One-Year Morbidity Following Videoscopic Inguinal Lymphadenectomy for Stage III Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes and Measurements
2.2. Surgical Procedure
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
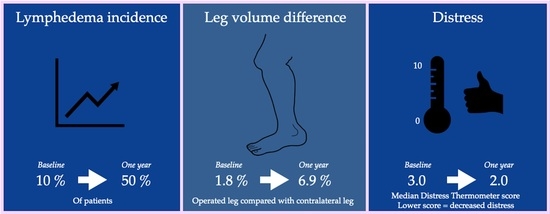
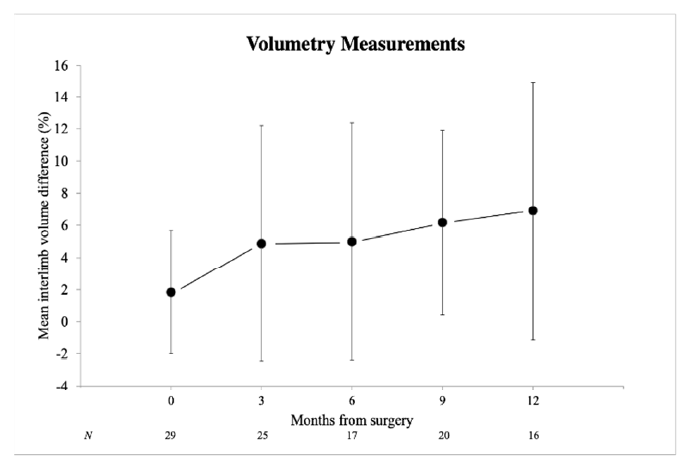
3.1. Lymphedema
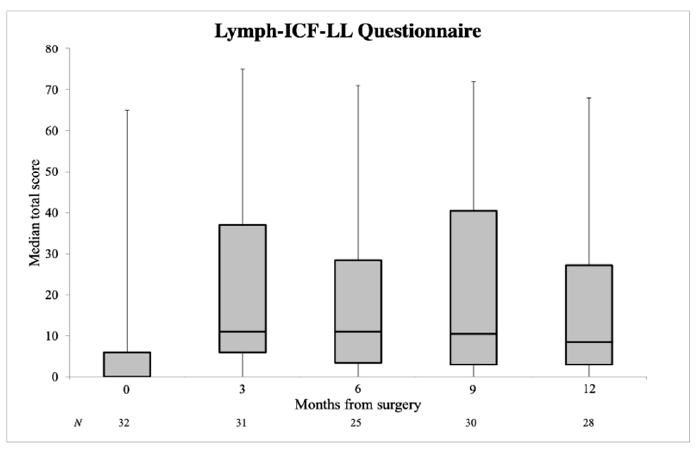
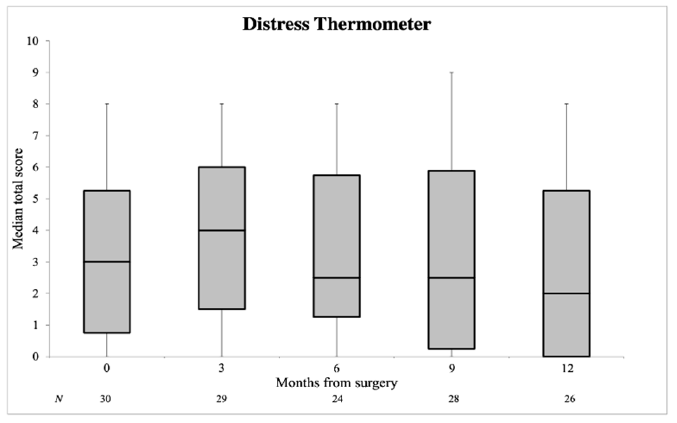
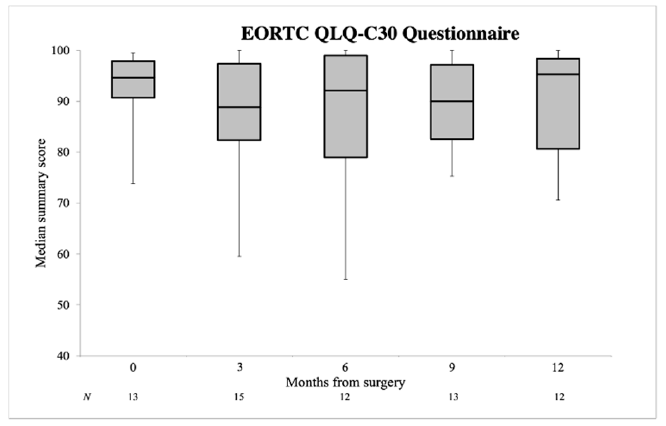
3.2. Quality of Life
4. Discussion
4.1. Lymphedema
4.2. Quality of Life
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Franke, V.; van Akkooi, A.C.J. The extent of surgery for stage III melanoma: How much is appropriate? Lancet Oncol. 2019, 20, e167–e174. [Google Scholar] [CrossRef]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schulte, K.W.; Lehmann, P.; et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 757–767. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- de Vries, M.; Vonkeman, W.G.; van Ginkel, R.J.; Hoekstra, H.J. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur. J. Surg. Oncol. 2006, 32, 785–789. [Google Scholar] [CrossRef]
- de Vries, M.; Hoekstra, H.J.; Hoekstra-Weebers, J.E. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann. Surg. Oncol. 2009, 16, 2840–2847. [Google Scholar] [CrossRef]
- Baas, P.C.; Schraffordt Koops, H.; Hoekstra, H.J.; van Bruggen, J.J.; van der Weele, L.T.; Oldhoff, J. Groin dissection in the treatment of lower-extremity melanoma. Short-term and long-term morbidity. Arch. Surg. 1992, 127, 281–286. [Google Scholar] [CrossRef]
- Poos, H.P.; Kruijff, S.; Bastiaannet, E.; van Ginkel, R.J.; Hoekstra, H.J. Therapeutic groin dissection for melanoma: Risk factors for short term morbidity. Eur. J. Surg. Oncol. 2009, 35, 877–883. [Google Scholar] [CrossRef]
- Guggenheim, M.M.; Hug, U.; Jung, F.J.; Rousson, V.; Aust, M.C.; Calcagni, M.; Künzi, W.; Giovanoli, P. Morbidity and recurrence after completion lymph node dissection following sentinel lymph node biopsy in cutaneous malignant melanoma. Ann. Surg. 2008, 247, 687–693. [Google Scholar] [CrossRef]
- Faut, M.; Heidema, R.M.; Hoekstra, H.J.; van Ginkel, R.J.; Been, S.L.; Kruijff, S.; van Leeuwen, B.L. Morbidity After Inguinal Lymph Node Dissections: It Is Time for a Change. Ann. Surg. Oncol. 2017, 24, 330–339. [Google Scholar] [CrossRef]
- Chang, S.B.; Askew, R.L.; Xing, Y.; Weaver, S.; Gershenwald, J.E.; Lee, J.E.; Royal, R.; Lucci, A.; Ross, M.I.; Cormier, J.N. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann. Surg. Oncol. 2010, 17, 2764–2772. [Google Scholar] [CrossRef]
