Real-World Evaluation of Modern Adjuvant Radiotherapy in Women with Stage IB Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Patient Selection
2.2. Surgery
2.3. Chemotherapy
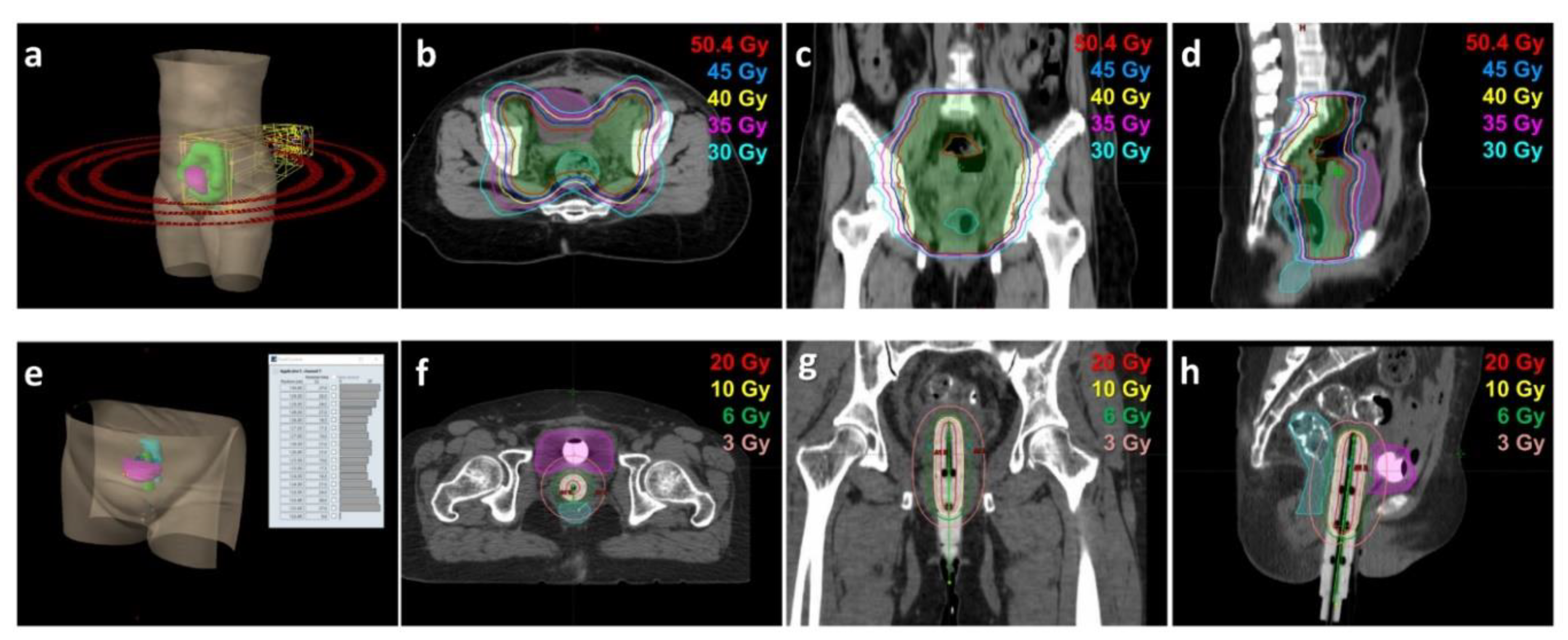
2.4. Radiotherapy
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
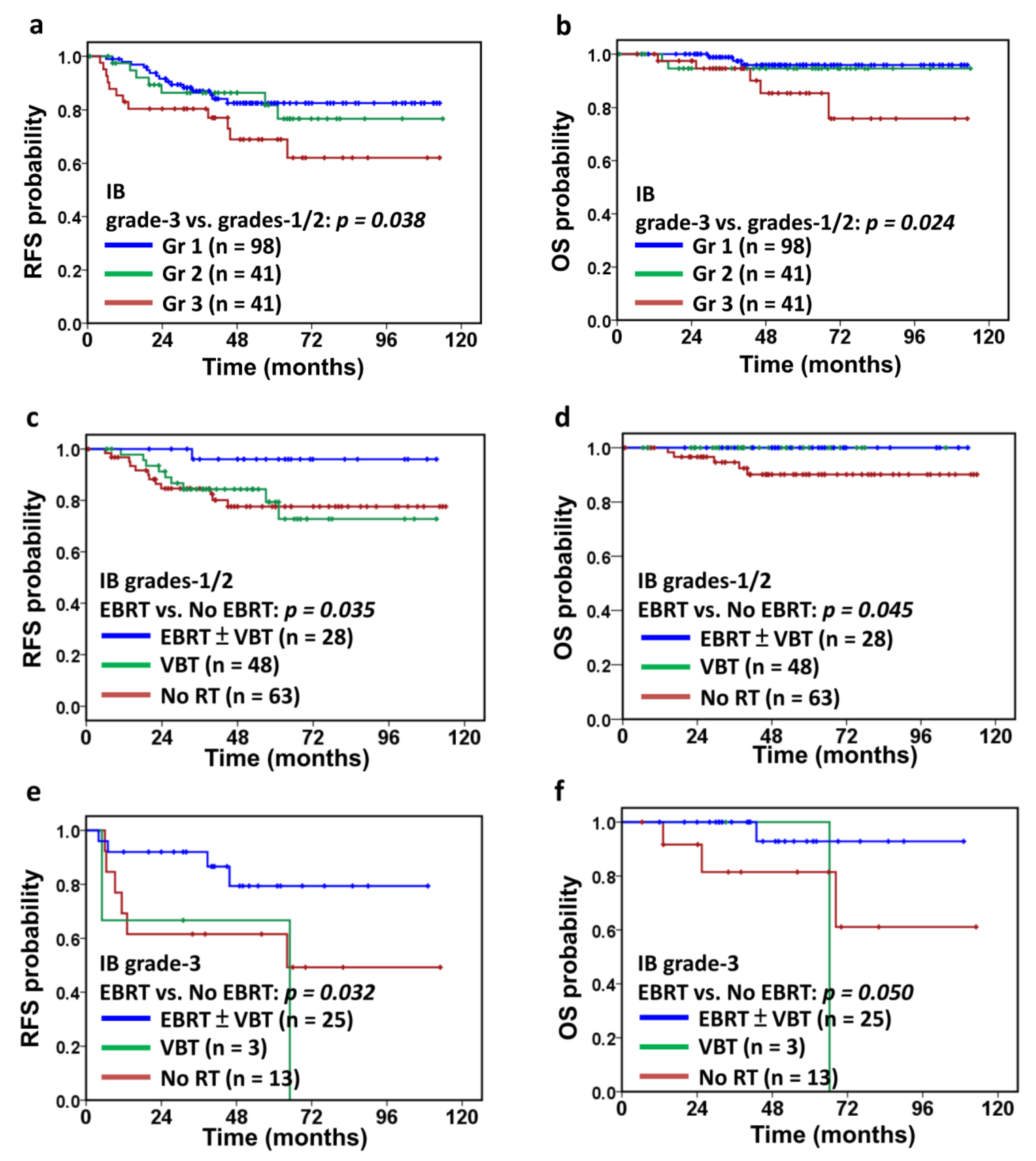
3.2. Adjuvant Treatments and Outcomes
3.3. Parameters Affecting Survivals in IB Patients
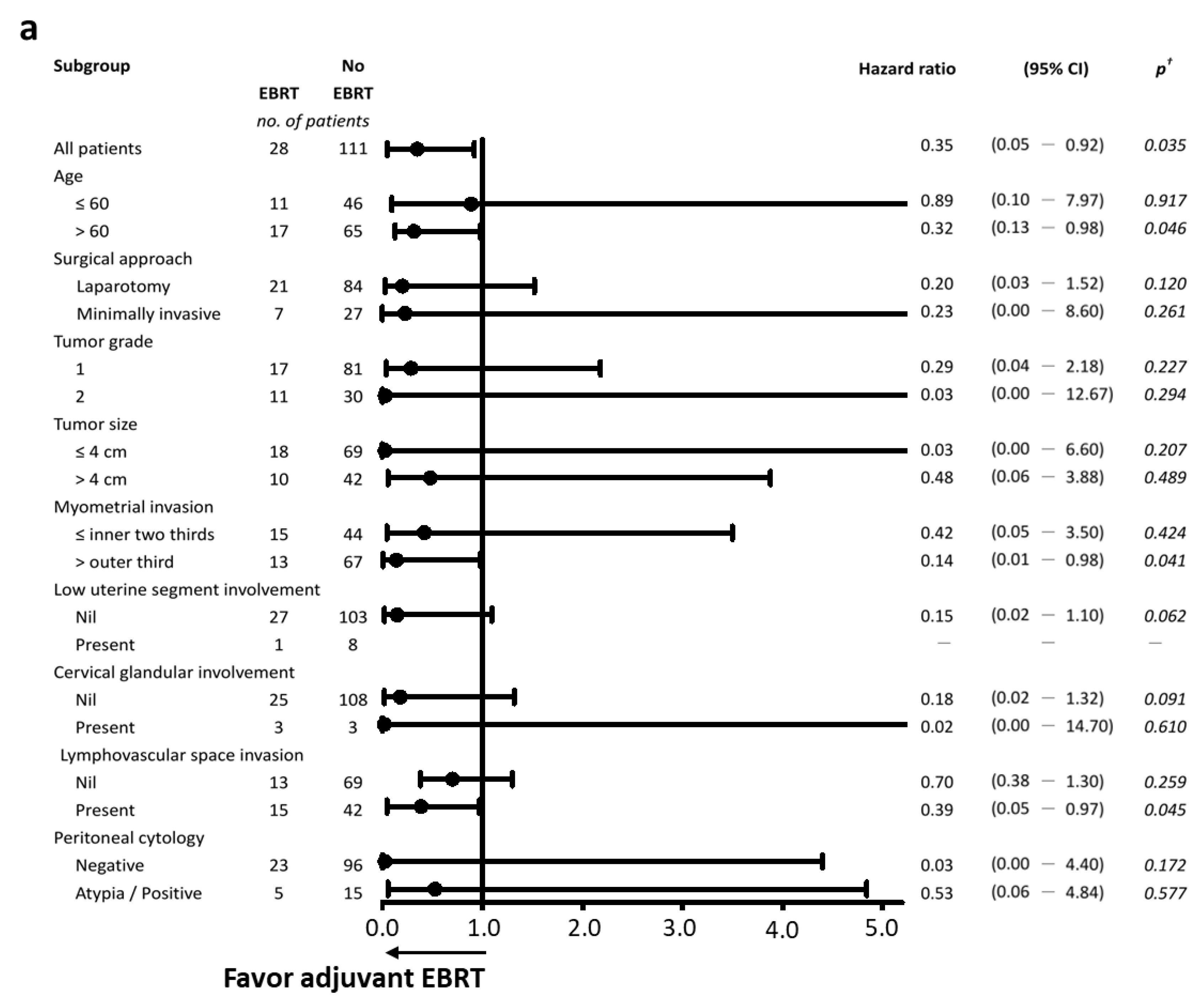
3.4. Effectiveness of Adjuvant External-Beam Radiotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Medenwald, D.; Langer, S.; Gottschick, C.; Vordermark, D. Effect of Radiotherapy in Addition to Surgery in Early Stage Endometrial Cancer: A Population-Based Study. Cancers 2020, 12, 3814. [Google Scholar] [CrossRef]
- Scholten, A.N.; van Putten, W.L.J.; Beerman, H.; Smit, V.T.H.B.M.; Koper, P.C.M.; Lybeert, M.L.M.; Jobsen, J.J.; Wárlám-Rodenhuis, C.C.; De Winter, K.A.J.; Lutgens, L.C.H.W.; et al. Postoperative radiotherapy for Stage 1 endometrial carcinoma: Long-term outcome of the randomized PORTEC trial with central pathology review. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef]
- Nout, R.A.; Smit, V.T.H.B.M.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Stenfert Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- Chen, J.L.Y.; Huang, Y.S.; Huang, C.Y.; Hsu, C.Y.; Lan, K.H.; Cheng, W.F.; Kuo, S.H. Impact of adjuvant radiotherapy on the survival of women with optimally resected stage III endometrial cancer in the era of modern radiotherapy: A retrospective study. Radiat. Oncol. 2020, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef]
- He, S.; Gill, B.S.; Heron, D.E.; Kelley, J.L.; Sukumvanich, P.; Olawaiye, A.B.; Edwards, R.P.; Comerci, J.; Beriwal, S. Long-term outcomes using adjuvant pelvic intensity modulated radiation therapy (IMRT) for endometrial carcinoma. Pract. Radiat. Oncol. 2017, 7, 19–25. [Google Scholar] [CrossRef]
