Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
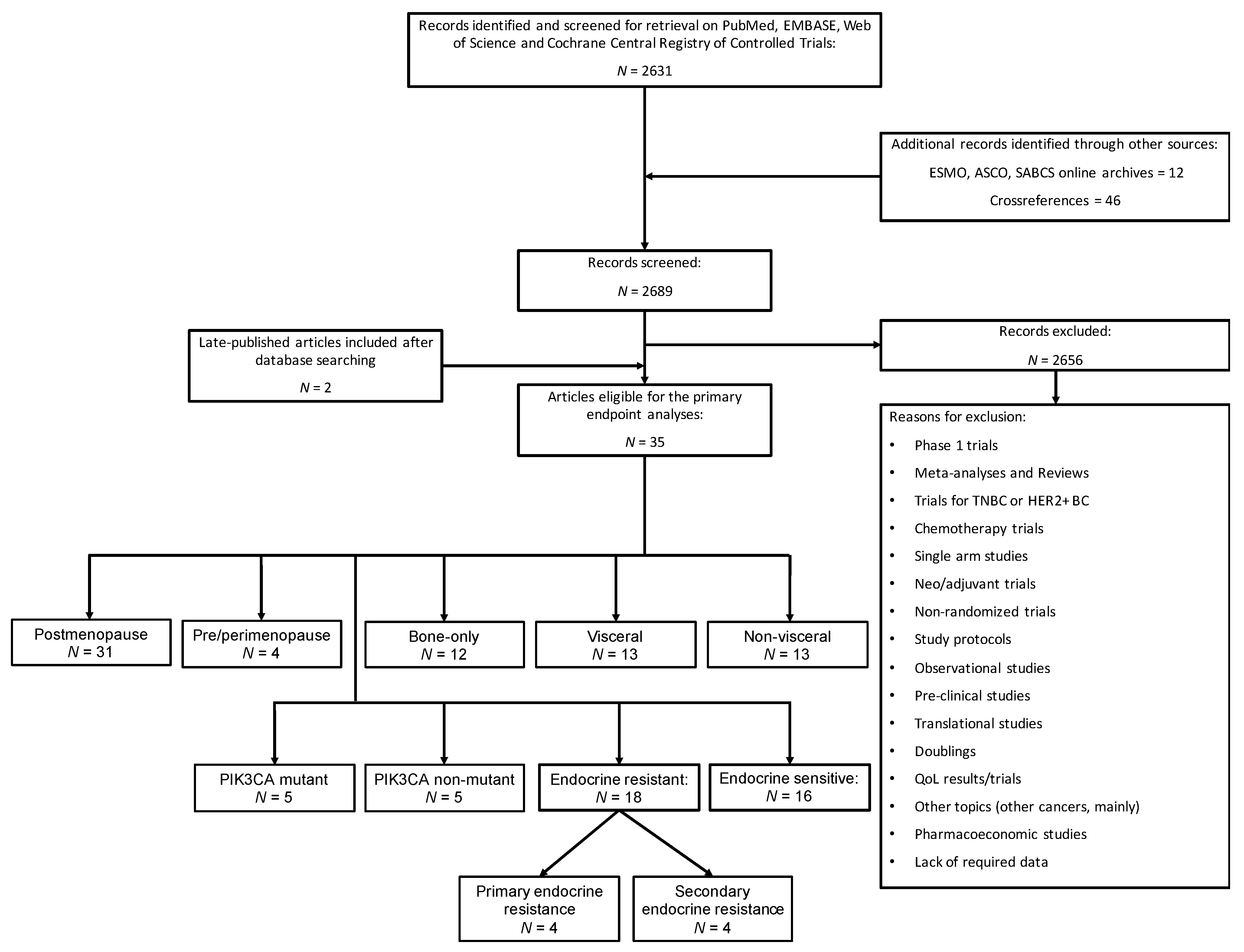
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Study Endpoints
2.4. Data Analysis
3. Results
3.1. Study Characteristics
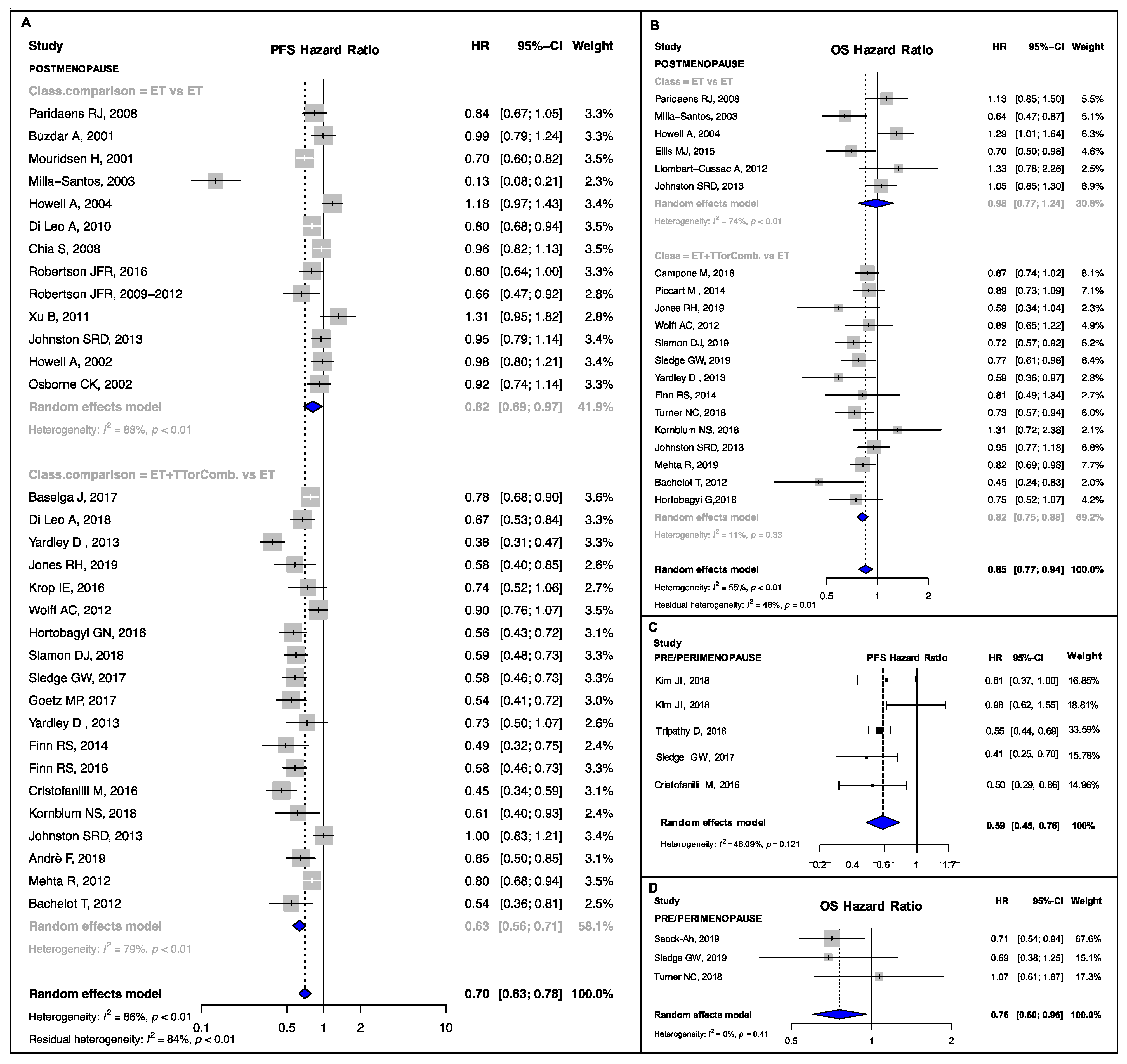
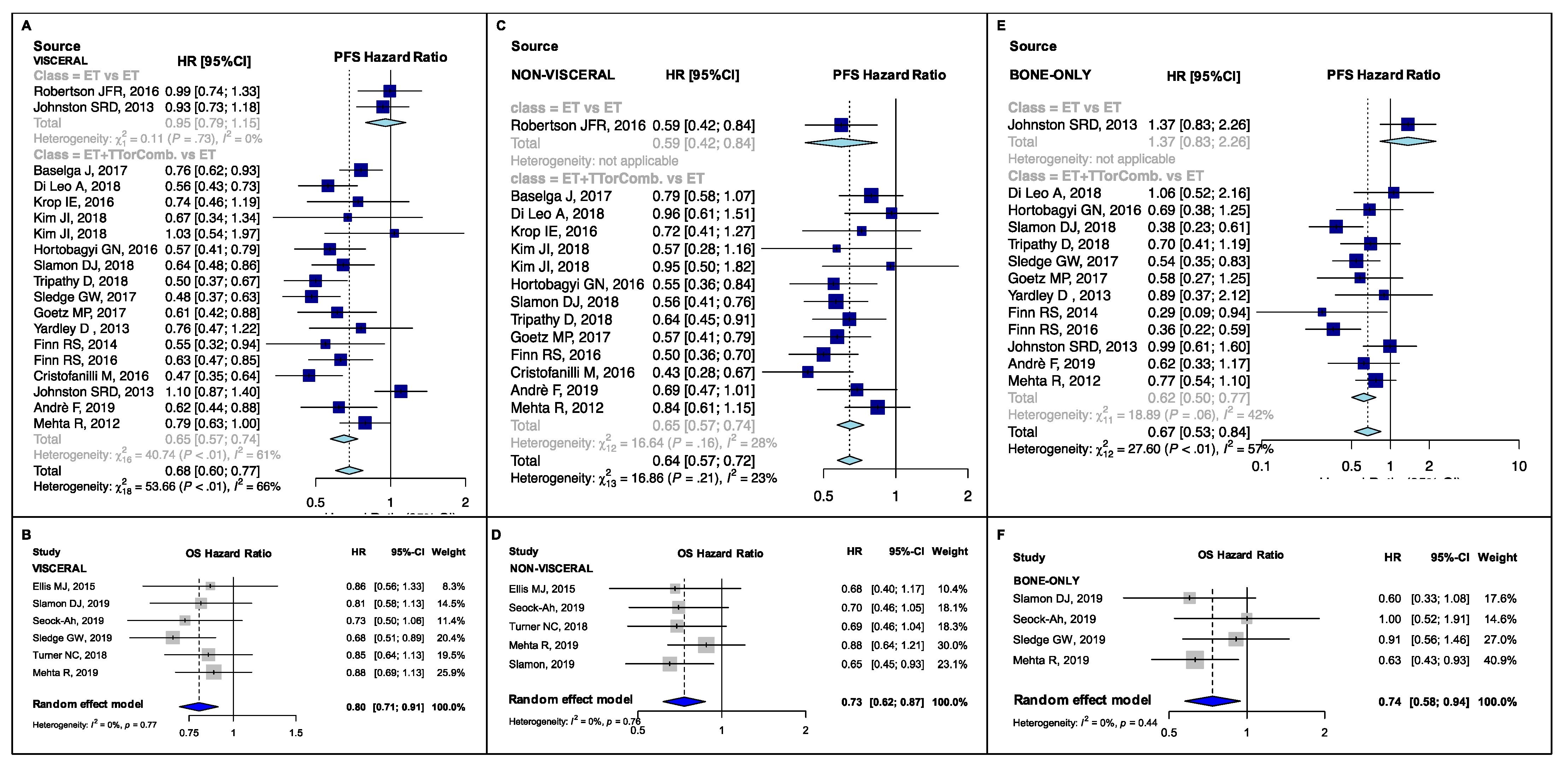
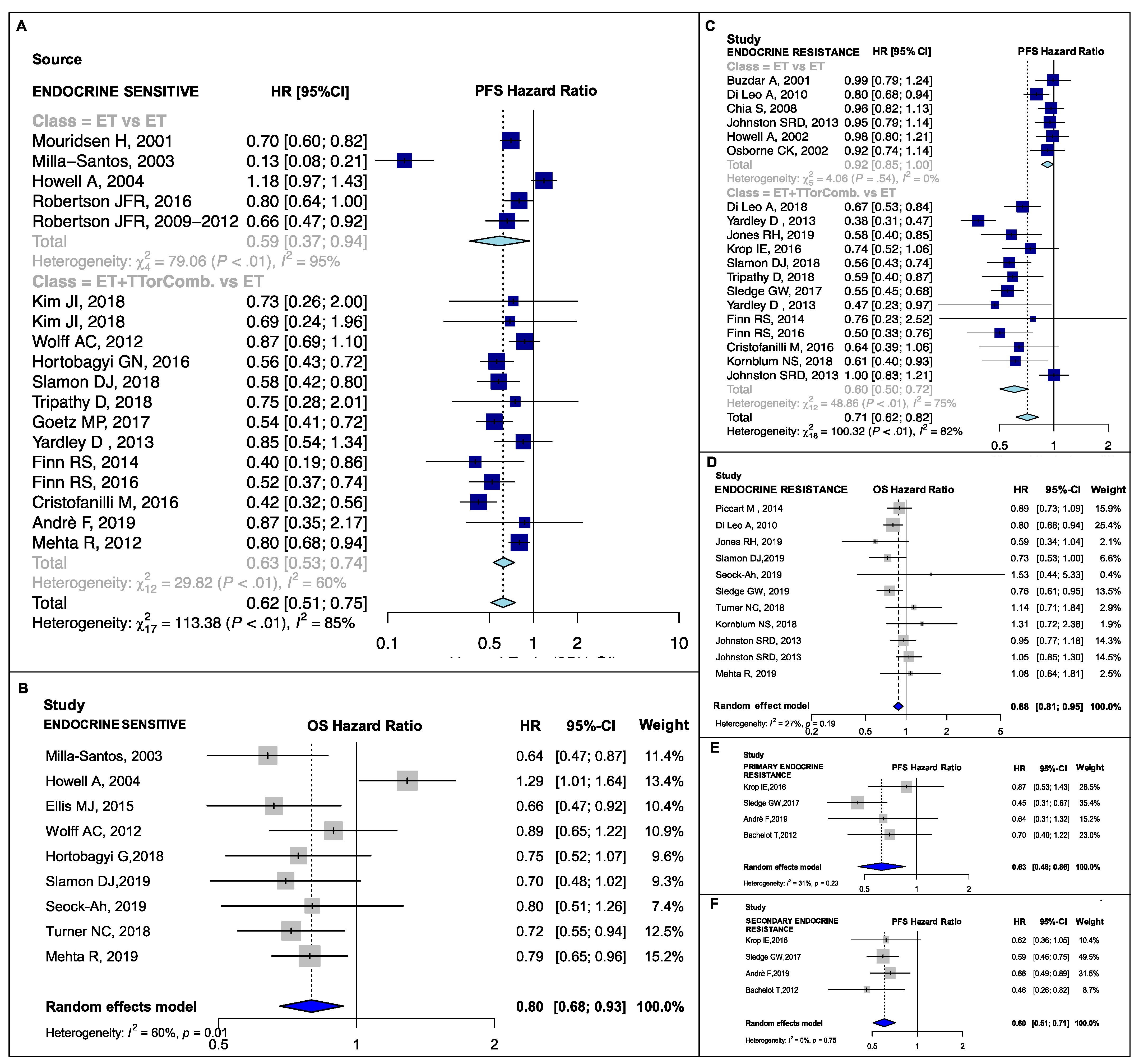
3.2. Pooled Estimates in Clinical Subsets
3.3. Subgroup Analyses and Meta-Regressions: Combination Studies versus Single Agent Studies
3.4. Subgroup Analyses and Meta-Regressions: Drug Class Comparisons
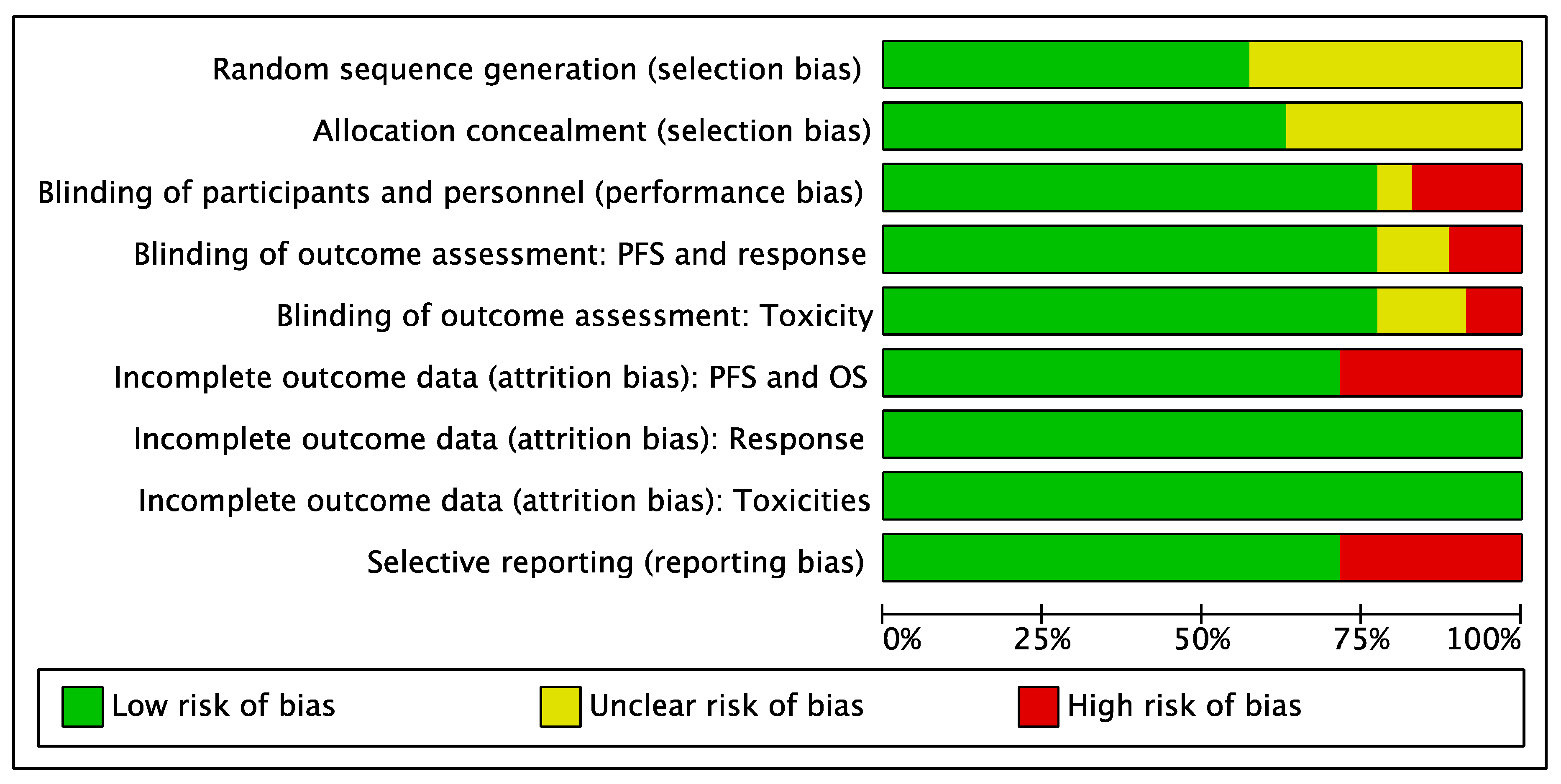
3.5. Risk of Bias Analysis
4. Discussion
