Resistance to Cell Death in Mucinous Colorectal Cancer—A Review
Simple Summary
Abstract
1. Introduction
2. Molecular Characteristics of Mucinous Colorectal Cancer
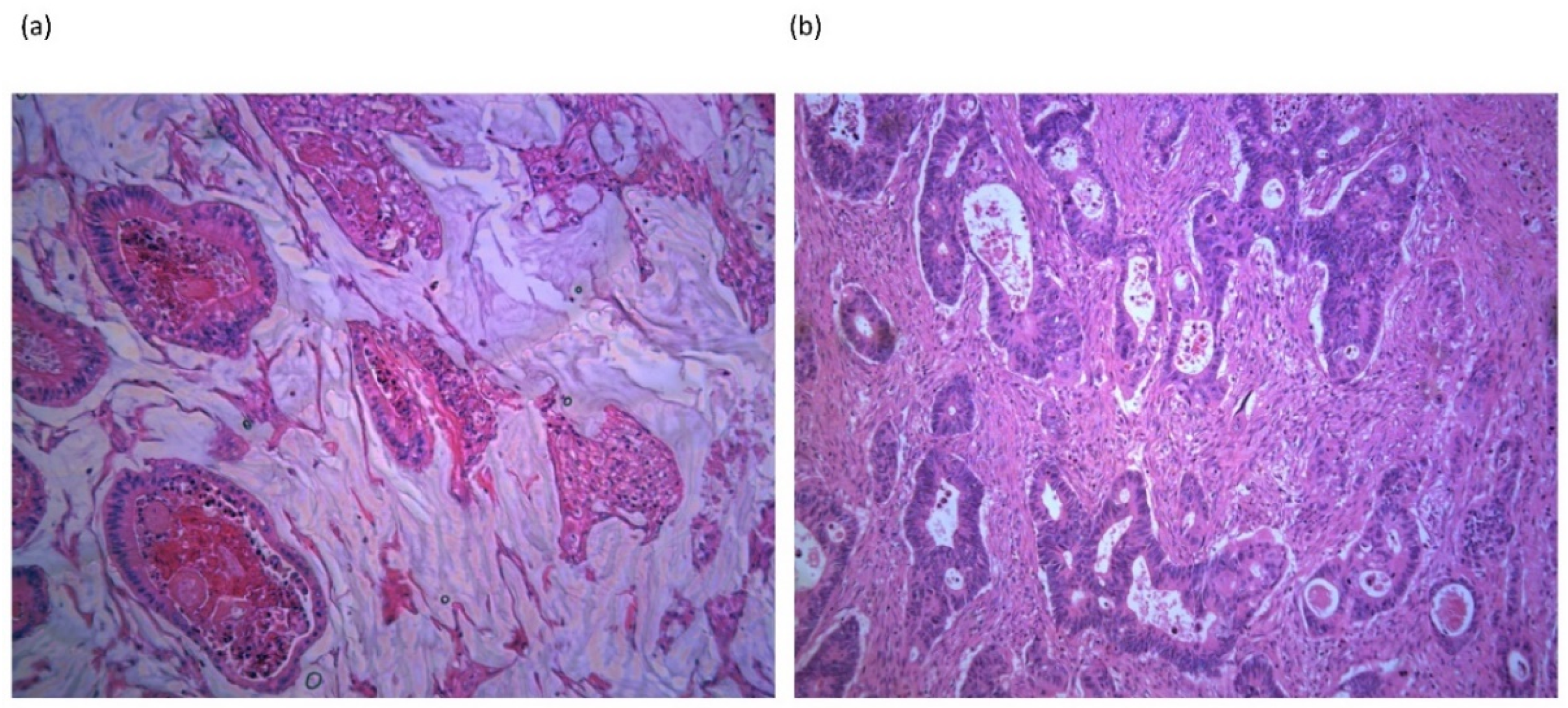
3. Expression of Mucins in Mucinous Colorectal Cancer
4. Acellular Mucin Pools Following Neoadjuvant Chemoradiotherapy
5. Apoptosis and Other Cell Death Signaling Pathways in Mucinous Colorectal Cancer
6. Mechanisms of Apoptosis Resistance in Mucinous Colorectal Cancer: Studies Exploring the Biological Functions of Individual Mucin Glycoproteins
6.1. MUC1 Glycoprotein
6.2. MUC2 Glycoprotein
6.3. MUC5AC Glycoprotein
6.4. MUC13 Glycoprotein
7. Chemotherapy Drug Metabolism and Resistance in Mucinous Colorectal Cancer
8. Radiotherapy Resistance in Mucinous Colorectal Cancer
9. Tumor Immune Microenvironment in Mucinous Colorectal Cancer
10. Clinical Approach to Mucinous Colorectal Cancer
11. Future Perspectives: Targeting Cell Death
Funding
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, M.K.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2019, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; Verhoeven, R.H.A.; Radema, S.A.; De Hingh, I.H.J.T.; Pruijt, J.F.M.; Nagtegaal, I.D.; Lemmens, V.E.P.P.; De Wilt, J.H.W. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann. Oncol. 2013, 24, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Hyngstrom, J.R.; Hu, C.-Y.; Xing, Y.; You, Y.N.; Feig, B.W.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Cormier, J.N.; Chang, G.J. Clinicopathology and Outcomes for Mucinous and Signet Ring Colorectal Adenocarcinoma: Analysis from the National Cancer Data Base. Ann. Surg. Oncol. 2012, 19, 2814–2821. [Google Scholar] [CrossRef]
- Kang, H.; O’Connell, J.B.; Maggard, M.A.; Sack, J.; Ko, C.Y. A 10-Year Outcomes Evaluation of Mucinous and Signet-Ring Cell Carcinoma of the Colon and Rectum. Dis. Colon Rectum 2005, 48, 1161–1168. [Google Scholar] [CrossRef]
- Aust, D.E.; Terdiman, J.P.; Willenbucher, R.F.; Chang, C.G.; Molinaro-Clark, A.; Baretton, G.B.; Loehrs, U.; Waldman, F.M. The APC/?-catenin pathway in ulcerative colitis-related colorectal carcinomas: A mutational analysis. Cancer 2002, 94, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R. Molecular Genetics of Colorectal Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M.; Carethers, J.M. Genomic and Epigenetic Instability in Colorectal Cancer Pathogenesis. Gastroenterology 2008, 135, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.S.; Furney, S.J.; Kay, E.W.; McNamara, D.A.; Prehn, J.H.M.; Burke, J.P. Meta-analysis of the molecular associations of mucinous colorectal cancer. BJS 2019, 106, 682–691. [Google Scholar] [CrossRef]
- Luo, C.; Cen, S.; Ding, G.; Wu, W. Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun. 2019, 39, 13. [Google Scholar] [CrossRef]
- Hugen, N.; Simons, M.; Halilović, A.; Van Der Post, R.S.; Bogers, A.J.; Zanten, M.A.M.-V.; De Wilt, J.H.; Nagtegaal, I.D. The molecular background of mucinous carcinoma beyond MUC2. J. Pathol. Clin. Res. 2014, 1, 3–17. [Google Scholar] [CrossRef]
