
4.6. Follow-Up and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| HCC | hepatocellular carcinoma |
| LTP | local tumor progression |
| MRI | magnetic resonance imaging |
| RFA | radiofrequency ablation |
| SD | standard deviation |
| TACE | transcatheter arterial chemoembolization |
| US | ultrasound |
References
- Chen, M.S.; Li, J.Q.; Zheng, Y.; Guo, R.P.; Liang, H.H.; Zhang, Y.Q.; Lin, X.J.; Lau, W.Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006, 243, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Makuuchi, M.; Hasegawa, K. Single HCC smaller than 2 cm: Surgery or ablation?: Surgeon’s perspective. J. Hepatobiliary Pancreat Sci. 2010, 17, 422–424. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, C.C.; Hung, C.H.; Chen, C.L.; Lu, S.N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012, 56, 412–418. [Google Scholar] [CrossRef]
- Peng, Z.W.; Lin, X.J.; Zhang, Y.J.; Liang, H.H.; Guo, R.P.; Shi, M.; Chen, M.S. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: A retrospective comparative study. Radiology 2012, 262, 1022–1033. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, Y.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef]
- Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Huang, Y.H.; Chiou, Y.Y.; Lin, H.C.; Huo, T.I. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann. Surg. 2016, 263, 538–545. [Google Scholar] [CrossRef]
- Liu, C.H.; Arellano, R.S.; Uppot, R.N.; Samir, A.E.; Gervais, D.A.; Mueller, P.R. Radiofrequency ablation of hepatic tumours: Effect of post-ablation margin on local tumour progression. Eur. Radiol. 2010, 20, 877–885. [Google Scholar] [CrossRef]
- Minami, Y.; Kudo, M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J. Radiol. 2010, 2, 417–424. [Google Scholar] [CrossRef]
- Tiong, L.; Maddern, G.J. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 2011, 98, 1210–1224. [Google Scholar] [CrossRef]
- Minami, Y.; Kudo, M. Imaging Modalities for Assessment of Treatment Response to Nonsurgical Hepatocellular Carcinoma Therapy: Contrast-Enhanced US, CT, and MRI. Liver Cancer 2015, 4, 106–114. [Google Scholar] [CrossRef]
- Nishikawa, H.; Inuzuka, T.; Takeda, H.; Nakajima, J.; Sakamoto, A.; Henmi, S.; Matsuda, F.; Eso, Y.; Ishikawa, T.; Saito, S.; et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: A proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J. Gastroenterol. 2011, 46, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Osaki, Y.; Iguchi, E.; Koshikawa, Y.; Ako, S.; Inuzuka, T.; Takeda, H.; Nakajima, J.; Matsuda, F.; Sakamoto, A.; et al. Radiofrequency ablation for hepatocellular carcinoma: The relationship between a new grading system for the ablative margin and clinical outcomes. J. Gastroenterol. 2013, 48, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, K.; Nouso, K.; Wakuta, A.; Oonishi, A.; Toyoda, H.; Tada, T.; Hiraoka, A.; Tsuji, K.; Itobayashi, E.; Ishikawa, T.; et al. Treatment of intermediate-stage hepatocellular carcinoma in Japan: Position of curative therapies. Liver Cancer 2020, 9, 41–49. [Google Scholar] [CrossRef]
- Lee, J.Y.; Minami, Y.; Choi, B.I.; Lee, W.J.; Chou, Y.H.; Jeong, W.K.; Park, M.S.; Kudo, N.; Lee, M.W.; Kamata, K.; et al. The AFSUMB Consensus Statements and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound using Sonazoid. Ultrasonography 2020, 39, 191–220. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, W.J.; Rhim, H.; Lim, H.K.; Choi, D.; Lee, J.Y. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am. J. Roentgenol. 2010, 195, 758–765. [Google Scholar]
- Kim, K.W.; Lee, J.M.; Klotz, E.; Kim, S.J.; Kim, S.H.; Kim, J.Y.; Han, J.K.; Choi, B.I. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am. J. Roentgenol. 2011, 196, W565–W572. [Google Scholar] [CrossRef]
- Minami, Y.; Kitai, S.; Kudo, M. Treatment response assessment of radiofrequency ablation for hepatocellular carcinoma: Usefulness of virtual CT sonography with magnetic navigation. Eur. J. Radiol. 2012, 81, e277–e280. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.M.; Kim, K.W.; Joo, I.; Han, J.K.; Choi, B.I.; Klotz, E. Postablation assessment using follow-up registration of CT images before and after radiofrequency ablation (RFA): Prospective evaluation of midterm therapeutic results of RFA for hepatocellular carcinoma. AJR Am. J. Roentgenol. 2014, 203, 70–77. [Google Scholar] [CrossRef]