- Chen, J.L.; Huang, Y.S.; Kuo, S.H.; Chen, Y.F.; Hong, R.L.; Ko, J.Y.; Lou, P.J.; Tsai, C.L.; Chen, W.Y.; Wang, C.W. Intensity-modulated radiation therapy for T4 nasopharyngeal carcinoma. Treatment results and locoregional recurrence. Strahlenther. Onkol. 2013, 189, 1001–1008. [Google Scholar] [CrossRef]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W., Jr.; Thompson, S.; et al. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Huang, Y.S.; Kuo, S.H.; Hong, R.L.; Ko, J.Y.; Lou, P.J.; Wang, C.W. Intensity-modulated radiation therapy achieves better local control compared to three-dimensional conformal radiation therapy for T4-stage nasopharyngeal carcinoma. Oncotarget 2017, 8, 14068–14077. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Uterine Neoplasms (Version1. 2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 22 October 2020).
- Klopp, A.; Smith, B.D.; Alektiar, K.; Cabrera, A.; Damato, A.L.; Erickson, B.; Fleming, G.; Gaffney, D.; Greven, K.; Lu, K.; et al. The role of postoperative radiation therapy for endometrial cancer: Executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract. Radiat. Oncol. 2014, 4, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Tai, Y.J.; Hsu, H.C.; Chiang, Y.C.; Chen, Y.L.; Chen, C.A.; Cheng, W.F. Impact of Adjuvant Modalities on Survival in Patients with Advanced Stage Endometrial Carcinoma: A Retrospective Analysis from a Tertiary Medical Center. Int. J. Environ. Res. Public Health 2019, 16, 2561. [Google Scholar] [CrossRef]
- Kizer, N.T.; Gao, F.; Guntupalli, S.; Thaker, P.H.; Powell, M.A.; Goodfellow, P.J.; Mutch, D.G.; Zighelboim, I. Lower uterine segment involvement is associated with poor outcomes in early-stage endometrioid endometrial carcinoma. Ann. Surg. Oncol. 2011, 18, 1419–1424. [Google Scholar] [CrossRef]