- Morikawa, T.; Kuchiba, A.; Qian, Z.R.; Mino-Kenudson, M.; Hornick, J.L.; Yamauchi, M.; Imamura, Y.; Liao, X.; Nishihara, R.; Meyerhardt, J.A.; et al. Prognostic Significance and Molecular Associations of Tumor Growth Pattern in Colorectal Cancer. Ann. Surg. Oncol. 2011, 19, 1944–1953. [Google Scholar] [CrossRef]
- Leystra, A.A.; Deming, D.A.; Zahm, C.D.; Farhoud, M.; Olson, T.J.P.; Hadac, J.N.; Nettekoven, L.A.; Albrecht, D.M.; Clipson, L.; Sullivan, R.; et al. Mice Expressing Activated PI3K Rapidly Develop Advanced Colon Cancer. Cancer Res. 2012, 72, 2931–2936. [Google Scholar] [CrossRef]
- Hugen, N.; Van Beek, J.J.P.; De Wilt, J.H.W.; Nagtegaal, I.D. Insight into Mucinous Colorectal Carcinoma: Clues from Etiology. Ann. Surg. Oncol. 2014, 21, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, S.; Lu, H.; Mi, S.; Shao, W.; Zuo, X.; Yin, H.; Zeng, S.; Shimamoto, F.; Qi, G. Expression of survivin, MUC2 and MUC5 in colorectal cancer and their association with clinicopathological characteristics. Oncol. Lett. 2017, 14, 1011–1016. [Google Scholar] [CrossRef]
- Li, L.; Huang, P.-L.; Yu, X.-J.; Bu, X.-D. Clinicopathological significance of mucin 2 immunohistochemical expression in colorectal cancer: A meta-analysis. Chin. J. Cancer Res. 2012, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-D. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J. Gastroenterol. 2010, 16, 4089–4094. [Google Scholar] [CrossRef] [PubMed]
- Debunne, H.; Ceelen, W. Mucinous Differentiation in Colorectal Cancer: Molecular, Histological and Clinical Aspects. Acta Chir. Belg. 2013, 113, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Losi, L.; Scarselli, A.; Benatti, P.; de Leon, M.P.; Roncucci, L.; Pedroni, M.; Borghi, F.; Lamberti, I.; Rossi, G.; Marino, M.; et al. Relationship between MUC5AC and altered expression of MLH1 protein in mucinous and non-mucinous colorectal carcinomas. Pathol. Res. Pract. 2004, 200, 371–377. [Google Scholar] [CrossRef]
- Imai, Y.; Yamagishi, H.; Fukuda, K.; Ono, Y.; Inoue, T.; Ueda, Y. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J. Gastroenterol. 2013, 19, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Okudaira, K.; Kakar, S.; Cun, L.; Choi, E.; DeCamillis, R.W.; Miura, S.; Sleisenger, M.H.; Deng, G.; Kim, Y. MUC2 gene promoter methylation in mucinous and non-mucinous colorectal cancer tissues. Int. J. Oncol. 2010, 36, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Bresalier, R.S. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Ookawa, K.; Kudo, T.; Aizawa, S.; Saito, H.; Tsuchida, S. Transcriptional Activation of the MUC2 Gene by p53. J. Biol. Chem. 2002, 277, 48270–48275. [Google Scholar] [CrossRef] [PubMed]
- Perrais, M.; Pigny, P.; Copin, M.-C.; Aubert, J.-P.; Van Seuningen, I. Induction of MUC2 and MUC5AC Mucins by Factors of the Epidermal Growth Factor (EGF) Family Is Mediated by EGF Receptor/Ras/Raf/Extracellular Signal-regulated Kinase Cascade and Sp1. J. Biol. Chem. 2002, 277, 32258–32267. [Google Scholar] [CrossRef] [PubMed]
