Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Database
2.2. Population
2.3. Outcomes
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
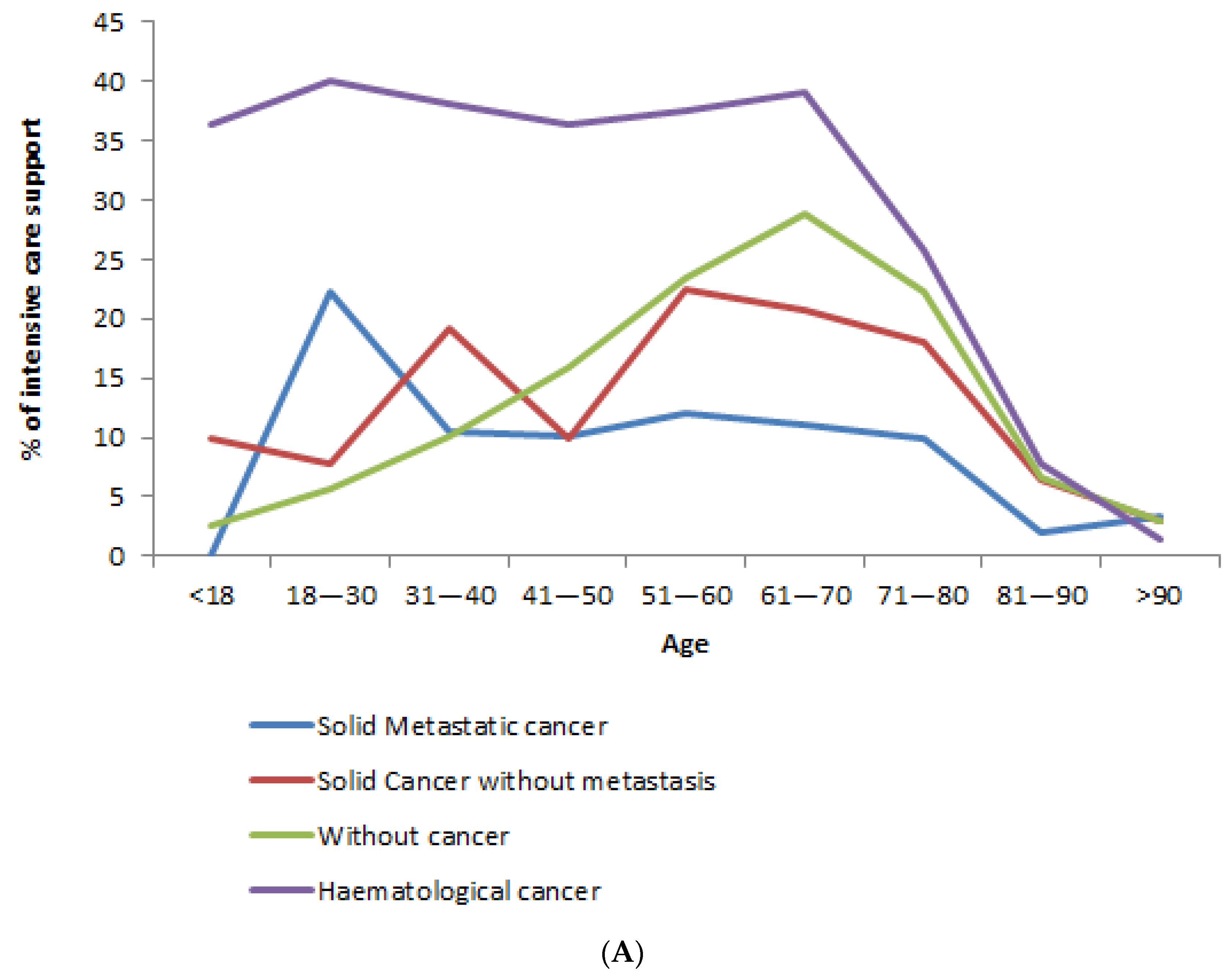
3.2. Admission to ICU
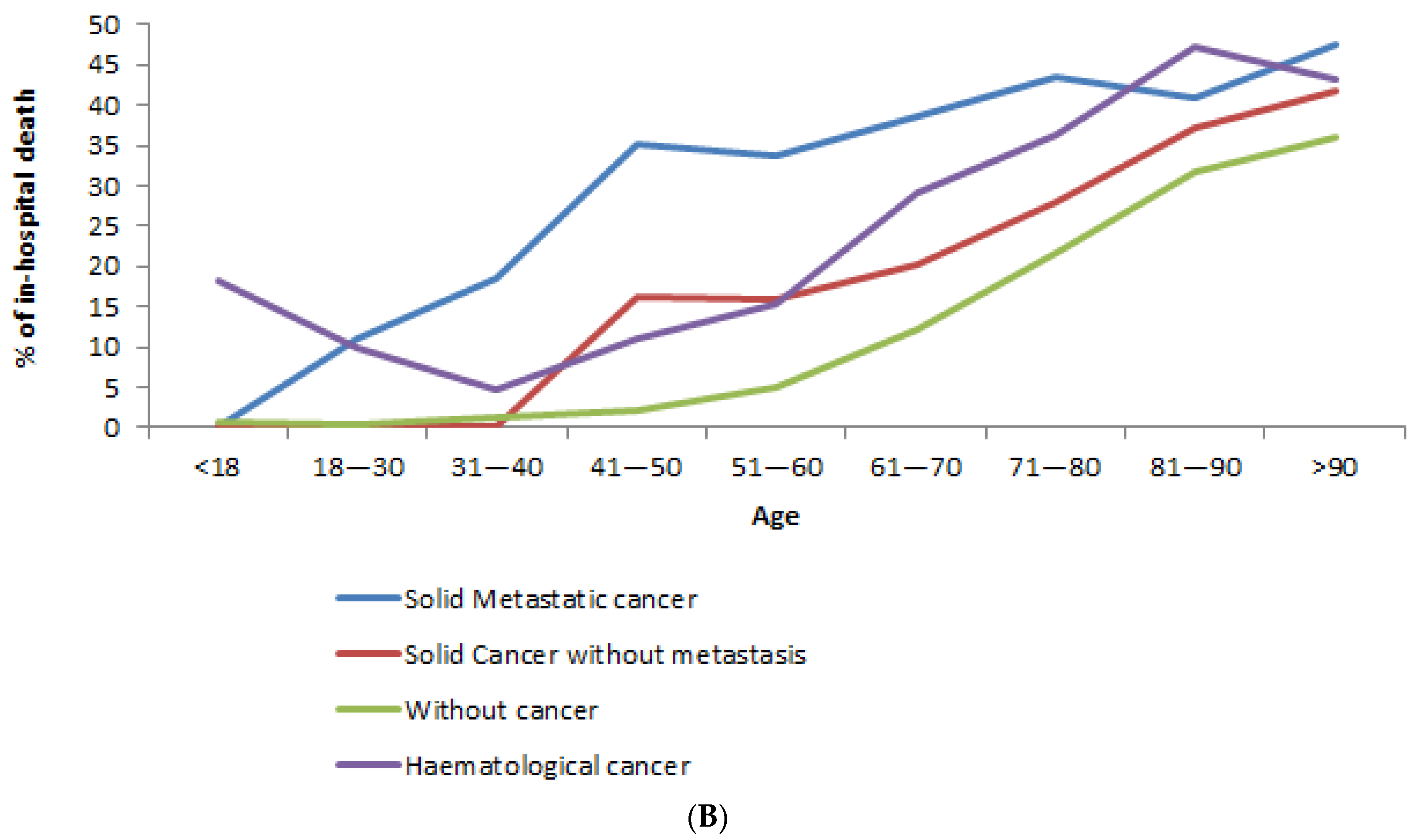
3.3. In-Hospital Mortality
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bénézit, F.; Loubet, P.; Galtier, F.; Pronier, C.; Lenzi, N.; Lesieur, Z.; Jouneau, S.; Lagathu, G.; L’Honneur, A.-S.; Foulongne, V.; et al. Non-influenza respiratory viruses in adult patients admitted with influenza-like illness: A 3-year prospective multicenter study. Infection 2020, 48, 489–495. [Google Scholar] [CrossRef]
- Bernard Stoecklin, S.; Rolland, P.; Silue, Y.; Mailles, A.; Campese, C.; Simondon, A.; Mechain, M.; Meurice, L.; Nguyen, M.; Bassi, C.; et al. First cases of coronavirus disease 2019 (COVID-19) in France: Surveillance, investigations and control measures, January 2020. Eurosurveillance 2020, 25, 2000094. [Google Scholar] [CrossRef]
- Bouadma, L.; Lescure, F.-X.; Lucet, J.-C.; Yazdanpanah, Y.; Timsit, J.-F. Severe SARS-CoV-2 infections: Practical considerations and management strategy for intensivists. Intensive Care Med. 2020, 46, 579–582. [Google Scholar] [CrossRef]
- Santé Publique France. L’épidémie de COVID-19 en Chiffres. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/articles/infection-au-nouveau-coronavirus-sars-cov-2-covid-19-france-et-monde#block-242818 (accessed on 4 October 2020).
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Compared characteristics, morbidity and mortality of COVID-19 and seasonal influenza: A nationwide population-based study. Lancet Respir. Med. 2020, 9, 51–259. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tao, Z.-W.; Lei, W.; Ming-Li, Y.; Kui, L.; Ling, Z.; Shuang, W.; Yan, D.; Jing, L.; Liu, H.-G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [PubMed]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging (Albany N. Y.) 