- Takeyama, N.; Mizobuchi, N.; Sakaki, M.; Shimozuma, Y.; Munechika, J.; Kajiwara, A.; Uchikoshi, M.; Uozumi, S.; Ohgiya, Y.; Gokan, T. Evaluation of hepatocellular carcinoma ablative margins using fused pre- and post-ablation hepatobiliary phase images. Abdom. Radiol. (NY) 2019, 44, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Solbiati, M.; Muglia, R.; Goldberg, S.N.; Ierace, T.; Rotilio, A.; Passera, K.M.; Marre, I.; Solbiati, L. A novel software platform for volumetric assessment of ablation completeness. Int. J. Hyperthermia 2019, 36, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, A.; Kai, S.; Iwashita, Y.; Hirano, S.; Ohta, M.; Kitano, S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 2005, 103, 299–306. [Google Scholar] [CrossRef]
- Okusaka, T.; Okada, S.; Ueno, H.; Ikeda, M.; Shimada, K.; Yamamoto, J.; Kosuge, T.; Yamasaki, S.; Fukushima, N.; Sakamoto, M. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002, 95, 1931–1937. [Google Scholar] [CrossRef]
- Nakashima, Y.; Nakashima, O.; Tanaka, M.; Okuda, K.; Nakashima, M.; Kojiro, M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol. Res. 2003, 26, 142–147. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kokubu, S.; Shibuya, A.; Ono, K.; Watanabe, M.; Hidaka, H.; Tsuchihashi, T.; Saigenji, K. Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin. AJR Am. J. Roentgenol. 2007, 188, 480–488. [Google Scholar] [CrossRef]
- Kudo, M. Local ablation therapy for hepatocellular carcinoma: Current status and future perspectives. J. Gastroenterol. 2004, 39, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, D.; Lim, H.K.; Rhim, H.; Kim, Y.S.; Kim, S.H.; Lee, W.J. Growth rate of new hepatocellular carcinoma after percutaneous radiofrequency ablation: Evaluation with multiphase CT. AJR Am. J. Roentgenol. 2008, 191, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, M.; Hayashi, K.; Kubota, K.; Sekine, S.; Okada, Y.; Ina, H.; Irie, T. Growth rate of locally recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: Comparing the growth rate of locally recurrent tumor with that of primary hepatocellular carcinoma. Dig. Dis. Sci. 2007, 52, 783–788. [Google Scholar] [CrossRef]
- Sainani, N.; Gervais, D.A.; Mueller, P.R.; Arellano, R.S. Imaging after percutaneous radiofrequency ablation of hepatic tumors: Part 1, Normal findings. AJR Am. J. Roentgenol. 2013, 200, 184–193. [Google Scholar] [CrossRef]
- Park, M.H.; Rhim, H.; Kim, Y.S.; Choi, D.; Lim, H.K.; Lee, W.J. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics 2008, 28, 379–390. [Google Scholar] [CrossRef]
- Chopra, S.; Dodd GD 3rd Chintapalli, K.N.; Leyendecker, J.R.; Karahan, O.I.; Rhim, H. Tumor recurrence after radiofrequency thermal ablation of hepatic tumors: Spectrum of findings on dual-phase contrast-enhanced CT. AJR Am. J. Roentgenol. 2001, 177, 381–387. [Google Scholar] [CrossRef]
- Dromain, C.; de Baere, T.; Elias, D.; Kuoch, V.; Ducreux, M.; Boige, V.; Petrow, P.; Roche, A.; Sigal, R. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology 2002, 223, 255–262. [Google Scholar] [CrossRef]
- Makino, Y.; Imai, Y.; Igura, T.; Hori, M.; Fukuda, K.; Sawai, Y.; Kogita, S.; Ohama, H.; Matsumoto, Y.; Nakahara, M.; et al. Utility of computed tomography fusion imaging for the evaluation of the ablative margin of radiofrequency ablation for hepatocellular carcinoma and the correlation to local tumor progression. Hepatol. Res. 2013, 43, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Imai, Y.; Igura, T.; Hori, M.; Fukuda, K.; Sawai, Y.; Kogita, S.; Fujita, N.; Takehara, T.; Murakami, T. Comparative evaluation of three-dimensional Gd-EOB-DTPA-enhanced MR fusion imaging with CT fusion imaging in the assessment of treatment effect of radiofrequency ablation of hepatocellular carcinoma. Abdom. Imaging 2015, 40, 102–111. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, K.; Su, Z.Z.; Huang, Z.P.; Wang, P.; Zheng, R.Q. Assessment of radiofrequency ablation margin by MRI-MRI image fusion in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 5345–5351. [Google Scholar] [CrossRef]