- Dilly, A.K.; Song, X.; Zeh, H.J.; Guo, Z.S.; Lee, Y.J.; Bartlett, D.L.; Choudry, H.A. Mitogen-activated protein kinase inhibition reduces mucin 2 production and mucinous tumor growth. Transl. Res. 2015, 166, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; Brown, G.; Glynne-Jones, R.; De Wilt, J.H.W.; Nagtegaal, I.D. Advances in the care of patients with mucinous colorectal cancer. Nat. Rev. Clin. Oncol. 2015, 13, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Cell Death: Apoptosis and Other Means to an End; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2018. [Google Scholar]
- Chand, H.S.; Montano, G.; Huang, X.; Randell, S.H.; Mebratu, Y.; Petersen, H.; Tesfaigzi, Y. A genetic variant of p53 restricts the mucous secretory phenotype by regulating SPDEF and Bcl-2 expression. Nat. Commun. 2014, 5, 5567. [Google Scholar] [CrossRef]
- Contu, P.C.; Contu, S.S.; Moreira, L.F. Bcl-2 expression in rectal cancer. Arq. Gastroenterol. 2006, 43, 284–287. [Google Scholar] [CrossRef][Green Version]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Agata, N.; Chen, D.; Li, Y.; Yu, W.-H.; Huang, L.; Raina, D.; Chen, W.; Kharbanda, S.; Kufe, D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 2004, 5, 163–175. [Google Scholar] [CrossRef]
- Agata, N.; Ahmad, R.; Kawano, T.; Raina, D.; Kharbanda, S.; Kufe, D. MUC1 Oncoprotein Blocks Death Receptor–Mediated Apoptosis by Inhibiting Recruitment of Caspase-8. Cancer Res. 2008, 68, 6136–6144. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.M.; O’Donovan, D.G.; Fitzmaurice, G.; O’Grady, A.; O’Donoghue, D.P.; Sheahan, K.; Byrne, M.F.; Conroy, R.M.; Kay, E.W.; Murray, F.E. Prognostic relevance of Fas (APO-1/CD95) ligand in human colorectal cancer. Eur. J. Gastroenterol. Hepatol. 2003, 15, 375–380. [Google Scholar] [CrossRef]
- Chen, Q.; Li, D.; Ren, J.; Li, C.; Xiao, Z.-X. MUC1 activates JNK1 and inhibits apoptosis under genotoxic stress. Biochem. Biophys. Res. Commun. 2013, 440, 179–183. [Google Scholar] [CrossRef]
- Gupta, B.K.; Maher, D.M.; Ebeling, M.C.; Stephenson, P.D.; Puumala, S.E.; Koch, M.R.; Aburatani, H.; Jaggi, M.; Chauhan, S.C. Functions and regulation of MUC13 mucin in colon cancer cells. J. Gastroenterol. 2013, 49, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.H.; Lourie, R.; Lindén, S.K.; Jeffery, P.L.; Roche, D.; Tran, T.V.; Png, C.W.; Waterhouse, N.; Sutton, P.; Florin, T.H.J.; et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 2011, 60, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Medema, J.P. BCL-2 family deregulation in colorectal cancer: Potential for BH3 mimetics in therapy. Apoptosis 2020, 25, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Evertsson, S.; Sun, X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int. J. Oncol. 1999, 14. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, S.C.; Yu, J.; Carvalho, L.P.; Shannon, W.D.; Fleshman, J.W.; McLeod, H.L. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br. J. Cancer 2005, 92, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.S.; O’Connell, E.; Fichtner, M.; McNamara, D.A.; Kay, E.W.; Prehn, J.H.M.; Furney, S.J.; Burke, J.P. Mucinous adenocarcinoma is a pharmacogenomically distinct subtype of colorectal cancer. Pharmacogenomics J. 2019, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Hansson, M.E.V.J.G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- De Campos-Lobato, L.F.; Dietz, D.W.; Stocchi, L.; Vogel, J.D.; Lavery, I.C.; Goldblum, J.R.; Skacel, M.; Pelley, R.J.; Kalady, M.F. Clinical implications of acellular mucin pools in resected rectal cancer with pathological complete response to neoadjuvant chemoradiation1. Colorectal Dis. 2010, 14, 62–67. [Google Scholar] [CrossRef]
- Smith, K.D.; Tan, D.; Das, P.; Chang, G.J.; Kattepogu, K.; Feig, B.W.; Skibber, J.M.; Rodriguez-Bigas, M.A. Clinical Significance of Acellular Mucin in Rectal Adenocarcinoma Patients With a Pathologic Complete Response to Preoperative Chemoradiation. Ann. Surg. 2010, 251, 261–264. [Google Scholar] [CrossRef]
- Bhatti, A.B.H.; Akbar, A.; Khattak, S.; Kazmi, A.S.; Jamshed, A.; Syed, A.A. Impact of acellular mucin pools on survival in patients with complete pathological response to neoadjuvant treatment in rectal cancer. Int. J. Surg. 2014, 12, 1123–1126. [Google Scholar] [CrossRef]
- Lim, S.-B.; Hong, S.-M.; Yu, C.S.; Hong, Y.S.; Kim, T.W.; Park, J.-H.; Kim, J.H.; Kim, J.C. Prevalence and Clinical Significance of Acellular Mucin in Locally Advanced Rectal Cancer Patients Showing Pathologic Complete Response to Preoperative Chemoradiotherapy. Am. J. Surg. Pathol. 2013, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.S.; McNamara, D.A.; Kay, E.W.; O’Neill, B.; Deasy, J.; Burke, J.P. The significance of mucin pools following neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J. Surg. Oncol. 2018, 118, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, X.; Zhang, Y.; Lin, H.; Lu, X.; Huang, Y.; Chi, P. Pathological complete response may underestimate distant metastasis in locally advanced rectal cancer following neoadjuvant chemoradiotherapy and radical surgery: Incidence, metastatic pattern, and risk factors. Eur. J. Surg. Oncol. 2019, 45, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guan, H.; Luo, Q.; Yuan, L.; Mao, Y.; Wu, X.; Pan, Z.; Lin, J.; Peng, J. Prognostic impact of acellular mucin pools towards the patients with locally advanced rectal cancer achieving pathological complete response after preoperative chemoradiotherapy. Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; pp. 252–254. [Google Scholar]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar] [CrossRef]
- Mardi, K.; Bhardwaj, M.; Kaushal, V.; Sharma, M.; Rao, M. Bcl-2 expression in colorectal carcinoma and its correlation with clinicopathological parameters. Clin. Cancer Investig. J. 2020, 9, 182. [Google Scholar] [CrossRef]