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multi-center study during the COVID-19 outbreak. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Deng, G.; Yin, M.; Chen, X.; Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care 2020, 24, 179. [Google Scholar] [CrossRef]
- Brar, G.; Pinheiro, L.C.; Shusterman, M.; Swed, B.; Reshetnyak, E.; Soroka, O.; Chen, F.; Yamshon, S.; Vaughn, J.; Martin, P.; et al. COVID-19 Severity and Outcomes in Patients With Cancer: A Matched Cohort Study. J. Clin. Oncol. 2020, 38, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Rey, G.; Jougla, E.; Fouillet, A.; Hémon, D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: Variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; De Lima Lopes, F.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 10241, 1907–1918. [Google Scholar] [CrossRef]
- Bryere, J.; Dejardin, O.; Launay, L.; Colonna, M.; Grosclaude, P.; Launoy, G. French Network of Cancer Registries (FRANCIM). Socioeconomic status and site-specific cancer incidence, a Bayesian approach in a French Cancer Registries Network study. Eur. J. Cancer Prev. 2018, 27, 391–398. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019. JAMA Internal Med. 2020, 180, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; Van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- McKinney, E.F.; Smith, K.G.C. Metabolic exhaustion in infection, cancer and autoimmunity. Nat. Immunol. 2018, 19, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Ligabue, G.; Fabbian, L.; Malagoli, C.; Gallo, G.; Lusenti, B.; Nasillo, V.; Manenti, A.; Maiorana, A. Type 3 hypersensitivity in COVID-19 vasculitis. Clin. Immunol. 2020, 108487. [Google Scholar] [CrossRef]
- Moore, D.; Aveyard, P.; Connock, M.; Wang, D.; Fry-Smith, A.; Barton, P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: Systematic review and meta-analysis. BMJ 2009, 338, b1024. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef]
