Biophysical Analysis of Acute and Late Toxicity of Radiotherapy in Gastric Marginal Zone Lymphoma—Impact of Radiation Dose and Planning Target Volume
Abstract
:Simple Summary
Abstract
1. Introduction
Purpose
2. Materials and Methods
2.1. Planning
2.2. Evaluation of Dose Burden for OAR
2.3. Estimation of NTCP
2.4. Here, di and vi Were Taken from the Differential DVH of the Patient
2.5. Estimation of NTCPs
2.6. Estimation of Dose NTCPs Using Data from the Literature
3. Results
3.1. Patients’ Treatment and Outcome
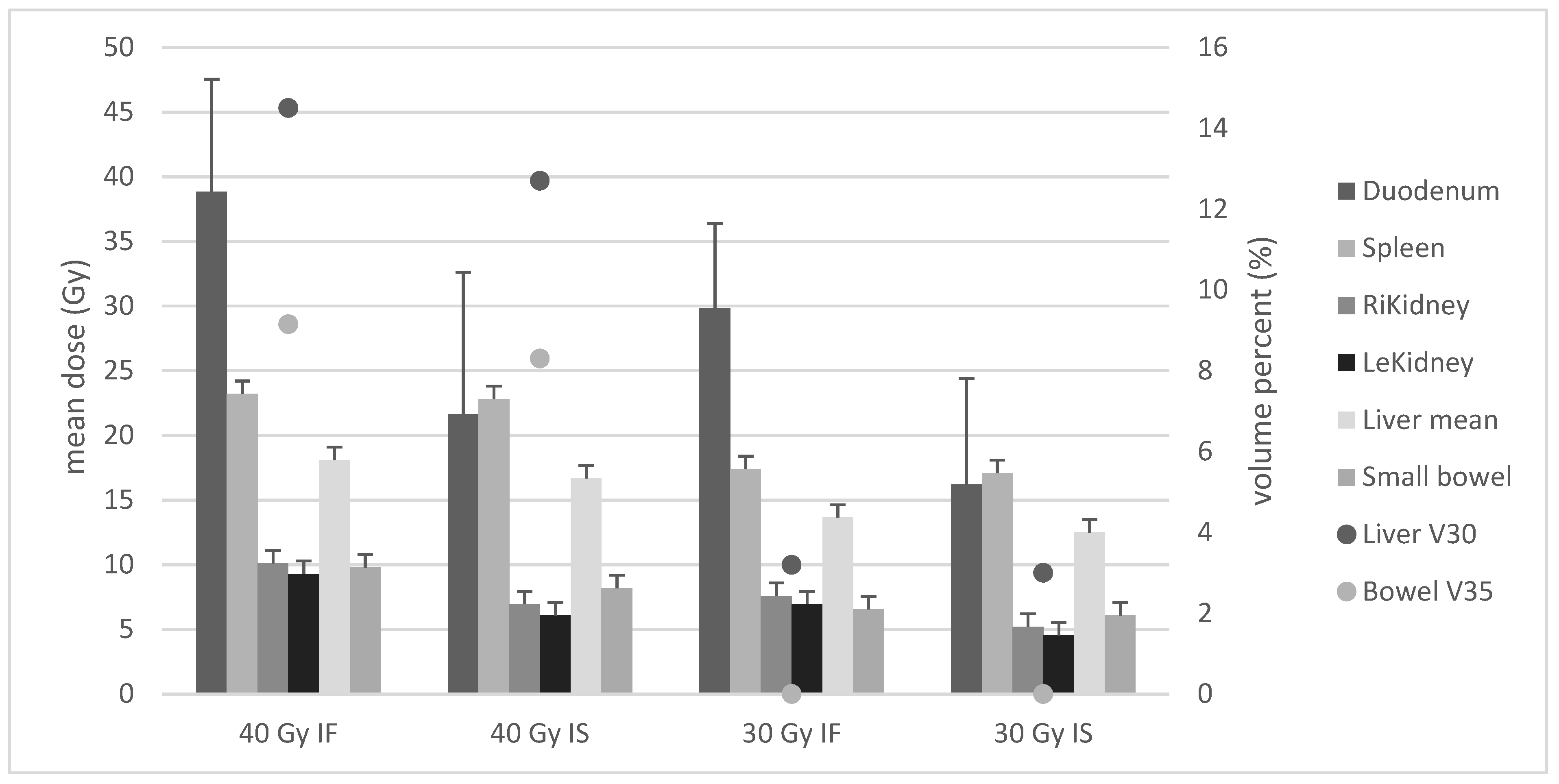
3.2. Dose Exposure According to Radiation Planning
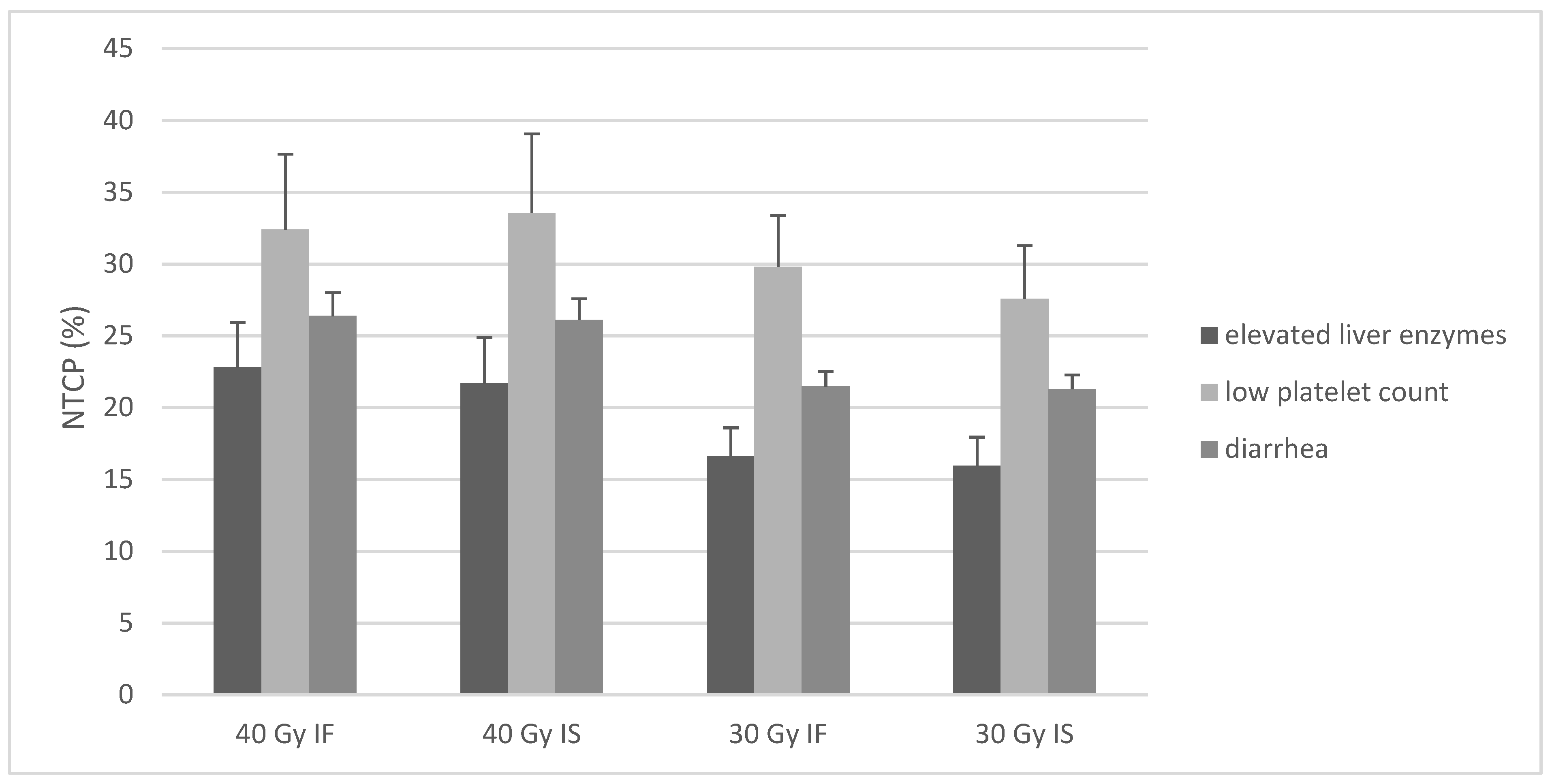
3.3. Estimation of NTCPs
3.4. Estimation of High-Grade Toxicities Using Data from the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahalom, J.; Illidge, T.; Specht, L.; Hoppe, R.T.; Li, Y.-X.; Tsang, R.; Wirth, A. Modern radiation therapy for extranodal lymphomas: Field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, G.; Pyra, R.P.; Lenz, G.; Liersch, R.; Stüben, G.; Micke, O.; Willborn, K.; Hess, C.F.; Probst, A.; Fietkau, R.; et al. Favorable radiation field decrease in gastric marginal zone lymphoma: Experience of the German Study Group on Gastrointestinal Lymphoma (DSGL). Strahlenther. Onkol. 2019, 195, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Schechter, N.R.; Portlock, C.S.; Yahalom, J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. JCO 1998, 16, 1916–1921. [Google Scholar] [CrossRef]
- Goda, J.S.; Gospodarowicz, M.; Pintilie, M.; Wells, W.; Hodgson, D.C.; Sun, A.; Crump, M.; Tsang, R.W. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010, 116, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Lowry, L.; Smith, P.; Qian, W.; Falk, S.; Benstead, K.; Illidge, T.; Linch, D.; Robinson, M.; Jack, A.; Hoskin, P. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomised phase III trial. Radiother. Oncol. 2011, 100, 86–92. [Google Scholar] [CrossRef]
- Teckie, S.; Qi, S.; Lovie, S.; Navarrett, S.; Hsu, M.; Noy, A.; Portlock, C.; Yahalom, J. Long-Term Outcomes and Patterns of Relapse of Early-Stage Extranodal Marginal Zone Lymphoma Treated With Radiation Therapy With Curative Intent. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 130–137. [Google Scholar] [CrossRef]
- Teckie, S.; Qi, S.; Chelius, M.; Lovie, S.; Hsu, M.; Noy, A.; Portlock, C.; Yahalom, J. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann. Oncol. 2017, 28, 1064–1069. [Google Scholar] [CrossRef]