- Miquel, C.; Borrini, F.; Grandjouan, S.; Aupérin, A.; Viguier, J.; Velasco, V.; Duvillard, P.; Praz, F.; Sabourin, J.-C. Role of bax Mutations in Apoptosis in Colorectal Cancers With Microsatellite Instability. Am. J. Clin. Pathol. 2005, 123, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.-S.; Park, Y.-L.; Chung, C.-Y.; Park, H.-C.; Kim, J.-S.; Cho, S.-B.; Lee, W.-S.; Lee, K.-H.; Lee, J.-H.; Joo, Y.-E. Expression of Livin in Colorectal Cancer and Its Relationship to Tumor Cell Behavior and Prognosis. PLoS ONE 2013, 8, e73262. [Google Scholar] [CrossRef]
- Faruk, M.; Ibrahim, S.; Aminu, S.M.; Adamu, A.; Abdullahi, A.; Suleiman, A.M.; Rafindadi, A.H.; Mohammed, A.; Iliyasu, Y.; Idoko, J.; et al. Prognostic significance of BIRC7/Livin, Bcl-2, p53, Annexin V, PD-L1, DARC, MSH2 and PMS2 in colorectal cancer treated with FOLFOX chemotherapy with or without aspirin. PLoS ONE 2021, 16, e0245581. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.; Kharbanda, S.; Kufe, D. The MUC1 Oncoprotein Activates the Anti-apoptotic Phosphoinositide 3-Kinase/Akt and Bcl-xL Pathways in Rat 3Y1 Fibroblasts. J. Biol. Chem. 2004, 279, 20607–20612. [Google Scholar] [CrossRef]
- Koornstra, J.J.; Jalving, M.; Rijcken, F.E.; Westra, J.; Zwart, N.; Hollema, H.; De Vries, E.G.; Hofstra, R.W.; Plukker, J.T.; De Jong, S.; et al. Expression of tumour necrosis factor-related apoptosis-inducing ligand death receptors in sporadic and hereditary colorectal tumours: Potential targets for apoptosis induction. Eur. J. Cancer 2005, 41, 1195–1202. [Google Scholar] [CrossRef]
- Dilly, A.K.; Honick, B.D.; Lee, Y.J.; Guo, Z.S.; Zeh, H.J.; Bartlett, D.L.; Choudry, H.A. Targeting G-protein coupled receptor-related signaling pathway in a murine xenograft model of appendiceal pseudomyxoma peritonei. Oncotarget 2017, 8, 106888–106900. [Google Scholar] [CrossRef] [PubMed]
- Dilly, A.K.; Honick, B.D.; Lee, Y.J.; Bartlett, D.L.; Choudry, H.A. Synergistic apoptosis following endoplasmic reticulum stress aggravation in mucinous colon cancer. Orphanet J. Rare Dis. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Dilly, A.; Honick, B.D.; Lee, Y.J.; Bartlett, D.L.; Choudry, H.A. Rational application of targeted therapeutics in mucinous colon/appendix cancers with positive predictive factors. Cancer Med. 2020, 9, 1753–1767. [Google Scholar] [CrossRef]
- Zhu, X.; Long, X.; Luo, X.; Song, Z.; Li, S.; Wang, H. Abrogation of MUC5AC Expression Contributes to the Apoptosis and Cell Cycle Arrest of Colon Cancer Cells. Cancer Biother. Radiopharm. 2016, 31, 261–267. [Google Scholar] [CrossRef]
- Pothuraju, R.; Rachagani, S.; Krishn, S.R.; Chaudhary, S.; Nimmakayala, R.K.; Siddiqui, J.A.; Ganguly, K.; Lakshmanan, I.; Cox, J.L.; Mallya, K.; et al. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol. Cancer 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Oh, S.; Lee, K.-M.; Yoo, S.-A.; Shin, I. CD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cells. Cell. Signal. 2015, 27, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.H.; He, Y.; Hasnain, S.Z.; Wang, R.; Tong, H.; Clarke, D.T.; Lourie, R.; Oancea, I.; Wong, K.Y.; Lumley, J.W.; et al. MUC13 protects colorectal cancer cells from death by activating the NF-κB pathway and is a potential therapeutic target. Oncogene 2016, 36, 700–713. [Google Scholar] [CrossRef]