- Abdihamid, O.; Cai, C.; Kapesa, L.; Zeng, S. The landscape of COVID-19 in cancer patients: Prevalence, impacts and recommendations. Cancer Manag. Res. 2020, 12, 8923–8933. [Google Scholar] [CrossRef]
| All Cancer n = 5722 | Solid Metastatic Cancer n = 1775 | Solid Cancer without Metastasis n = 2558 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Hematological | 1.89 | 24.3 | - | - | - | - |
| Lung | 873 | 15.3 | 461 | 26.0 | 412 | 16.1 |
| Digestive (non-colorectal) | 626 | 10.9 | 244 | 13.8 | 382 | 14.9 |
| Prostate | 621 | 10.9 | 196 | 11.0 | 425 | 16.6 |
| Breast | 561 | 9.8 | 241 | 13.6 | 320 | 12.5 |
| Colorectal | 518 | 9.1 | 244 | 13.8 | 274 | 10.7 |
| Urinary tract | 363 | 6.3 | 128 | 7.2 | 235 | 9.2 |
| Other cancers * | 303 | 5.3 | 54 | 3.0 | 249 | 9.7 |
| Female genital organs | 185 | 3.2 | 119 | 6.7 | 66 | 2.6 |
| Lip, oral cavity and pharynx | 162 | 2.8 | 45 | 2.5 | 117 | 4.6 |
| Skin | 121 | 2.1 | 43 | 2.4 | 78 | 3.1 |
| Hematological Cancer | Solid Metastatic Cancer | Solid Cancer without Metastasis | Without Cancer | p-Value * | |
|---|---|---|---|---|---|
| Number of patients | 1389 | 1775 | 2558 | 83,329 | |
| Male gender (n, %) | 797 (57.4) ** | 998 (56.2) ** | 1627 (63.6) ** | 43,787 (52.6) | <0.01 |
| Age | <0.01 | ||||
| Mean +/− std | 72 +/− 15 ** | 70 +/− 13 ** | 74 +/− 13 ** | 65 +/− 20 | |
| Med (Q1–Q3) | 74 (65–83) | 71 (62–80) | 75 (66–84) | 67 (51–81) | |
| Social deprivation score | |||||
| Mean +/− std | −0.44 +/− 1.81 $ | −0.45 +/− 1.72 $ | −0.44 +/− 1.79 $ | −0.26 +/− 1.78 | <0.01 |
| Med [Q1–Q3] | −0.17 [−1.41–0.82] | −0.20 [−1.40–0.75] $ | −0.20 [−1.38–0.78] $ | −0.14 [−1.22–0.93] | |
| Lowest (<−1.233) | 383 (27.3) ** | 504 (28.1) ** | 702 (26.9) ** | 19,661 (24.8) | <0.01 |