- Oertel, M.; Elsayad, K.; Weishaupt, C.; Steinbrink, K.; Eich, H.T. De-escalated radiotherapy for indolent primary cutaneous B-cell lymphoma. Strahlenther. Onkol. 2020, 196, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, C.; de Jong, D.; Boot, H.; De Boer, J.P.; Wegman, F.; Aleman, B.M. Long-term results of stomach-conserving therapy in gastric MALT lymphoma. Radiother. Oncol. 2008, 87, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Gospodarowicz, M.; Aleman, B.M.P.; Bressel, M.; Ng, A.; Chao, M.; Hoppe, R.T.; Thieblemont, C.; Tsang, R.; Moser, L.; et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: A retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann. Oncol. 2013, 24, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; del Valle, F.; Berdel, W.E.; Willich, N.A.; Reers, B.; Hiddemann, W.; Grothaus-Pinke, B.; Reinartz, G.; Brockmann, J.; Temmesfeld, A.; et al. Primary gastrointestinal non-Hodgkin’s lymphoma: II. Combined surgical and conservative or conservative management only in localized gastric lymphoma—Results of the prospective German Multicenter Study GIT NHL 01/92. J. Clin. Oncol. 2001, 19, 3874–3883. [Google Scholar] [CrossRef]
- Chen, L.; Guerin, A.; Marynchenko, M.; Ionescu-Ittu, R.; Hiscock, R.; Nitulescu, R.; Pooja, C.; Hsu, L.-I.; Keir, C.; Wu, E.Q. Impact Of Low-Grade Adverse Events (AEs) On Health-Related Quality Of Life (HRQoL) In Adult Patients With Newly Diagnosed Philadelphia Chromosome-Positive Chronic Myelogenous Leukemia In Chronic Phase (Ph+ CML-CP) From The Enestnd Trial: 48-Month Follow-Up. Blood 2013, 122, 4038. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.; Shank, B.; Solin, L.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Haken, R.K.T.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of Normal Tissue Complication Probability Models in the Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutcher, G.J.; Burman, C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method gerald. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1623–1630. [Google Scholar] [CrossRef]
- Lyman, J.T. Complication Probability as Assessed from Dose-Volume Histograms. Radiat. Res. Suppl. 1985, 8, S13. [Google Scholar] [CrossRef] [PubMed]