- Evertsson, S.; Sun, X.F. Protein expression of NF-kappaB in human colorectal adenocarcinoma. Int. J. Mol. Med. 2002, 10, 547–550. [Google Scholar]
- McCawley, N.; Clancy, C.; O’Neill, B.D.P.; Deasy, J.; McNamara, D.A.; Burke, J.P. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis. Colon Rectum 2016, 59, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Shin, U.S.; Yu, C.S.; Kim, J.H.; Kim, T.W.; Lim, S.-B.; Yoon, S.N.; Yoon, Y.S.; Kim, C.W.; Kim, J.C. Mucinous Rectal Cancer: Effectiveness of Preoperative Chemoradiotherapy and Prognosis. Ann. Surg. Oncol. 2011, 18, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; Van De Velde, C.J.; Bosch, S.L.; Fütterer, J.J.; Elferink, M.A.; Marijnen, C.A.; Rutten, H.J.; De Wilt, J.H.; Nagtegaal, I.D. Modern Treatment of Rectal Cancer Closes the Gap Between Common Adenocarcinoma and Mucinous Carcinoma. Ann. Surg. Oncol. 2015, 22, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Gash, K.; Baser, O.; Kiran, R. Factors associated with degree of tumour response to neo-adjuvant radiotherapy in rectal cancer and subsequent corresponding outcomes. Eur. J. Surg. Oncol. 2017, 43, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Nguyen, N.; Mohammadianpanah, M.; Mirzaei, S.; Bananzadeh, A.M. Predictive Significance of Mucinous Histology on Pathologic Complete Response Rate Following Capecitabine-Based Neoadjuvant Chemoradiation in Rectal Cancer: A Comparative Study. J. Gastrointest. Cancer 2018, 50, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, M.; Renkonen-Sinisalo, L.; Mustonen, H.; Lepistö, A. Is there a need for neoadjuvant short-course radiotherapy in T3 rectal cancer with positive lymph node involvement? A single-center retrospective cohort study. World J. Surg. Oncol. 2019, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vernmark, K.; Sun, X.-F.; Holmqvist, A. Mucinous and Non-Mucinous Rectal Adenocarcinoma—Differences in Treatment Response to Preoperative Radiotherapy. J. Personal. Med. 2020, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, K.; Imam, I.; Mezheyeuski, A.; Ekström, J.; Sjöblom, T.; Glimelius, B. A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo) Radiotherapy in Rectal Cancer. Cancers 2020, 13, 16. [Google Scholar] [CrossRef]
- Tozawa, E.; Ajioka, Y.; Watanabe, H.; Nishikura, K.; Mukai, G.; Suda, T.; Kanoh, T.; Hatakeyama, K. Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: Is mucinous carcinoma a distinct histological entity? Pathol. Res. Pract. 2007, 203, 567–574. [Google Scholar] [CrossRef]