- Chen, J.L.Y.; Wang, M.C.; Huang, Y.S.; Huang, C.Y.; Pan, C.K.; Hsu, C.Y.; Lan, K.H.; Kuo, S.H. Extended-field bone marrow sparing radiotherapy for primary chemoradiotherapy in cervical cancer patients with para-aortic lymphadenopathy: Volumetric-modulated Arc therapy versus helical tomotherapy. J. X-ray Sci. Technol. 2020, 28, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Bosch, W.R.; Harkenrider, M.M.; Strauss, J.B.; Abu-Rustum, N.; Albuquerque, K.V.; Beriwal, S.; Creutzberg, C.L.; Eifel, P.J.; Erickson, B.A.; et al. NRG Oncology/RTOG Consensus Guidelines for Delineation of Clinical Target Volume for Intensity Modulated Pelvic Radiation Therapy in Postoperative Treatment of Endometrial and Cervical Cancer: An Update. Int. J. Radiat. Oncol. Biol. Phys. 2020, 109, 413–424. [Google Scholar] [CrossRef]
- Beadle, B.M.; Liao, K.P.; Elting, L.S.; Buchholz, T.A.; Ang, K.K.; Garden, A.S.; Guadagnolo, B.A. Improved survival using intensity-modulated radiation therapy in head and neck cancers: A SEER-Medicare analysis. Cancer 2014, 120, 702–710. [Google Scholar] [CrossRef]
- Chen, J.L.Y.; Cheng, J.C.H.; Kuo, S.H.; Chen, C.A.; Lin, M.C.; Huang, C.Y. Outcome analysis of cervical adenosquamous carcinoma compared with adenocarcinoma. Acta Obstet. Gynecol. Scand. 2012, 91, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.Y.; Huang, C.Y.; Huang, Y.S.; Chen, R.J.; Wang, C.W.; Chen, Y.H.; Cheng, J.C.H.; Cheng, A.L.; Kuo, S.H. Differential clinical characteristics, treatment response and prognosis of locally advanced adenocarcinoma/adenosquamous carcinoma and squamous cell carcinoma of cervix treated with definitive radiotherapy. Acta Obstet. Gynecol. Scand. 2014, 93, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, S.; Van Rompuy, A.S.; Van Gorp, T.; Vanden Bempt, I.; Brems, H.; Van Nieuwenhuysen, E.; Han, S.N.; Neven, P.; Victoor, J.; Laenen, A.; et al. Analysis of 108 patients with endometrial carcinoma using the PROMISE classification and additional genetic analyses for MMR-D. Gynecol. Oncol. 