4.1. Postmenopausal, Visceral and Endocrine-Resistant Disease Subgroups
4.2. Bone-Only, Endocrine-Sensitive and Non-Visceral Disease Subgroups
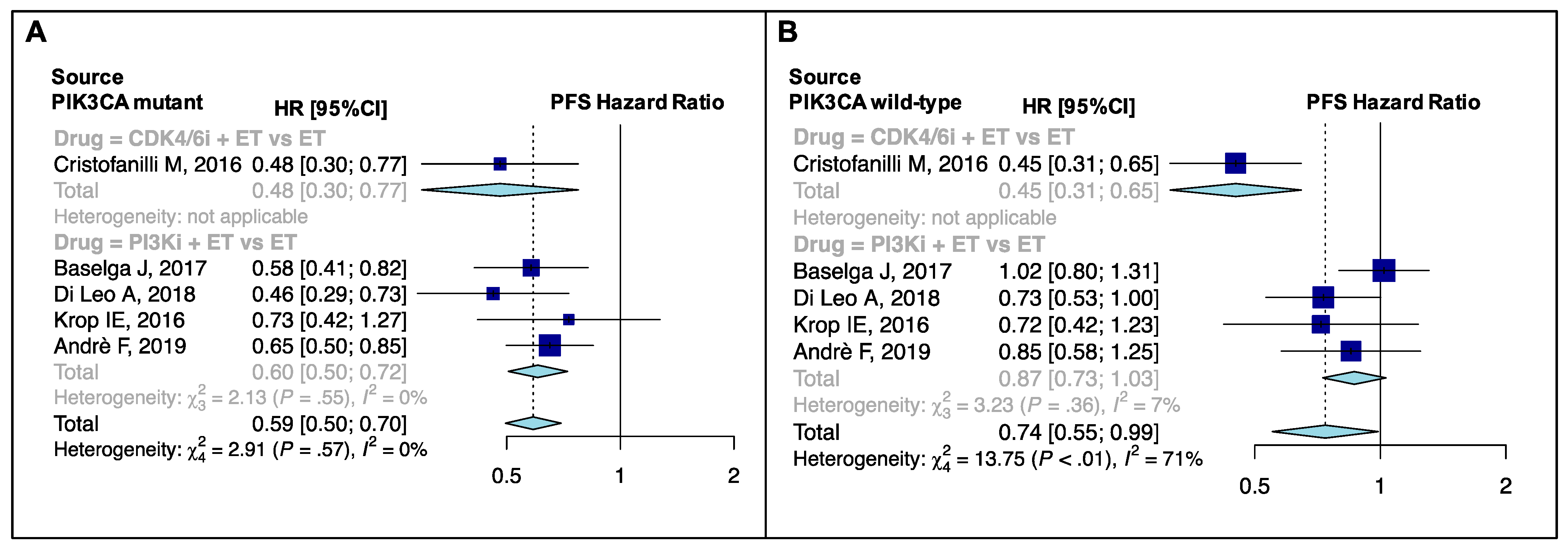
4.3. PIK3CA Mutational Status
4.4. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. S2), S26–S35. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart-Gebhart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III randomized study of Ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Schettini, F.; Giudici, F.; Giuliano, M.; Cristofanilli, M.; Arpino, G.; Del Mastro, L.; Puglisi, F.; De Placido, S.; Paris, I.; De Placido, P.; et al. Overall survival of CDK4/6-inhibitor–based treatments in clinically relevant subgroups of metastatic breast cancer: Systematic review and meta-analysis. J. Natl. Cancer Inst. 2020, 112, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W. Taxanes for the treatment of metastatic breast cancer. Breast Cancer Basic Clin. Res. 2012, 6, 159–171. [Google Scholar] [CrossRef]
- Twelves, C.C.; Cortes, J.; Vahdat, L.L.; Olivo, M.M.; He, Y.Y.; Kaufman, P.P.; Awada, A. Efficacy of eribulin in women with metastatic breast cancer: A pooled analysis of two phase 3 studies. Breast Cancer Res. Treat. 2014, 148, 553–561. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER Statistics for Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 9 January 2020).
- Caswell-Jin, J.L.; Plevritis, S.K.; Tian, L.; Cadham, C.J.; Xu, C.; Stout, N.K.; Sledge, G.W.; Mandelblatt, J.S.; Kurian, A.W. Change in survival in metastatic breast cancer with treatment advances: Meta-analysis and systematic review. JNCI Cancer Spectr. 2018, 2, pky062. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American society of clinical oncology guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines for Breast Cancer. Version 6.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 10 September 2020).