- Eich, H.T.; Haverkamp, U.; Engert, A.; Kocher, M.; Skripnitchenko, R.; Brillant, C.; Sehlen, S.; Dühmke, E.; Diehl, V.; Müller, R.-P. Biophysical analysis of the acute toxicity of radiotherapy in Hodgkin’s lymphoma—A comparison between extended field and involved field radiotherapy based on the data of the German Hodgkin Study Group. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 860–865. [Google Scholar] [CrossRef]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Haken, R.K.T. Radiation-Associated Liver Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S94–S100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.C.-H.; Liu, H.-S.; Wu, J.-K.; Chung, H.-W.; Jan, G.-J. Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Normolle, D.; Balter, J.M.; McGinn, C.J.; Lawrence, T.S.; Haken, R.K.T. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 810–821. [Google Scholar] [CrossRef]
- Kavanagh, B.D.; Pan, C.C.; Dawson, L.A.; Das, S.K.; Li, X.A.; Haken, R.K.T.; Miften, M. Radiation Dose–Volume Effects in the Stomach and Small Bowel. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S101–S107. [Google Scholar] [CrossRef]
- Pan, C.C.; Dawson, L.A.; McGinn, C.J.; Lawrence, T.; Haken, R.T. Analysis of radiation-induced gastric and duodenal bleeds using the Lyman-Kutcher-Burman model. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, S217–S218. [Google Scholar] [CrossRef]
- Holyoake, D.L.P.; Aznar, M.; Mukherjee, S.; Partridge, M.; Hawkins, M.A. Modelling duodenum radiotherapy toxicity using cohort dose-volume-histogram data. Radiother. Oncol. 2017, 123, 431–437. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); Version 5.0; HHS: Washington, DC, USA, 2017.
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 123–135. [Google Scholar] [CrossRef]
- Reinartz, G.; Kardels, B.; Koch, P.; Willich, N. Analysis of failures after whole abdominal irradiation in gastrointestinal lymphomas. Is prophylactic irradiation of inguinal lymph nodes required? German Multicenter Study Group on GI-NHL, University of Muenster. Strahlenther. Onkol. 1999, 175, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Probst, A.; Berdel, W.E.; Willich, N.A.; Reinartz, G.; Brockmann, J.; Liersch, R.; Del Valle, F.; Clasen, H.; Hirt, C.; et al. Treatment Results in Localized Primary Gastric Lymphoma: Data of Patients Registered Within the German Multicenter Study (GIT NHL 02/96). JCO 2005, 23, 7050–7059. [Google Scholar] [CrossRef]