- Serpell, J.W.; Carne, P.W.; Bailey, M. Radical lymph node dissection for melanoma. ANZ J. Surg. 2003, 73, 294–299. [Google Scholar] [CrossRef]
- Stuiver, M.M.; Westerduin, E.; ter Meulen, S.; Vincent, A.D.; Nieweg, O.E.; Wouters, M.W. Surgical wound complications after groin dissection in melanoma patients—A historical cohort study and risk factor analysis. Eur. J. Surg. Oncol. 2014, 40, 1284–1290. [Google Scholar] [CrossRef]
- Sommariva, A.; Pasquali, S.; Cona, C.; Ciccarese, A.A.; Saadeh, L.; Campana, L.G.; Meroni, M.; Rossi, C.R. Videoscopic ilioinguinal lymphadenectomy for groin lymph node metastases from melanoma. Br. J. Surg. 2016, 103, 1026–1032. [Google Scholar] [CrossRef]
- Solari, N.; Gipponi, M.; Franco, D.S.; Rè, F.; Sonia, O.; Bertoglio, S.; Cafiero, F. Videoscopic Inguinal-iliac-obturator Lymph-node Dissection: New Videoscopic Technique for Regional Lymphadenectomy in Patients with Melanoma. Anticancer Res. 2016, 36, 6579–6583. [Google Scholar] [CrossRef]
- Martin, B.M.; Etra, J.W.; Russell, M.C.; Rizzo, M.; Kooby, D.A.; Staley, C.A.; Master, V.A.; Delman, K.A. Oncologic outcomes of patients undergoing videoscopic inguinal lymphadenectomy for metastatic melanoma. J. Am. Coll. Surg. 2014, 218, 620–626. [Google Scholar] [CrossRef]
- Vrielink, O.M.; Faut, M.; Deckers, E.A.; van Leeuwen, B.L.; Been, L.B. Evaluation of the videoscopic inguinal lymphadenectomy in melanoma patients. Eur. J. Surg. Oncol. 2019, 45, 1712–1716. [Google Scholar] [CrossRef]
- Martin, B.M.; Master, V.A.; Delman, K.A. Videoscopic inguinal lymphadenectomy for metastatic melanoma. Cancer Control. 2013, 20, 255–260. [Google Scholar] [CrossRef]
- Delman, K.A.; Kooby, D.A.; Rizzo, M.; Ogan, K.; Master, V. Initial experience with videoscopic inguinal lymphadenectomy. Ann. Surg. Oncol. 2011, 18, 977–982. [Google Scholar] [CrossRef]
- Delman, K.A.; Kooby, D.A.; Ogan, K.; Hsiao, W.; Master, V. Feasibility of a novel approach to inguinal lymphadenectomy: Minimally invasive groin dissection for melanoma. Ann. Surg. Oncol. 2010, 17, 731–737. [Google Scholar] [CrossRef]
- Postlewait, L.M.; Farley, C.R.; Diller, M.L.; Martin, B.; Squires, M.H.; Russell, M.C.; Rizzo, M.; Ogan, K.; Master, V.; Delman, K. A Minimally Invasive Approach for Inguinal Lymphadenectomy in Melanoma and Genitourinary Malignancy: Long-Term Outcomes in an Attempted Randomized Control Trial. Ann. Surg. Oncol. 2017, 24, 3237–3244. [Google Scholar] [CrossRef]