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.; André, F.; Barrios, C.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Wilson, F.R.; Varu, A.; Mitra, D.; Cameron, C.; Iyer, S. Systematic review and network meta-analysis comparing Palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res. Treat. 2017, 166, 167–177. [Google Scholar] [CrossRef]
- Generali, D.; Venturini, S.; Rognoni, C.; Ciani, O.; Pusztai, L.; Loi, S.; Jerusalem, G.; Bottini, A.; Tarricone, R. A network meta-analysis of everolimus plus exemestane versus chemotherapy in the first- and second-line treatment of estrogen receptor-positive metastatic breast cancer. Breast Cancer Res. Treat. 2015, 152, 95–117. [Google Scholar] [CrossRef][Green Version]
- Brandão, M.; Maurer, C.; Ziegelmann, P.K.; Pondé, N.F.; Ferreira, A.; Martel, S.; Piccart, M.; De Azambuja, E.; De Biasi, M.; Lambertini, M. Endocrine therapy-based treatments in hormone receptor-positive/HER2-negative advanced breast cancer: Systematic review and network meta-analysis. ESMO Open 2020, 5, e000842. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO Study. J. Clin. Oncol. 2012, 30, 2718–2724. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.A.S.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.C.; Jonat, W.; Clemons, M.J.; Ito, Y.Y.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Bonneterre, J.; Thürlimann, B.; Robertson, J.R.; Krzakowski, M.; Mauriac, L.; Koralewski, P.; Vergote, I.; Webster, A.; Steinberg, M.; Von Euler, M. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: Results of the tamoxifen or arimidex randomized group efficacy and tolerability study. J. Clin. Oncol. 2000, 18, 3748–3757. [Google Scholar] [CrossRef]
- Buzdar, A.; Douma, J.; Davidson, N.; Elledge, R.; Morgan, M.; Smith, R.; Porter, L.; Nabholtz, J.; Xiang, X.; Brady, C. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J. Clin. Oncol. 2001, 19, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.; Gradishar, W.J.; Mauriac, L.; Bines, J.; Amant, F.; Federico, M.; Fein, L.; Romieu, G.; Buzdar, A.; Robertson, J.F.; et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor–positive, advanced breast cancer: Results from EFECT. J. Clin. Oncol. 2008, 26, 1664–1670. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.-A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor–positive advanced breast cancer. J. Clin. Oncol. 2010, 28, 4594–4600. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boér, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Howell, A.; Robertson, J.F.; Abram, P.; Lichinitser, M.R.; Elledge, R.; Bajetta, E.; Watanabe, T.; Morris, C.; Webster, A.; Dimery, I.; et al. Comparison of Fulvestrant Versus Tamoxifen for the Treatment of Advanced Breast Cancer in Postmenopausal Women Previously Untreated with Endocrine Therapy: A Multinational, Double-Blind, Randomized Trial. J. Clin. Oncol. 2004, 22, 1605–1613. [Google Scholar] [CrossRef]
- Howell, A.; Robertson, J.; Quaresma Albano, J.; Aschermannova, A.; Mauriac, L.; Kleeberg, U.; Vergote, I.; Erikstein, B.; Webster, A.; Morris, C. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J. Clin. Oncol. 2002, 20, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.; Kilburn, L.S.; Ellis, P.; Dodwell, D.; Cameron, D.; Hayward, L.; Im, Y.-H.; Braybrooke, J.P.; Brunt, A.M.; Cheung, K.-L.; et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): A composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013, 14, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Carucci, M.; Casbard, A.C.; Butler, R.; Alchami, F.; Bale, C.J.; Bezecny, P.; Joffe, J.; Moon, S.; Twelves, C.; et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): A randomized, double-blind, placebo-controlled, phase II trial. J. Clin. Oncol. 2019, 37, 1005. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Im, S.-A.; Jung, K.H.; Ro, J.; Sohn, J.; Kim, J.H.; Park, Y.H.; Kim, T.-Y.; Kim, S.-B.; Lee, K.S.; et al. Fulvestrant plus goserelin versus anastrozole plus goserelin versus goserelin alone for hormone receptor-positive, HER2-negative tamoxifen-pretreated premenopausal women with recurrent or metastatic breast cancer (KCSG BR10-04): A multicentre, open-label, three-arm, randomised phase II trial (FLAG study). Eur. J. Cancer 2018, 103, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized phase II Trial of Fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer resistant to aromatase inhibitor therapy: Results of PrE0102. J. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Krop, E.I.E.; Mayer, I.I.; Ganju, V.V.; Dickler, M.M.; Johnston, S.; Morales, S.S.; Yardley, D.D.; Melichar, B.B.; Forero-Torres, A.A.; Lee, S.C.S.; et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Livingston, R.B.; et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N. Engl. J. Med. 2012, 367, 435–444. [Google Scholar] [CrossRef]
- Milla-Santos, A.; Milla, L.; Portella, J.; Rallo, L.; Pons, M.; Rodes, E.; Casanovas, J.; Puig-Gali, M. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: A prospective, randomized, Phase III Study. Am. J. Clin. Oncol. 2003, 26, 317–322. [Google Scholar] [CrossRef]
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Pérez-Carrión, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Jänicke, F.; et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III Study of the international letrozole breast cancer group. J. Clin. Oncol. 2001, 19, 2596–2606. [Google Scholar] [CrossRef]