2020, 157, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Kidd, E.A. Survival benefit of radiation in high-risk, early-stage endometrioid carcinoma. J. Gynecol. Oncol. 2020, 31, e39. [Google Scholar] [CrossRef] [PubMed]
- Adishesh, M.; Hapangama, D.K. Enriching Personalized Endometrial Cancer Research with the Harmonization of Biobanking Standards. Cancers 2019, 11, 1734. [Google Scholar] [CrossRef]
- ASTEC/EN.5 Study Group; Blake, P.; Swart, A.M.; Orton, J.; Kitchener, H.; Whelan, T.; Lukka, H.; Eisenhauer, E.; Bacon, M.; Tu, D.; et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009, 373, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Onsrud, M.; Cvancarova, M.; Hellebust, T.P.; Tropé, C.G.; Kristensen, G.B.; Lindemann, K. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J. Clin. Oncol. 2013, 31, 3951–3956. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.W.; Lee, T.S.; Zang, R.; Chen, X.; Yang, J.; Wang, K.L.; Sugiyama, T. Difference in Practice Patterns in the Management of Endometrial Cancer: A Survey of the Members of 4 East Asian Gynecologic Oncology Groups. Int. J. Gynecol. Cancer 2017, 27, 1888–1894. [Google Scholar] [CrossRef]
- Leu, M.; Possiel, J.; Schirmer, M.A.; Hille, A.; Rieken, S.; Dröge, L.H. Evaluation of Prognosticators and Treatment-Related Side Effects in Patients Irradiated Postoperatively for Endometrial Cancer. Cancers 2020, 12, 3613. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- Rabinovich, A. Minimally invasive surgery for endometrial cancer. Curr. Opin. Obstet. Gynecol. 2015, 27, 302–307. [Google Scholar] [CrossRef]