- Laimer, G.; Schullian, P.; Jaschke, N.; Putzer, D.; Eberle, G.; Alzaga, A.; Odisio, B.; Bale, R. Minimal ablative margin (MAM) assessment with image fusion: An independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur. Radiol. 2020, 30, 2463–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Tang, A.; Bashir, M.R.; Corwin, M.T.; Cruite, I.; Dietrich, C.F.; Do, R.K.G.; Ehman, E.C.; Fowler, K.J.; Hussain, H.K.; Jha, R.C.; for the LI-RADS Evidence Working Group; et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018, 286, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baère, T.; Dodd, G.D., 3rd; et al. International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [PubMed]
- Wei, Y.; Ye, Z.; Yuan, Y.; Huang, Z.; Wei, X.; Zhang, T.; Wan, S.; Tang, H.; He, X.; Song, B. A new diagnostic criterion with gadoxetic acid-enhanced MRI may improve the diagnostic performance for hepatocellular carcinoma. Liver Cancer 2020, 9, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Rhim, H.; Lee, J.; Song, K.D.; Lee, M.W.; Kim, Y.S.; Lim, H.K.; Jang, K.M.; Kim, S.H.; Gwak, G.Y.; et al. Magnetic resonance imaging with gadoxetic acid for local tumour progression after radiofrequency ablation in patients with hepatocellular carcinoma. Eur. Radiol. 2016, 26, 3437–3446. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Grassi, C.J.; Cardella, J.F.; Charboneau, J.W.; Dodd, G.D., 3rd; Dupuy, D.E.; Gervais, D.A.; Gillams, A.R.; Kane, R.A.; Lee, F.T., Jr.; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J. Vasc. Interv. Radiol. 2009, 20 (Suppl. 7), S377–S390. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Values |
|---|---|
| Sex | |
| Male/Female | 13/4 |
| Age (year) | |
| Mean ± SD | 68.3 ± 5.7 |
| Range | 58–79 |
| Etiologic cause of HCC | |
| Hepatitis B/Hepatitis C/nonBnonC | 2/12/3 |
| Mean serum albumin level (g/dL) * | 3.6 ± 0.4 |
| Mean serum total bilirubin level (g/dL) * | 0.9 ± 0.5 |
| Child-Pugh class | |
| A/B/C | 15/2/0 |
| Serum AFP level (ng/mL) | |
| <20/20-200/>200 | 9/6/2 |
| Number of HCCs | 17 |
| Tumor location | |
| Left lateral/Left medial/4/3/Right medial/Right lateral/Segment 1 | 4/3/5/5/0 |
| Tumor size before ablation (cm) | |
| Mean ± SD | 1.4 ± 0.5 |
| Range | 0.7–2.7 |
| Coagulation size after ablation (cm) | |
| Mean LAD ± SD | 3.2 ± 0.9 |
| Range | 1.6–5.4 |
| Mean SAD ± SD | 2.5 ± 0.5 |
| Range | 1.4–3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, Y.; Minami, T.; Ueshima, K.; Yagyu, Y.; Tsurusaki, M.; Okada, T.; Hori, M.; Kudo, M.; Murakami, T. Three-Dimensional Radiological Assessment of Ablative Margins in Hepatocellular Carcinoma: Pilot Study of Overlay Fused CT/MRI Imaging with Automatic Registration. Cancers 2021, 13, 1460. https://doi.org/10.3390/cancers13061460
Minami Y, Minami T, Ueshima K, Yagyu Y, Tsurusaki M, Okada T, Hori M, Kudo M, Murakami T. Three-Dimensional Radiological Assessment of Ablative Margins in Hepatocellular Carcinoma: Pilot Study of Overlay Fused CT/MRI Imaging with Automatic Registration. Cancers. 2021; 13(6):1460. https://doi.org/10.3390/cancers13061460
Chicago/Turabian StyleMinami, Yasunori, Tomohiro Minami, Kazuomi Ueshima, Yukinobu Yagyu, Masakatsu Tsurusaki, Takuya Okada, Masatoshi Hori, Masatoshi Kudo, and Takamichi Murakami. 2021. "Three-Dimensional Radiological Assessment of Ablative Margins in Hepatocellular Carcinoma: Pilot Study of Overlay Fused CT/MRI Imaging with Automatic Registration" Cancers 13, no. 6: 1460. https://doi.org/10.3390/cancers13061460
APA StyleMinami, Y., Minami, T., Ueshima, K., Yagyu, Y., Tsurusaki, M., Okada, T., Hori, M., Kudo, M., & Murakami, T. (2021). Three-Dimensional Radiological Assessment of Ablative Margins in Hepatocellular Carcinoma: Pilot Study of Overlay Fused CT/MRI Imaging with Automatic Registration. Cancers, 13(6), 1460. https://doi.org/10.3390/cancers13061460