- Nabholtz, J.; Buzdar, A.; Pollak, M.; Harwin, W.; Burton, G.; Mangalik, A.; Steinberg, M.; Webster, A.; Von Euler, M. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: Results of a North American multicenter randomized trial. J. Clin. Oncol. 2000, 18, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.; Pippen, J.; Jones, S.E.; Parker, L.; Ellis, M.J.C.; Come, S.E.; Gertler, S.Z.; May, J.; Burton, G.V.; Dimery, I.W.; et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J. Clin. Oncol. 2002, 20, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Paridaens, R.J.; Dirix, L.Y.; Beex, L.V.; Nooij, M.; Cameron, D.A.; Cufer, T.; Piccart-Gebhart, M.; Bogaerts, J.; Therasse, P. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The european organisation for research and treatment of cancer breast Cancer Cooperative Group. J. Clin. Oncol. 2008, 26, 4883–4890. [Google Scholar] [CrossRef]
- Robertson, J.F.; Llombart-Cussac, A.; Rolski, J.; Feltl, D.; Dewar, J.; MacPherson, E.; Lindemann, J.; Ellis, M.J. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: Results from the FIRST Study. J. Clin. Oncol. 2009, 27, 4530–4535. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Wolff, A.C.; Lazar, A.A.; Bondarenko, I.; Garin, A.M.; Brincat, S.; Chow, L.; Sun, Y.; Neskovic-Konstantinovic, Z.; Guimaraes, R.C.; Fumoleau, P.; et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2013, 31, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, Z.; Shao, Z.; Wang, J.; Feng, J.; Song, S.; Chen, Z.; Gu, K.; Yu, S.; Zhang, Y.; et al. Fulvestrant 250 mg versus anastrozole for Chinese patients with advanced breast cancer: Results of a multicentre, double-blind, randomised phase III trial. Cancer Chemother. Pharmacol. 2011, 67, 223–230. [Google Scholar] [CrossRef]
- Yardley, D.A.; Ismail-Khan, R.R.; Melichar, B.; Lichinitser, M.; Munster, P.N.; Klein, P.M.; Cruickshank, S.; Miller, K.D.; Lee, M.J.; Trepel, J.B. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 2013, 31, 2128–2135. [Google Scholar] [CrossRef]
- Yardley, D.A.; Noguchi, S.S.; Pritchard, K.I.; Burris, H.H.; Baselga, J.; Gnant, M.; Hortobagyi, G.N.; Campone, M.; Pistilli, B.B.; Piccart-Gebhart, M.; et al. Everolimus plus exemestane in postmenopausal patients with HR+ breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013, 30, 870–884. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Spicer, D.; Lu, J. Overcoming endocrine resistance in metastatic hormone receptor-positive breast cancer. J. Hematol. Oncol. 2018, 11, 80. [Google Scholar] [CrossRef]
- Schettini, F.; Buono, G.; Trivedi, M.V.; De Placido, S.; Arpino, G.; Giuliano, M. PI3K/mTOR Inhibitors in the treatment of luminal breast cancer. why, when and to whom. Breast Care 2017, 12, 290–294. [Google Scholar] [CrossRef] [PubMed]
- McCartney, A.; Migliaccio, I.; Bonechi, M.; Biagioni, C.; Romagnoli, D.; De Luca, F.; Galardi, F.; Risi, E.; De Santo, I.; Benelli, M.; et al. Mechanisms of resistance to CDK4/6 inhibitors: Potential implications and biomarkers for clinical practice. Front. Oncol. 2019, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: A secondary analysis of the BOLERO-2 Clinical Trial. JAMA Oncol. 2016, 2, 1310–1315. [Google Scholar] [CrossRef]
- Connolly, R.M.; Zhao, F.; Miller, K.D.; Lee, M.-J.; Piekarz, R.L.; Smith, K.L.; Brown-Glaberman, U.; Winn, J.S.; Faller, B.A.; Onitilo, A.A.; et al. Abstract GS4-02: E2112: Randomized phase 3 trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. A trial of the ECOG-ACRIN cancer research group. Gen. Sess. Abstr. 2020, 81, GS4–GS02. [Google Scholar]
- Toss, A.; Venturelli, M.; Sperduti, I.; Molinaro, E.; Isca, C.; Barbieri, E.; Piacentini, F.; Omarini, C.; Cortesi, L.; Cascinu, S.; et al. First-line treatment for endocrine-sensitive bone-only metastatic breast cancer: Systematic review and meta-analysis. Clin. Breast Cancer 2019, 19, e701–e716. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.; Ahn, H.K.; Yi, J.H.; Cho, E.Y.; Sun, J.M.; Lee, J.E.; Nam, S.J.; Yang, J.-H.; Park, Y.H.; et al. Implications of bone-only metastases in breast cancer: Favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res. Treat. 2011, 43, 89–95. [Google Scholar] [CrossRef]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): BYLieve study results. J. Clin. Oncol. 2020, 38, 1006. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.; Wilson, T.; Cui, N.; Schimmoller, F.; Hsu, J.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- Tudur Smith, C.; Clarke, M.; Marson, T.; Riley, S.; Tierney, J. A framework for deciding if individual participant data are likely to be worthwhile. In Proceedings of the Cochrane Colloquium, Vienna, Austria, 3–7 October 2015. [Google Scholar]
- Tierney, J.F.; Fisher, D.J.; Burdett, S.; Stewart, L.A.; Parmar, M.K.B. Comparison of aggregate and individual participant data approaches to meta-analysis of randomised trials: An observational study. PLoS Med. 2020, 17, e1003019. [Google Scholar] [CrossRef]
- Tang, J.-L.; Liu, J.L. Misleading funnel plot for detection of bias in meta-analysis. J. Clin. Epidemiol. 2000, 53, 477–484. [Google Scholar] [CrossRef]
- Lau, J.; Ioannidis, J.P.A.; Terrin, N.; Schmid, C.H.; Olkin, I. The case of the misleading funnel plot. BMJ 2006, 333, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: https://training.cochrane.org/handbook/archive/v5.1/ (accessed on 10 September 2020).