- Sommariva, A.; Cona, C.; Tonello, M.; Pilati, P.; Rossi, C.R. Oncological outcome of videoscopic groin dissection for lymph node metastasis from melanoma. Surg. Endosc. 2020, 1–7. [Google Scholar] [CrossRef]
- Henderson, M.A.; Gyorki, D.; Burmeister, B.H.; Ainslie, J.; Fisher, R.; Di Iulio, J.; Smithers, B.M.; Hong, A.; Shannon, K.; Scolyer, R.A.; et al. Inguinal and Ilio-inguinal Lymphadenectomy in Management of Palpable Melanoma Lymph Node Metastasis: A Long-Term Prospective Evaluation of Morbidity and Quality of Life. Ann. Surg. Oncol. 2019, 26, 4663–4672. [Google Scholar] [CrossRef]
- Hyngstrom, J.R.; Chiang, Y.J.; Cromwell, K.D.; Ross, M.I.; Xing, Y.; Mungovan, K.S.; Lee, J.E.; Gershenwald, J.E.; Royal, R.E.; Lucci, A.; et al. Prospective assessment of lymphedema incidence and lymphedema-associated symptoms following lymph node surgery for melanoma. Melanoma Res. 2013, 23, 290–297. [Google Scholar] [CrossRef]
- Baur, J.; Mathe, K.; Gesierich, A.; Weyandt, G.; Wiegering, A.; Germer, C.T.; Gasser, M.; Pelz, J.O.W. Morbidity and oncologic outcome after saphenous vein-sparing inguinal lymphadenectomy in melanoma patients. World J. Surg. Oncol. 2017, 15, 99. [Google Scholar] [CrossRef]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H.; van Laarhoven, H.W.; Nijhuis-van der Sanden, M.W.; van der Wees, P.J. Measurement Properties of Instruments for Measuring of Lymphedema: Systematic Review. Phys. Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef]
- Devoogdt, N.; De Groef, A.; Hendrickx, A.; Damstra, R.; Christiaansen, A.; Geraerts, I.; Vervloesem, N.; Vergote, I.; Van Kampen, M. Lymphoedema Functioning, Disability and Health Questionnaire for Lower Limb Lymphoedema (Lymph-ICF-LL): Reliability and validity. Phys. Ther. 2014, 94, 705–721. [Google Scholar] [CrossRef]
- Tuinman, M.A.; Gazendam-Donofrio, S.M.; Hoekstra-Weebers, J.E. Screening and referral for psychosocial distress in oncologic practice: Use of the Distress Thermometer. Cancer 2008, 113, 870–878. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Hopwood, P.; Fletcher, I.; Lee, A.; Al Ghazal, S. A body image scale for use with cancer patients. Eur. J. Cancer 2001, 37, 189–197. [Google Scholar] [CrossRef]