- Ørtoft, G.; Lausten-Thomsen, L.; Høgdall, C.; Hansen, E.S.; Dueholm, M. Lymph-vascular space invasion (LVSI) as a strong and independent predictor for non-locoregional recurrences in endometrial cancer: A Danish Gynecological Cancer Group Study. J. Gynecol. Oncol. 2019, 30, e84. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef] [PubMed]

| Characteristic No. of Patients (%) | All (n = 180) | Grade 1–2 (n = 139) | Grade 3 (n = 41) | p-Value |
|---|---|---|---|---|
| Age (years), mean (range) | 61.9 (35.5–86.6) | 62.1 (37.0–86.6) | 61.3 (35.5–83.1) | 0.673 * |
| Surgical approach | 0.838 † | |||
| Laparotomy | 135 (75%) | 105 (76%) | 30 (73%) | |
| Minimally invasive * | 45 (25%) | 34 (24%) | 11 (27%) | |
| Tumor size (cm) | 0.669 * | |||
| Mean (range) | 4.2 (1.0–14.0) | 4.1 (1.0–14.0) | 4.3 (1.0–10.0) | |
| Myometrial invasion | 0.468 * | |||
| Mean (range) | 0.70 (0.50–1.00) | 0.70 (0.50–1.00) | 0.72 (0.50–1.00) | |
| Low uterine segment involvement | 0.046 † | |||
| Nil | 164 (91%) | 130 (94%) | 34 (83%) | |
| Present | 16 (9%) | 9 (6%) | 7 (17%) | |
| Cervical glandular involvement | 1.000 † | |||
| Nil | 172 (96%) | 133 (96%) | 39 (95%) | |
| Present | 8 (4%) | 6 (4%) | 2 (5%) | |
| Lymphovascular space invasion | 0.042 † | |||
| Nil | 99 (55%) | 82 (59%) | 17 (41%) | |
| Present | 81 (45%) | 57 (41%) | 24 (59%) | |
| Peritoneal washing cytology | 0.603 † | |||
| Negative | 156 (87%) | 119 (86%) | 37 (90%) | |
| Atypia/Positive | 24 (13%) | 20 (14%) | 4 (10%) | |
| Adjuvant chemotherapy | 0.012 † | |||
| No | 164 (91%) | 131 (94%) | 33 (80%) | |
| Yes | 16 (9%) | 8 (6%) | 8 (20%) | |
| Adjuvant radiotherapy | <0.001 † | |||
| No | 76 (42%) | 63 (45%) | 13 (32%) | |
| VBT | 51 (28%) | 48 (35%) | 3 (7%) | |
| EBRT ± VBT boost | 53 (30%) | 28 (20%) | 25 (61%) |
| 5-Year RFS | HR (95% CI) | p-Value † | 5-Year OS | HR (95% CI) | p-Value † | |
|---|---|---|---|---|---|---|
| Age (years) | 0.074 | 0.040 | ||||
| ≤60 | 89 | — | 100 | — | ||
| >60 | 78 | 2.4 (0.9–6.5) | 93 | 4.8 (1.1–7.5) | ||