| Second ([−1.23; −0.146]) | 332 (23.7) | 443 (24.7) | 672 (25.7) | 19,621 (24.8) | |
| Third ([−0.146; −0.917]) | 369 (26.3) | 463 (25.8) | 658 (25.2) | 19,983 (25.2) | |
| Highest (≥0.917) | 319 (22.7) $ | 382 (21.3) $ | 582 (22.3) $ | 19,980 (25.2) | |
| Hospital type admission | |||||
| Public | 1288 (92.7) ** | 1492 (84.1) $ | 2259 (88.3) | 74,248 (89.1) | <0.01 |
| Private | 101 (7.3) $ | 283 (15.9) ** | 299 (11.7) | 9081 (10.9) | |
| Comorbidities | |||||
| Hypertension | 514 (37.0) ** | 502 (28.3) $ | 1016 (39.7) ** | 27,406 (32.9) | <0.01 |
| Dementia | 81 (5.8) $ | 59 (3.3) $ | 213 (8.3) | 6361 (7.6) | <0.01 |
| HIV | 9 (0.7) | 6 (0.3) | 16 (0.6) | 400 (0.5) | 0.39 |
| Heart failure | 161 (11.6) ** | 128 (7.2) | 246 (9.6) ** | 6553 (7.9) | <0.01 |
| Chronic respiratory disease | 22 (1.6) | 37 (2.1) | 49 (1.9) | 1313 (1.6) | 0.10 |
| Chronic kidney disease | 161 (11.6) ** | 103 (5.8) $ | 303 (11.9) ** | 6838 (8.2) | <0.01 |
| Cirrhosis | 10 (0.7) | 23 (1.3) ** | 90 (3.5) ** | 584 (0.7) | <0.01 |
| Diabetes | 237 (17.1) | 301 (17.0) $ | 587 (23.0) ** | 15,841 (19.0) | < 0.01 |
| Peripheral vascular disease | 47 (3.4) | 67 (3.8) | 159 (6.2) ** | 2572 (3.1) | <0.01 |
| Obese or overweight | 129 (9.3) $ | 73 (4.1) $ | 205 (8.0) $ | 9691 (11.6) | <0.01 |
| Obese | 104 (7.5) $ | 66 (3.7) $ | 170 (6.7) $ | 8257 (9.9) | <0.01 |
| Dyslipidemia | 96 (6.9) ** | 78 (4.4) | 173 (6.8) ** | 4103 (4.9) | <0.01 |
| Deficiency Anemia | 82 (5.9) ** | 73 (4.1) | 159 (6.2) ** | 3162 (3.8) | <0.01 |
| COPD | 68 (4.9) | 116 (6.5) ** | 250 (9.8) ** | 4385 (5.3) | <0.01 |
| Pulmonary bacterial infection | 137 (9.9) ** | 98 (5.5) $ | 177 (6.9) | 5845 (7.0) | 0.05 |
| Complications | |||||
| Acute respiratory failure | 476 (34.3) ** | 519 (29.2) ** | 753 (29.4) ** | 22,436 (26.9) | <0.01 |
| Pulmonary embolism | 59 (4.3) | 86 (4.9) ** | 105 (4.1) ** | 2813 (3.4) | <0.01 |
| Venous thrombosis | 89 (6.5) ** | 115 (6.5) ** | 144 (5.6) ** | 3988 (4.8) | <0.01 |