- van Verschuer, V.M.; Vrijland, W.W.; Mares-Engelberts, I.; Klem, T.M. Reliability and validity of the Dutch-translated Body Image Scale. Qual. Life Res. 2015, 24, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Master, V.; Ogan, K.; Kooby, D.; Hsiao, W.; Delman, K. Leg endoscopic groin lymphadenectomy (LEG procedure): Step-by-step approach to a straightforward technique. Eur. Urol. 2009, 56, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.A.; Burmeister, B.H.; Ainslie, J.; Fisher, R.; Di Iulio, J.; Smithers, B.M.; Hong, A.; Shannon, K.; Scolyer, R.A.; Carruthers, S.; et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015, 16, 1049–1060. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Cromwell, K.D.; Chiang, Y.J.; Armer, J.; Heppner, P.P.; Mungovan, K.; Ross, M.I.; Gershenwald, J.E.; Lee, J.E.; Royal, R.E.; Lucci, A.; et al. Is surviving enough? Coping and impact on activities of daily living among melanoma patients with lymphoedema. Eur. J. Cancer Care 2015, 24, 724–733. [Google Scholar] [CrossRef]
- Stoffels, I.; Jansen, P.; Petri, M.; Goerdt, L.; Brinker, T.J.; Griewank, K.G.; Poeppel, T.D.; Schadendorf, D.; Klode, J. Assessment of Nonradioactive Multispectral Optoacoustic Tomographic Imaging with Conventional Lymphoscintigraphic Imaging for Sentinel Lymph Node Biopsy in Melanoma. JAMA Netw. Open 2019, 2, e199020. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
| Patient and Melanoma Characteristics | |
|---|---|
| Gender | |
| Male | 10 (29.4%) |
| Female | 24 (70.6%) |
| Age, years a | 56 (29–78) |
| BMI, kg/m2 | 26.5 (18.0–43.1) |
| Diabetes Mellitus | |
| Yes | 2 (5.9%) |
| No | 32 (94.1%) |
| Smoking | |
| Yes | 6 (17.6%) |
| No | 28 (82.4%) |
| Histologic typing | |
| Superficial spreading | 19 (55.9%) |
| Nodular | 7 (20.6%) |
| Other | 3 (8.8%) |
| Unknown primary | 3 (8.8%) |
| Unknown | 2 (5.9%) |
| Breslow thickness, mm | 2.5 (0.3–10.0) |
| T stage | |
| Tis | 2 (5.9%) |
| T1 (≤1.00 mm) | 3 (8.8%) |
| T2 (1.01–2.00 mm) | 9 (26.5%) |
| T3 (2.01–4.00 mm) | 10 (29.4%) |
| T4 (>4.00 mm) | 7 (20.6%) |
| Unknown primary | 3 (8.8%) |
| Ulceration | |
| Yes | 10 (29.4%) |
| No | 19 (55.9%) |
| Unknown primary | 3 (8.8%) |
| Unknown | 2 (5.9%) |
| Surgical and Postoperative Characteristics | |
| Operation | |
| Videoscopic inguinal lymphadenectomy | 15 (44.1%) |
| Additional open lymphadenectomy | 19 (55.9%) |
| Indication | |
| Micrometastases | 13 (38.2%) |
| Macrometastases | 21 (61.8%) |
| Wound complication | |
| Yes | 24 (70.6%) |
| No | 10 (29.4%) |
| Adjuvant radiotherapy b | |
| Yes | 10 (29.4%) |
| No | 24 (70.6%) |
| Variable | Baseline | 12 months | p-Value |
|---|---|---|---|
| Interlimb volume difference (%) | 1.8 (3.8) | 6.9 (8.1) | 0.041 |
| Lymph-ICF-LL | 0.0 (0.0–6.0) | 8.5 (3.0–27.3) | 0.007 |