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.A.; Van Kampen, R.; Voogd, A.; Dercksen, M.; van den Berkmortel, F.; Smilde, T.; Van De Wouw, A.; Peters, F.; Van Riel, J.; Peters, N.; et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: A study of the Southeast Netherlands Breast Cancer Consortium. Ann. Oncol. 2015, 27, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Mustacchi, G.; Giordano, M.; Garrone, O.; Donadio, M.; Del Mastro, L.; Livi, L.; Natoli, C.; Michelotti, A.; Turletti, A.; et al. 259P adherence to International ESO-ESMO (ABC) guide-lines in HER2-ve metastatic breast cancer (MBC) patients (pts): Preliminary results of the GIM 13—AMBRA Study. Ann. Oncol. 2017, 28, v83–v84. [Google Scholar] [CrossRef]
- André, F.; Neven, P.; Marinsek, N.; Zhang, J.; Baladi, J.-F.; Degun, R.; Benelli, G.; Saletan, S.; Jerusalem, G. Disease management patterns for postmenopausal women in Europe with hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer. Curr. Med. Res. Opin. 2014, 30, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Di Maio, M.; De Angelis, C.; Cinausero, M.; Moroso, S.; Milano, M.; Stanzione, B.; Gargiulo, P.; Iacono, D.; et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: A propensity score analysis. Breast 2017, 31, 114–120. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.; Jonsson, P.-E.; Lidbrink, E.K.; Trudeau, M.; Eiermann, W.; Brattström, D.; Lindemann, J.P.; Wiklund, F.; Henriksson, R. FACT: An Open-Label Randomized Phase III Study of Fulvestrant and Anastrozole in Combination Compared With Anastrozole Alone As First-Line Therapy for Patients With Receptor-Positive Postmenopausal Breast Cancer. J. Clin. Oncol. 2012, 30, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Final Overall Survival: Fulvestrant 500 mg vs 250 mg in the Randomized CONFIRM Trial. J. Natl. Cancer Inst. 2014, 106, djt337. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Llombart-Cussac, A.; Feltl, D.; Dewar, J.A.; Jasiówka, M.; Hewson, N.; Rukazenkov, Y.; Robertson, J.F. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J. Clin. Oncol. 2015, 33, 3781–3787. [Google Scholar] [CrossRef]
- Iwata, H.; Masuda, N.; Ohno, S.; Rai, Y.; Sato, Y.; Ohsumi, S.; Hashigaki, S.; Nishizawa, Y.; Hiraoka, M.; Morimoto, T.; et al. A randomized, double-blind, controlled study of exemestane versus anastrozole for the first-line treatment of postmenopausal Japanese women with hormone-receptor-positive advanced breast cancer. Breast Cancer Res. Treat. 2013, 139, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Bajetta, E.; Dirix, L.Y.; Fein, L.E.; Jones, S.E.; Zilembo, N.; Dugardyn, J.-L.; Nasurdi, C.; Mennel, R.G.; Cervek, J.; et al. Exemestane Is Superior to Megestrol Acetate After Tamoxifen Failure in Postmenopausal Women With Advanced Breast Cancer: Results of a Phase III Randomized Double-Blind Trial. J. Clin. Oncol. 2000, 18, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Klijn, J.G.M.; Beex, L.V.A.M.; Mauriac, L.; Van Zijl, J.A.; Veyret, C.; Wildiers, J.; Jassem, J.; Piccart, M.; Burghouts, J.; Becquart, D.; et al. Combined Treatment With Buserelin and Tamoxifen in Premenopausal Metastatic Breast Cancer: A Randomized Study. J. Natl. Cancer Inst. 2000, 92, 903–911. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Cortés, J.; Paré, L.; Galván, P.; Bermejo, B.; Martínez, N.; Vidal, M.; Pernas, S.; López, R.; Muñoz, M.; et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): An open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017, 18, 545–554. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Lindemann, J.P.O.; Llombart-Cussac, A.; Rolski, J.; Feltl, D.; Dewar, J.; Emerson, L.; Dean, A.; Ellis, M.J. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: Follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res. Treat. 2012, 136, 503–511. [Google Scholar] [CrossRef]
- Rose, C.; Vtoraya, O.; Pluzanska, A.; Davidson, N.; Gershanovich, M.; Thomas, R.; Johnson, S.; Caicedo, J.; Gervasio, H.; Manikhas, G.; et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer. Eur. J. Cancer 2003, 39, 2318–2327. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
| PFS/TTP | ||||||
|---|---|---|---|---|---|---|
| Subset | N. Comparisons b | Pooled HR (95% CI) | I2 (%) | ppooled | pheterogeneity | Publication Bias |
| (Egger’s Test p) | ||||||
| Pre/perimenopausal | 4 | 0.59 (0.45–0.76) | 46.09% | <0.001 | 0.121 | NE |
| Postmenopausal a | 32 | 0.70 (0.63–0.78) | 86.2% | <0.001 | <0.001 | 0.009 |
| Visceral | 19 | 0.68 (0.60–0.77) | 66.50% | <0.001 | <0.001 | 0.47 |
| Non-visceral | 14 | 0.64 (0.57–0.72) | 22.90% | <0.001 | 0.21 | 0.74 |
| Bone-only | 13 | 0.67 (0.53–0.84) | 56.50% | <0.001 | 0.006 | 0.81 |
| Endocrine Sensitive | 18 | 0.62 (0.51–0.75) | 85.40% | <0.001 | <0.001 | 0.15 |
| Endocrine Resistant | 19 | 0.71 (0.62–0.82) | 82.10% | <0.001 | <0.001 | 0.09 |
| Primary Resistance | 4 | 0.63 (0.46–0.86) | 30.90% | 0.004 | 0.23 | NE |
| Secondary Resistance | 4 | 0.60 (0.51–0.71) | 0.00% | <0.001 | 0.75 | NE |
| PIK3CA-mutant | 5 | 0.59 (0.50–0.70) | 0.00% | <0.001 | 0.57 | NE |
| PIK3CA-wild-type | 5 | 0.74 (0.55–0.99) | 70.90% | 0.042 | <0.001 | NE |
| OS | ||||||
| Pre/perimenopausal | 3 | 0.76 (0.60–0.96) | 0.00% | 0.02 | 0.41 | NE |
| Postmenopausal a | 21 | 0.85 (0.77–0.94) | 55.0% | <0.001 | 0.003 | 0.47 |
| Visceral | 6 | 0.80 (0.71–0.91) | 0.00% | <0.001 | 0.77 | NE |
| Non-visceral | 5 | 0.73 (0.62–0.87) | 0.00% | <0.001 | 0.76 | NE |
| Bone-only | 4 | 0.74 (0.58–0.94) | 0.00% | 0.01 | 0.44 | NE |
| Endocrine Sensitive | 9 | 0.80 (0.68–0.93) | 59.9% | 0.005 | 0.01 | 0.44 |
| Endocrine Resistant | 11 | 0.88 (0.81–0.95) | 26.7% | 0.002 | 0.19 | 0.35 |
| PIK3CA-mutant | 2 | 0.80 (0.59–1.03) | 0.00% | 0.08 | 0.76 | NE |
| PIK3CA-wild-type | 2 | 0.99 (0.79–1.24) | 34.70% | 0.94 | 0.21 | NE |
| Subsets | Comparisons | N. Comparisons | Pooled HR | psubgroup | I2 (%) | βmeta-regression | pmeta-regression |