- Nazemalhosseini-Mojarad, E.; Mohammadpour, S.; Esafahani, A.T.; Gharib, E.; Larki, P.; Moradi, A.; Porhoseingholi, M.A.; Aghdaei, H.A.; Kuppen, P.J.K.; Zali, M.R. Intratumoral infiltrating lymphocytes correlate with improved survival in colorectal cancer patients: Independent of oncogenetic features. J. Cell. Physiol. 2018, 234, 4768–4777. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, X.; Xu, X.; Yu, S.; Wang, J.; Sun, L. Prognostic value and clinicopathological roles of phenotypes of tumour-associated macrophages in colorectal cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 3005–3019. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Gong, J.; Wang, C.; Lee, P.P.; Chu, P.; Fakih, M. Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring aPOLEMutation. J. Natl. Compr. Cancer Netw. 2017, 15, 142–147. [Google Scholar] [CrossRef]
- Khan, M.; Loree, J.M.; Advani, S.M.; Ning, J.; Li, W.; Pereira, A.; Lam, M.; Raghav, K.; Morris, V.K.; Broaddus, R.; et al. Prognostic Implications of Mucinous Differentiation in Metastatic Colorectal Carcinoma Can Be Explained by Distinct Molecular and Clinicopathologic Characteristics. Clin. Colorectal. Cancer 2018, 17, e699–e709. [Google Scholar] [CrossRef]
- Jung, D.H.; Park, H.J.; Jang, H.H.; Kim, S.-H.; Jung, Y.; Lee, W.-S. Clinical Impact of PD-L1 Expression for Survival in Curatively Resected Colon Cancer. Cancer Investig. 2020, 38, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Luber, B.; Siegel, N.; Awan, A.H.; Oke, T.; Zhu, Q.; Bartlett, B.R.; Aulakh, L.K.; Thompson, E.D.; Jaffee, E.M.; et al. Immunopathologic Stratification of Colorectal Cancer for Checkpoint Blockade Immunotherapy. Cancer Immunol. Res. 2019, 7, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Bouillez, A.; Rajabi, H.; Jin, C.; Samur, M.; Tagde, A.; Alam, M.; Hiraki, M.; Maeda, T.; Hu, X.; Adeegbe, D.; et al. MUC1-C integrates PD-L1 induction with repression of immune effectors in non-small-cell lung cancer. Oncogene 2017, 36, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Bouillez, A.; Adeegbe, D.; Jin, C.; Hu, X.; Tagde, A.; Alam, M.; Rajabi, H.; Wong, K.-K.; Kufe, D. MUC1-C promotes the suppressive immune microenvironment in non-small cell lung cancer. OncoImmunology 2017, 6, e1338998. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, M.-H.; Jiang, Y.; Chen, Z.-P.; Wang, Q.; Wei, J.-C.; Li, W.-L.; Cao, J. Outcomes of Laparoscopic Surgery for Mucinous Colorectal Adenocarcinoma. J. Laparoendosc. Adv. Surg. Tech. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sengul, N.; Wexner, S.D.; Woodhouse, S.; Arrigain, S.; Xu, M.; Larach, J.A.; Ahn, B.K.; Weiss, E.G.; Nogueras, J.J.; Berho, M. Effects of radiotherapy on different histopathological types of rectal carcinoma. Colorectal. Dis. 2006, 8, 283–288. [Google Scholar] [CrossRef]