| Distress Thermometer | 3.0 (0.8–5.3) | 2.0 (0.0–5.3) | 0.747 |
| Problem List | 4.0 (1.0–6.0) | 3.5 (0.0–10.5) | 0.362 |
| EORTC summary score | 94.7 (90.7–97.9) | 95.3 (80.6–98.3) | 0.203 |
| EORTC quality of life score | 91.6 (83.3–100.0) | 83.3 (75.0–100.0) | 0.206 |
| Body Image Scale | 0.0 (0.0–2.0) | 0.0 (0.0–5.75) | 0.400 |
| Variable | Mean Interlimb Volume Difference in % | B (95% CI) | p-Value |
|---|---|---|---|
| Gender | |||
| Men | 10.6 (12.2) | 5.4 (−3.8–14.5) | 0.230 |
| Women | 5.2 (5.3) | Reference | |
| BMI | |||
| <25 kg/m2 | 7.8 (10.3) | Reference | |
| ≥25 kg/m2 | 5.7 (4.1) | −2.1 (−11.1–6.8) | 0.619 |
| Surgical procedure | |||
| Inguinal | 8.4 (9.9) | Reference | |
| Iliac and inguinal | 5.4 (6.0) | −3.0 (−11.8–5.8) | 0.475 |
| Adjuvant radiotherapy | |||
| Yes | 7.0 (3.6) | 0.1 (−11.4–11.5) | 0.991 |
| No | 6.9 (8.9) | Reference |
| Variable | Median Lymph-ICF-LL Total Score (IQR) | B (95% CI) | p-Value |
|---|---|---|---|
| Gender | |||
| Men | 4.0 (0.75–6.75) | −20.0 (−36.7–−3.2) | 0.021 |
| Women | 18.00 (6.0–50.25) | Reference | |
| BMI | |||
| <25 kg/m2 | 14.0 (2.0–28.0) | Reference | |
| ≥25 kg/m2 | 7.0 (3.0–35.0) | 0.7 (−16.1–17.6) | 0.931 |
| Surgical procedure | |||
| Inguinal | 6.0 (2.5–23.0) | Reference | |
| Iliac and inguinal | 14.0 (6.0–52.0) | 10.5 (−5.8–26.8) | 0.198 |
| Adjuvant radiotherapy | |||
| Yes | 45.0 (7.0–57.0) | 22.3 (5.1–39.5) | 0.013 |
| No | 6.0 (2.5–18.0) | Reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, M.R.; Vrielink, O.M.; Faut, M.; Deckers, E.A.; Been, L.B.; van Leeuwen, B.L. One-Year Morbidity Following Videoscopic Inguinal Lymphadenectomy for Stage III Melanoma. Cancers 2021, 13, 1450. https://doi.org/10.3390/cancers13061450
Jansen MR, Vrielink OM, Faut M, Deckers EA, Been LB, van Leeuwen BL. One-Year Morbidity Following Videoscopic Inguinal Lymphadenectomy for Stage III Melanoma. Cancers. 2021; 13(6):1450. https://doi.org/10.3390/cancers13061450
Chicago/Turabian StyleJansen, Marnix R., Otis M. Vrielink, Marloes Faut, Eric A. Deckers, Lukas B. Been, and Barbara L. van Leeuwen. 2021. "One-Year Morbidity Following Videoscopic Inguinal Lymphadenectomy for Stage III Melanoma" Cancers 13, no. 6: 1450. https://doi.org/10.3390/cancers13061450
APA StyleJansen, M. R., Vrielink, O. M., Faut, M., Deckers, E. A., Been, L. B., & van Leeuwen, B. L. (2021). One-Year Morbidity Following Videoscopic Inguinal Lymphadenectomy for Stage III Melanoma. Cancers, 13(6), 1450. https://doi.org/10.3390/cancers13061450