- Reinartz, G.; Tabrizi, C.M.; Liersch, R.; Ullerich, H.; Hering, D.; Willborn, K.; Schultze, J.; Micke, O.; Ruebe, C.; Fischbach, W.; et al. Renaissance of Radiotherapy in Intestinal Lymphoma? 10-Year Efficacy and Tolerance in Multimodal Treatment of 134 Patients: Follow-up of Two German Multicenter Consecutive Prospective Phase II Trials. Oncologist 2020, 25, e816–e832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinartz, G.; Haverkamp, U.; Wullenkord, R.; Lehrich, P.; Kriz, J.; Büther, F.; Schäfers, K.; Schäfers, M.; Eich, H.T. 4D-Listmode-PET-CT and 4D-CT for optimizing PTV margins in gastric lymphoma. Strahlenther. Onkol. 2016, 192, 322–332. [Google Scholar] [CrossRef]
- McCulloch, M.M.; Muenz, D.G.; Schipper, M.J.; Velec, M.; Dawson, L.A.; Brock, K.K. A simulation study to assess the potential impact of developing normal tissue complication probability models with accumulated dose. Adv. Radiat. Oncol. 2018, 3, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Duan, J.; Yu, S.; Ma, C. Dosimetric study of three-dimensional static and dynamic SBRT radiotherapy for hepatocellular carcinoma based on 4DCT image deformable registration. J. Appl. Clin. Med. Phys. 2020, 21, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.; Dennis, K.; De Angelis, C.; Chung, H.; Stinson, J.; Zhang, L.; Bedard, G.; Popović, M.; Lao, N.; Pulenzas, N.; et al. A prospective study of gastrointestinal radiation therapy-induced nausea and vomiting. Support. Care Cancer 2014, 22, 1493–1507. [Google Scholar] [CrossRef]
- Feyer, P.; Jahn, F.; Jordan, K. Radiation induced nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 165–171. [Google Scholar] [CrossRef]
- Katz, M.S. Bystander Effects and Unintended Consequences: Time to Include the Spleen in Radiation Therapy Planning. Front. Oncol. 2020, 10, 1171. [Google Scholar] [CrossRef]
- Dailey, M.O.; Coleman, C.N.; Kaplan, H.S. Radiation-Induced Splenic Atrophy in Patients with Hodgkin’s Disease and Non-Hodgkin’s Lymphomas. N. Engl. J. Med. 1980, 302, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Paule, B.; Cosset, J.M.; Le Bourgeois, J.P.L. The possible role of radiotherapy in chronic lymphocytic leukaemia: A critical review. Radiother. Oncol. 1985, 4, 45–54. [Google Scholar] [CrossRef]