| Surgical approach | 0.415 | 0.736 | ||||
| Laparotomy | 81 | — | 96 | — | ||
| Minimally invasive * | 87 | 0.6 (0.2–1.9) | 96 | 0.7 (0.1–6.2) | ||
| Tumor size | 0.120 | 0.183 | ||||
| ≤4 cm | 83 | — | 100 | — | ||
| >4 cm | 81 | 1.2 (0.4–2.5) | 93 | 3.0 (0.0–19.3) | ||
| Myometrial invasion | 0.084 | 0.333 | ||||
| ≤Inner two thirds | 84 | — | 94 | — | ||
| >Outer third | 78 | 1.5 (0.6–3.6) | 97 | 2.8 (0.3–15.3) | ||
| Low uterine segment involvement | 0.076 | 0.552 | ||||
| Nil | 84 | — | 100 | — | ||
| Present | 78 | 2.5 (1.0–5.0) | 95 | 2.0 (0.0–16.7) | ||
| Cervical glandular involvement | 0.946 | 0.593 | ||||
| Nil | 83 | — | 95 | — | ||
| Present | 82 | 1.1 (0.1–8.0) | 100 | 2.2 (0.0–7.5) | ||
| Lymphovascular space invasion | 0.076 | 0.048 | ||||
| Nil | 84 | — | 92 | — | ||
| Present | 78 | 2.5 (1.0–5.0) | 100 | 4.2 (1.0–14.5) | ||
| Peritoneal cytology | 0.042 | 0.046 | ||||
| Negative | 84 | — | 97 | — | ||
| Atypia/Positive | 64 | 2.1 (1.0–5.6) | 90 | 4.4 (1.1–16.5) | ||
| Adjuvant chemotherapy | 0.611 | 0.575 | ||||
| No | 82.6 | — | 95 | — | ||
| Yes | 75.0 | 1.5 (0.3–6.2) | 100 | 0.1 (0.0–8.1) | ||
| Adjuvant radiotherapy | 0.048 | 0.040 | ||||
| No | 78 | — | 90 | — | ||
| VBT | 79 | 0.9 (0.4–2.3) | 100 | 0.4 (0.0–8.2) | ||
| EBRT ± VBT boost | 96 | 0.3 (0.0–0.9) | 100 | 0.3 (0.0–9.3) |
| 5-Year RFS | HR (95% CI) | p-Value † | 5-Year OS | HR (95% CI) | p-Value † | |
|---|---|---|---|---|---|---|
| Age (years) | 0.380 | 0.445 | ||||
| ≤60 | 70 | — | 86 | — | ||
| >60 | 68 | 1.7 (0.5–5.3) | 84 | 1.9 (0.3–12.1) | ||
| Surgical approach | 0.600 | 0.675 | ||||
| Laparotomy | 70 | — | 79 | — | ||
| Minimally invasive * | 68 | 1.4 (0.4–4.6) | 100 | 1.6 (0.2–14.4) | ||
| Tumor size | 0.557 | 0.979 | ||||
| ≤4 cm | 72 | — | 87 | — | ||
| >4 cm | 67 | 1.4 (0.4–4.8) | 83 | 1.0 (0.2–5.9) | ||
| Myometrial invasion | 0.913 | 0.316 | ||||