| Septic shock | 59 (5.0) ** | 26 (1.5) $ | 86 (3.3) | 2356 (2.8) | <0.01 |
| Myocardial infarction | 10 (0.7) | 2 (0.1) $ | 11 (0.4) | 531 (0.6) | 0.01 |
| Atrial fibrillation | 239 (17.2) ** | 217 (12.2) | 440 (17.2) ** | 10,155 (12.2) | <0.01 |
| Stroke | 12 (0.9) | 15 (0.9) | 37 (1.5) | 997 (1.2) | 0.20 |
| Hemorrhagic stroke | 5 (0.4) | 6 (0.3) | 6 (0.2) | 234 (0.3) | 0.82 |
| Ischemic stroke | 5 (0.4) | 6 (0.3) $ | 24 (0.9) | 674 (0.8) | 0.07 |
| Transient Ischemic Attack | 2 (0.1) | 5 (0.3) | 8 (0.3) | 146 (0.2) | 0.17 |
| Acute kidney failure | 157 (11.3) ** | 107 (6.0) | 202 (7.9) * | 5258 (6.3) | <0.01 |
| Intensive care support | 345 (24.8) ** | 157 (8.9) $ | 373 (14.6) $ | 13,655 (16.4) | <0.01 |
| In-hospital death | 470 (33.8) ** | 693 (39.0) ** | 690 (27.0) ** | 13,057 (15.7) | <0.01 |
| <40 | 41–50 | 51–80 | 81–90 | >90 | |
|---|---|---|---|---|---|
| Cancer | 3.6 [2.4–5.6] | 1.2 [0.8–1.7] | 0.7 [0.6–0.8] | 0.8 [0.6–0.9] | 0.8 [0.4–1.7] |
| Without cancer | ref | ref | ref | ref | ref |
| Solid Cancer with metastasis | 1.5 [0.6–4.0] | 0.7 [0.3–1.5] | 0.4 [0.3–0.5] | 0.2 [0.1–0.5] | 1.2 [0.3–4.9] |
| Solid Cancer without metastasis | 2.2 [0.9–5.7] | 0.6 [0.2–1.4] | 0.7 [0.6–0.8] | 0.9 [0.7–1.3] | 1.0 [0.4–2.3] |
| Hematological cancer | 10.4 [5.5–19.9] | 3.7 [2.0–6.7] | 1.5 [1.3–1.8] | 1.0 [0.7–1.5] | 0.5 [0.1–3.4] |
| Without cancer | ref | ref | ref | ref | ref |
| Death | Hospital Mortality Rate | OR | p-Value | aOR * | p-Value | |
|---|---|---|---|---|---|---|
| Cancer | 2047 | 33.0 | 2.7 [2.5–2.8] | <0.01 | 2.2 [2.0–2.3] | <0.01 |
| Withour cancer | 13,057 | 15.7 | ref | ref | ||
| Solid cancer with metastasis | 693 | 39.0 | 3.4 [3.1–3.8] | <0.01 | 3.6 [3.2–4.0] | <0.01 |
| Solid cancer without metastasis | 690 | 27.0 | 2.0 [1.8–2.2] | <0.01 | 1.4 [1.3–1.5] | <0.01 |
| Hematological cancer | 470 | 33.8 | 2.8 [2.5–3.1] | <0.01 | 2.2 [2.0–2.5] | <0.01 |