- McFarland, J.T.; Kuzma, C.; Millard, F.E.; Johnstone, P.A.S. Palliative Irradiation of the Spleen. Am. J. Clin. Oncol. 2003, 26, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, M.; Becker, G.; Einsele, H.; Bamberg, M. Clinical indications and biological mechanisms of splenic irradiation in chronic leukaemias and myeloproliferative disorders. Radiother. Oncol. 2001, 58, 235–246. [Google Scholar] [CrossRef]
- Markus, H.; Forfar, J.C. Splenic irradiation in treating warm autoimmune haemolytic anaemia. BMJ 1986, 293, 839–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhao, Q.; Deng, W.; Lu, J.; Xu, X.; Wang, R.; Li, X.; Yue, J. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat. Oncol. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Haken, R.K.T.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An Introduction to the Scientific Issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Tho, L.M.; Glegg, M.; Paterson, J.; Yap, C.; MacLeod, A.; McCabe, M.; McDonald, A.C. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: Investigating dose–volume relationships and role for inverse planning. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 505–513. [Google Scholar] [CrossRef] [PubMed]
| Sex, Number of Patients (n) | |
| Female | 6 |
| Male | 12 |
| Age (Years) | |
| Median | 62.4 |
| Range | 31–85 |
| Stage | |
| I | 9 |
| II1 | 9 |
| II2 | 0 |
| PTV Definition (Original Treatment) | |
| Extended field (abdomen) | 1 |
| Extended field short (abdomen without pelvis) | 5 |
| Involved field | 10 |
| Involved site | 2 |
| Follow-Up (Months) | |
| Mean | 81.6 |
| Range | 13.5–182 |
| Patients deceased (n) | 2 (11%) |
| Overall survival | 89% |
| Lymphoma relapse (n) | 0 (0%) |
| Mean lymphoma-free survival (months) | 81.6 |
| Five-year-overall survival | 94% |
| Five-year lymphoma-specific survival | 100% |
| Early Toxicities, Grade 1–2 (n) | |
|---|---|
| Gastrointestinal Tract | |
| Nausea | 13 (72%) |
| Emesis | 2 (11%) |
| Constipation | 3 (16.7%) |
| Diarrhea | 3 (16.7%) |
| Abdominal pain | 1 (5.5%) |
| Hematopoietic system | |
| Anemia | 2 (11%) |
| Low lymphocyte count | 2 (11%) |
| Low platelet count | 4 (22%) |
| Urinary tract | |
| Increased urinary frequency | 1 (5.5%) |
| Acute infection | 1 (5.5%) |
| Other | |
| Fatigue | 6 (33.3%) |
| Hypokalemia | 1 (5.5%) |
| Early Toxicities, Grade 3–4 (n) | |
| Hematopoietic system | |
| Low platelet count | 1 (5.5%) |
| Low lymphocyte count | 1 (5.5%) |
| Late Toxicities Grade 1–2 (n) | |
| Hepatobiliary system | |
| Elevated transaminases | 5 (27.7%) |