- Yu, S.K.; Chand, M.; Tait, D.M.; Brown, G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur. J. Cancer 2014, 50, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Park, J.S.; Park, S.I.; Kim, N.K.; Kim, J.H.; Moon, H.J.; Park, Y.N.; Kim, W.H. Accuracy in Differentiation of Mucinous and Nonmucinous Rectal Carcinoma on MR Imaging. J. Comput. Assist. Tomogr. 2003, 27, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, J.; Ferdinande, L.; Demetter, P.; Ceelen, W. Mucinous subtype as prognostic factor in colorectal cancer: A systematic review and meta-analysis. J. Clin. Pathol. 2012, 65, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Ping, J.; Li, Y.; Holmqvist, A.; Adell, G.; Arbman, G.; Zhang, H.; Zhou, Z.-G.; Sun, X.-F. Prognostic Significance and Molecular Features of Colorectal Mucinous Adenocarcinomas: A Strobe-Compliant Study. Medicine 2015, 94, e2350. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Mathieson, A. Epidemiology of Mucinous Adenocarcinomas. Cancers 2020, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
- Soderquist, R.S.; Crawford, L.; Liu, E.; Lu, M.; Agarwal, A.; Anderson, G.R.; Lin, K.H.; Winter, P.S.; Cakir, M.; Wood, K.C. Systematic mapping of BCL-2 gene dependencies in cancer reveals molecular determinants of BH3 mimetic sensitivity. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]

| Mechanism | Effect | Reference |
|---|---|---|
| Physical tumor structure | Mucin barrier occluding cells | [29] |
| Compressive effect of mucin | ||
| Mucin glycoproteins | Inhibition of mitochondrial apoptotic signaling | [30,31,32] |
| Inhibition of death receptor apoptotic signaling. | [33,34,35] | |
| Downregulation of p53 | [36,37] | |
| Activation of JNK1 | ||
| Activation of PI3K/Akt pathway Activation of MAPK pathway | ||
| Activation of NF-κB pathway | ||
| BCL-2 proteins | Increased expression of BCL-XL | [31,37,38] |
| Bax G(8) frameshift mutation | [39] | |
| Increased Livin (IAP) expression | ||
| Death receptors | Reduced Fas ligand expression | [40,41] |
| Reduced DR4 expression | ||
| Chemo-resistance genes | Overexpression of TYMS | [42,43] |
| Altered mutation rate of genes responsible for chemotherapy metabolism | ||
| Tumor immune microenvironment | Adhesion ligands and suppressive cytokines inhibiting immune cell activity | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, E.; Reynolds, I.S.; McNamara, D.A.; Burke, J.P.; Prehn, J.H.M. Resistance to Cell Death in Mucinous Colorectal Cancer—A Review. Cancers 2021, 13, 1389. https://doi.org/10.3390/cancers13061389
O’Connell E, Reynolds IS, McNamara DA, Burke JP, Prehn JHM. Resistance to Cell Death in Mucinous Colorectal Cancer—A Review. Cancers. 2021; 13(6):1389. https://doi.org/10.3390/cancers13061389
Chicago/Turabian StyleO’Connell, Emer, Ian S. Reynolds, Deborah A. McNamara, John P. Burke, and Jochen H. M. Prehn. 2021. "Resistance to Cell Death in Mucinous Colorectal Cancer—A Review" Cancers 13, no. 6: 1389. https://doi.org/10.3390/cancers13061389
APA StyleO’Connell, E., Reynolds, I. S., McNamara, D. A., Burke, J. P., & Prehn, J. H. M. (2021). Resistance to Cell Death in Mucinous Colorectal Cancer—A Review. Cancers, 13(6), 1389. https://doi.org/10.3390/cancers13061389