| Without cancer | 13,057 | 15.7 | ref | ref |
| n | Death | Hospital Mortality Rate | OR | p-Value | aOR * | p-Value | |
|---|---|---|---|---|---|---|---|
| All Cancer | |||||||
| Colorectal | 518 | 142 | 27.4 | ref | – | ref | – |
| Digestive (non-colorectal) | 626 | 233 | 37.2 | 1.6 [1.2–2.0] | <0.01 | 1.6 [1.3–2.1] | <0.01 |
| Breast | 561 | 133 | 23.7 | 0.8 [0.6–1.1] | 0.16 | 1.0 [0.8–1.4] | 0.76 |
| Prostate | 621 | 188 | 30.3 | 1.2 [0.9–1.5] | 0.29 | 0.9 [0.7–1.2] | 0.36 |
| Lung | 873 | 359 | 41.1 | 1.8 [1.5–2.3] | <0.01 | 2.0 [1.6–2.6] | <0.01 |
| Urinary tract | 363 | 122 | 33.6 | 1.3 [1.0–1.8] | 0.049 | 1.2 [0.9–1.7] | 0.15 |
| Female genital organs | 185 | 54 | 29.2 | 1.1 [0.8–1.6] | 0.64 | 1.4 [0.9–2.1] | 0.07 |
| Lip, oral cavity and pharynx | 162 | 38 | 23.5 | 0.8 [0.5–1.2] | 0.32 | 0.9 [0.6–1.4] | 0.73 |
| Skin | 121 | 32 | 26.5 | 1.0 [0.6–1.5] | 0.83 | 0.8 [0.5–1.3] | 0.44 |
| Other cancers ** | 303 | 82 | 27.1 | 1.0 [0.7–1.3] | 0.91 | 1.2 [0.8–1.6] | 0.37 |
| Hematological | 1389 | 470 | 33.8 | 1.4 [1.1–1.7] | 0.01 | 1.4 [1.1–1.8] | <0.01 |
| Solid Cancer with metastasis | |||||||
| Colorectal | 244 | 84 | 34.4 | ref | – | ref | – |
| Digestive (non-colorectal) | 244 | 109 | 44.7 | 1.5 [1.1–2.2] | 0.02 | 1.6 [1.1–2.3] | 0.02 |
| Breast | 241 | 77 | 32.0 | 0.9 [0.6–1.3] | 0.56 | 1.1 [0.8–1.7] | 0.52 |
| Prostate | 196 | 74 | 37.8 | 1.2 [0.8–1.7] | 0.47 | 0.9 [0.6–1.3] | 0.73 |
| Lung | 461 | 215 | 46.6 | 1.7 [1.2–2.3] | <0.01 | 1.7 [1.2–2.4] | <0.01 |
| Urinary tract | 128 | 54 | 42.2 | 1.4 [0.9–2.2] | 0.14 | 1.4 [0.9–2.1] | 0.15 |
| Female genital organs | 119 | 41 | 34.5 | 1.0 [0.6–1.6] | 0.99 | 1.2 [0.7–2.0] | 0.44 |
| Lip, oral cavity and pharynx | 45 | 12 | 26.7 | 0.7 [0.3–1.4] | 0.31 | 0.7 [0.4–1.5] | 0.37 |
| Skin | 43 | 12 | 27.9 | 0.7 [0.4–1.5] | 0.41 | 0.7 [0.4–1.5] | 0.41 |