| Hematopoietic system | |
| Low platelet count | 1 (5.5%) |
| Low lymphocyte count | 2 (11%) |
| Gastrointestinal tract | |
| Diarrhea | 1 (5.5%) |
| Heartburn | 2 (11%) |
| Constipation | 2 (11%) |
| Loss of appetite | 1 (5.5%) |
| other | |
| Fatigue | 2 (11%) |
| Late Toxicities, Grade 3–4 (n) | |
| Gastrointestinal tract | |
| Gastric bleeding | 1 (5.5%) |
| Organ | Endpoint | n | m | TD50 (Gy) |
|---|---|---|---|---|
| Spleen | low platelet count | 0.5 | 0.85 | 35.0 |
| Small bowel | diarrhea | 0.15 | 0.79 | 55.0 |
| Small bowel [25] | perforation/obstruction | 0.15 | 0.16 | 55.0 |
| Liver | elevated transaminases | 0.32 | 0.61 | 39.6 |
| Liver [20] | RILD | 0.97 | 0.12 | 45.8 |
| Endpoint | Cohort Toxicity Rate | Estimated NTCP |
|---|---|---|
| Low platelet count | 33% | 32% (SD 5.25) |
| Diarrhea | 22.2% | 26.4% (SD 1.57) |
| Elevated transaminases | 27.7% | 22.8% (SD 3.15) |
| Organ at Risk (OAR) | Endpoint | IF 40 Gy | IS 40 Gy | IF 30 Gy | IS 30 Gy |
|---|---|---|---|---|---|
| Small bowel | ulceration/perforation | 0.06% | 0.04% | 0% | 0% |
| Liver | RILD | 0% | 0% | 0% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinartz, G.; Baehr, A.; Kittel, C.; Oertel, M.; Haverkamp, U.; Eich, H.T. Biophysical Analysis of Acute and Late Toxicity of Radiotherapy in Gastric Marginal Zone Lymphoma—Impact of Radiation Dose and Planning Target Volume. Cancers 2021, 13, 1390. https://doi.org/10.3390/cancers13061390
Reinartz G, Baehr A, Kittel C, Oertel M, Haverkamp U, Eich HT. Biophysical Analysis of Acute and Late Toxicity of Radiotherapy in Gastric Marginal Zone Lymphoma—Impact of Radiation Dose and Planning Target Volume. Cancers. 2021; 13(6):1390. https://doi.org/10.3390/cancers13061390
Chicago/Turabian StyleReinartz, Gabriele, Andrea Baehr, Christopher Kittel, Michael Oertel, Uwe Haverkamp, and Hans Th. Eich. 2021. "Biophysical Analysis of Acute and Late Toxicity of Radiotherapy in Gastric Marginal Zone Lymphoma—Impact of Radiation Dose and Planning Target Volume" Cancers 13, no. 6: 1390. https://doi.org/10.3390/cancers13061390
APA StyleReinartz, G., Baehr, A., Kittel, C., Oertel, M., Haverkamp, U., & Eich, H. T. (2021). Biophysical Analysis of Acute and Late Toxicity of Radiotherapy in Gastric Marginal Zone Lymphoma—Impact of Radiation Dose and Planning Target Volume. Cancers, 13(6), 1390. https://doi.org/10.3390/cancers13061390