|---|---|---|---|---|---|---|---|
| Postmenopausal a | ET vs. ET | 13 | 0.82 (0.69–0.97) | 0.01 | 87.9% | Reference | 0.01 |
| ET + TTorComb. vs. ET | 19 | 0.63 (0.56–0.71) | 78.8% | −0.16 | |||
| Postmenopausal a | CDK4/6i + ET vs. ET | 7 | 0.55 (0.50–0.61) | 0.01 | 0.0% | Reference | - |
| AI vs. Progestins | 1 | 0.99 (0.79–1.24) | NE | 0.60 | 0.04 | ||
| AI vs. SERM | 3 | 0.44 (0.21–0.92) | 96.2% | −0.08 | 0.70 | ||
| AKTi + ET vs. ET | 1 | 0.58 (0.40–0.85) | NE | 0.07 | 0.84 | ||
| HDACi + ET vs. ET | 1 | 0.73 (0.50–1.07) | NE | 0.30 | 0.38 | ||
| mTORi + ET vs. ET | 4 | 0.58 (0.36–0.94) | 92.1% | 0.07 | 0.68 | ||
| PI3Ki + ET vs. ET | 4 | 0.73 (0.66–0.81) | 0.0% | 0.27 | 0.13 | ||
| SERD + AI vs. AI | 1 | 0.80 (0.68–0.94) | NE | 0.39 | 0.17 | ||
| SERD + AI vs. SERD | 1 | 1.00 (0.83–1.21) | NE | 0.61 | 0.03 | ||
| SERD vs. AI/SERM | 8 | 0.96 (0.85–1.08) | 55.0% | 0.56 | <0.001 | ||
| Visceral | ET vs. ET | 2 | 0.95 (0.79–1.15) | <0.001 | 0.00% | 0.028 | |
| ET+TT or ET comb. vs. ET | 17 | 0.65 (0.57–0.74) | 60.70% | −0.30 | |||
| Visceral | CDK4/6i + ET vs. ET | 8 | 0.55 (0.49–0.61) | <0.001 | 0.00% | Reference | |
| HDACi + ET vs. ET | 1 | 0.76 (0.47–1.22) | NE | 0.36 | 0.19 | ||
| PI3Ki + ET vs. ET | 4 | 0.67 (0.57–0.79) | 16.00% | 0.21 | 0.02 | ||
| SERD + AI vs. AI | 1 | 0.79 (0.63–0.99) | NE | 0.36 | 0.006 | ||
| SERD + AI vs. SERD | 1 | 1.10 (0.87–1.40) | NE | 0.69 | 0.001 | ||
| SERD vs. AI/SERM | 2 | 0.95 (0.79–1.15) | 0.00% | 0.55 | 0.001 | ||
| Non-visceral | ET vs. ET | 1 | 0.59 (0.42–0.84) | 0.63 | NE | Reference | |
| ET+TT or ET comb. vs. ET | 13 | 0.65 (0.57–0.74) | 27.90% | 0.09 | 0.69 | ||
| Non-visceral | CDK4/6i + ET vs. ET | 6 | 0.55 (0.47–0.63) | 0.008 | 0.00% | Reference | |
| PI3Ki + ET vs. ET | 4 | 0.78 (0.64–0.95) | 0.00% | 0.36 | 0.004 | ||
| SERD + AI vs. AI | 1 | 0.84 (0.61–1.15) | NE | 0.43 | 0.01 | ||
| SERD vs. AI/SERM | 1 | 0.59 (0.42–0.84) | NE | 0.08 | 0.67 | ||
| Bone-only | ET vs. ET | 1 | 1.37 (0.83–2.26) | 0.004 | NE | Reference | |
| ET+TT or ET comb. vs. ET | 12 | 0.62 (0.50–0.77) | 41.80% | −0.79 | 0.03 | ||
| Bone-only | CDK4/6i + ET vs. ET | 7 | 0.50 (0.40–0.62) | 0.002 | 10.70% | Reference | |
| HDACi + ET vs. ET | 1 | 0.89 (0.37–2.12) | NE | 0.58 | 0.21 | ||
| PI3Ki + ET vs. ET | 2 | 0.79 (0.47–1.34) | 17.70% | 0.46 | 0.09 | ||
| SERD +AI vs. AI | 1 | 0.77 (0.54–1.10) | NE | 0.44 | 0.07 | ||
| SERD+AI vs. SERD | 1 | 0.99 (0.61–1.60) | NE | 0.69 | 0.018 | ||
| SERD vs. AI/SERM | 1 | 1.37 (0.83–2.56) | NE | 1.01 | 0.001 | ||
| Endocrine Sensitive | ET vs. ET | 5 | 0.59 (0.37–0.94) | 0.79 | 94.90% | Reference | |
| ET + TT or ET comb. vs. ET | 13 | 0.63 (0.53–0.74) | 59.80% | 0.039 | 0.85 | ||
| Endocrine Sensitive | CDK4/6i + ET vs. ET | 7 | 0.52 (0.46–0.59) | <0.001 | 0.00% | Reference | |
| AI vs. SERM | 2 | 0.31 (0.06–1.59) | 97.8% | −0.43 | 0.21 | ||
| HDACi + ET vs. ET | 1 | 0.85 (0.54–1.34) | NE | 0.43 | 0.31 | ||
| mTORi + ET vs. ET | 1 | 0.87 (0.69–1.10) | NE | 0.51 | 0.24 | ||
| PI3Ki + ET vs. ET | 1 | 0.87 (0.35–2.17) | NE | 0.51 | 0.41 | ||
| SERD + AI vs. AI | 1 | 0.80 (0.68–0.94) | NE | 0.43 | 0.32 | ||
| SERD vs. AI/SERM | 3 | 0.87 (0.62–1.22) | 82.9% | 0.50 | 0.08 | ||
| Endocrine Resistance | ET vs. ET | 6 | 0.92 (0.85–1.00) | <0.001 | 0.00% | Reference | |
| ET + TT or ET comb. vs. ET | 13 | 0.60 (0.50–0.72) | 75.40% | −0.43 | 0.001 | ||
| Endocrine Resistance | CDK4/6i + ET vs. ET | 6 | 0.56 (0.49–0.65) | <0.001 | 0.00% | Reference | |
| AKTi + ET vs. ET | 1 | 0.58 (0.40–0.85) | NE | 0.03 | 0.88 | ||
| AI vs. Progestins | 1 | 0.99 (0.79–1.24) | NE | 0.56 | 0.001 | ||
| HDACi + ET vs. ET | 1 | 0.47 (0.23–0.97) | NE | −0.18 | 0.62 | ||
| mTORi + ET vs. ET | 2 | 0.47 (0.29–0.74) | 74.30% | −0.29 | 0.016 | ||
| PI3Ki + ET vs. ET | 2 | 0.69 (0.57–0.84) | 0.00% | 0.2 | 0.092 | ||
| SERD + AI vs. SERD | 1 | 1.00 (0.83–1.21) | NE | 0.57 | 0.001 | ||
| SERD vs. AI/SERM | 4 | 0.95 (0.87–1.05) | 0.00% | 0.53 | 0.001 | ||
| PIK3CA-mutant | CDK4/6i + ET vs. ET | 1 | 0.48 (0.30–0.77) | 0.38 | NE | NE | NE |
| PI3Ki + ET vs. ET | 4 | 0.60 (0.50–0.72) | 0.00% | ||||
| PIK3CA-wild-type | CDK4/6i + ET vs. ET | 1 | 0.45 (0.31–0.65) | 0.001 | NE | NE | NE |
| PI3Ki + ET vs. ET | 4 | 0.87 (0.72–1.03) | 7.20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schettini, F.; Giuliano, M.; Giudici, F.; Conte, B.; De Placido, P.; Venturini, S.; Rognoni, C.; Di Leo, A.; Locci, M.; Jerusalem, G.; et al. Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis. Cancers 2021, 13, 1458. https://doi.org/10.3390/cancers13061458
Schettini F, Giuliano M, Giudici F, Conte B, De Placido P, Venturini S, Rognoni C, Di Leo A, Locci M, Jerusalem G, et al. Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis. Cancers. 2021; 13(6):1458. https://doi.org/10.3390/cancers13061458
Chicago/Turabian StyleSchettini, Francesco, Mario Giuliano, Fabiola Giudici, Benedetta Conte, Pietro De Placido, Sergio Venturini, Carla Rognoni, Angelo Di Leo, Mariavittoria Locci, Guy Jerusalem, and et al. 2021. "Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis" Cancers 13, no. 6: 1458. https://doi.org/10.3390/cancers13061458
APA StyleSchettini, F., Giuliano, M., Giudici, F., Conte, B., De Placido, P., Venturini, S., Rognoni, C., Di Leo, A., Locci, M., Jerusalem, G., Del Mastro, L., Puglisi, F., Conte, P., De Laurentiis, M., Pusztai, L., Rimawi, M. F., Schiff, R., Arpino, G., De Placido, S., ... Generali, D. (2021). Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer: Systematic Review and Meta-Analysis. Cancers, 13(6), 1458. https://doi.org/10.3390/cancers13061458