| Other cancers ** | 54 | 15 | 27.8 | 0.7 [0.4–1.4] | 0.35 | 0.8 [0.4–1.6] | 0.53 |
| Solid Cancer without metastasis | |||||||
| Colorectal | 274 | 58 | 21.2 | ref | – | ref | – |
| Digestive (non-colorectal) | 382 | 124 | 32.5 | 1.8 [1.2–2.6] | <0.01 | 2.0 [1.3–3.0] | <0.01 |
| Breast | 320 | 56 | 17.5 | 0.8 [0.5–1.2] | 0.26 | 1.1 [0.7–1.7] | 0.63 |
| Prostate | 425 | 114 | 26.8 | 1.4 [0.9–2.0] | 0.09 | 1.0 [0.7–1.4] | 0.93 |
| Lung | 412 | 144 | 35.0 | 2.0 [1.4–2.9] | <0.01 | 2.4 [1.7–3.5] | <0.01 |
| Urinary tract | 235 | 68 | 28.9 | 1.5 [1.0–2.3] | 0.04 | 1.3 [0.9–2.0] | 0.20 |
| Female genital organs | 66 | 13 | 19.7 | 0.9 [0.5–1.8] | 0.79 | 1.4 [0.7–2.9] | 0.32 |
| Lip, oral cavity and pharynx | 117 | 26 | 22.2 | 1.1 [0.6–1.8] | 0.82 | 1.5 [0.9–2.6] | 0.12 |
| Skin | 78 | 20 | 25.6 | 1.3 [0.7–2.3] | 0.40 | 1.0 [0.5–1.8] | 0.91 |
| Other cancers ** | 249 | 67 | 26.9 | 1.4 [0.9–2.1] | 0.13 | 2.0 [1.3–3.0] | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, A.; Cottenet, J.; Bonniaud, P.; Piroth, L.; Arveux, P.; Tubert-Bitter, P.; Quantin, C. Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level. Cancers 2021, 13, 1436. https://doi.org/10.3390/cancers13061436
Bernard A, Cottenet J, Bonniaud P, Piroth L, Arveux P, Tubert-Bitter P, Quantin C. Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level. Cancers. 2021; 13(6):1436. https://doi.org/10.3390/cancers13061436
Chicago/Turabian StyleBernard, Alain, Jonathan Cottenet, Philippe Bonniaud, Lionel Piroth, Patrick Arveux, Pascale Tubert-Bitter, and Catherine Quantin. 2021. "Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level" Cancers 13, no. 6: 1436. https://doi.org/10.3390/cancers13061436
APA StyleBernard, A., Cottenet, J., Bonniaud, P., Piroth, L., Arveux, P., Tubert-Bitter, P., & Quantin, C. (2021). Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level. Cancers, 13(6), 1436. https://doi.org/10.3390/cancers13061436