| ≤Inner two thirds | 73 | — | 89 | — | ||
| >Outer third | 63 | 1.1 (0.3–3.4) | 82 | 2.9 (0.3–27.0) | ||
| Low uterine segment involvement | 0.820 | 0.783 | ||||
| Nil | 69 | — | 83 | — | ||
| Present | 86 | 1.2 (0.3–5.6) | 50 | 1.4 (0.1–12.5) | ||
| Cervical glandular involvement | 0.421 | 0.637 | ||||
| Nil | 72 | — | 85 | — | ||
| Present | 50 | 2.3 (0.3–18.1) | 100 | 0.1 (0.0–37.3) | ||
| Lymphovascular space invasion | 0.666 | 0.418 | ||||
| Nil | 75 | — | 81 | — | ||
| Present | 65 | 1.3 (0.4–4.3) | 94 | 2.4 (0.3–21.6) | ||
| Peritoneal cytology | 0.004 | 0.171 | ||||
| Negative | 76 | — | 87 | — | ||
| Atypia/Positive | 25 | 5.9 (1.5–23.5) | 67 | 4.3 (0.5–24.8) | ||
| Adjuvant chemotherapy | 0.387 | 0.114 | ||||
| No | 76 | — | 84 | — | ||
| Yes | 50 | 1.8 (0.5–6.8) | 50 | 3.9 (0.8–14.7) | ||
| Adjuvant radiotherapy | 0.039 | 0.097 | ||||
| No | 62 | — | 82 | — | ||
| VBT | 67 | 2.6 (0.5–13.6) | 100 | 2.6 (0.7–10.3) | ||
| EBRT ± VBT boost | 79 | 0.4 (0.1–1.5) | 93 | 0.3 (0.1–2.9) |
| IB Grade 1–2 | IB Grade 3 | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value * | HR (95% CI) | p-Value * | |
| Peritoneal cytology Atypia/Positive vs. Negative | 2.3 (0.8–6.2) | 0.110 | 6.5 (1.6–26.5) | 0.010 |
| Adjuvant radiotherapy EBRT ± VBT boost vs. No/VBT | 0.1 (0.0–1.0) | 0.049 | 0.3 (0.1–0.9) | 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.L.-Y.; Huang, C.-Y.; Huang, Y.-S.; Hsu, C.-Y.; Lan, K.-H.; Shih, I.-L.; Cheng, W.-F.; Chen, C.-A.; Sheu, B.-C.; Kuo, S.-H. Real-World Evaluation of Modern Adjuvant Radiotherapy in Women with Stage IB Endometrial Cancer. Cancers 2021, 13, 1386. https://doi.org/10.3390/cancers13061386
Chen JL-Y, Huang C-Y, Huang Y-S, Hsu C-Y, Lan K-H, Shih I-L, Cheng W-F, Chen C-A, Sheu B-C, Kuo S-H. Real-World Evaluation of Modern Adjuvant Radiotherapy in Women with Stage IB Endometrial Cancer. Cancers. 2021; 13(6):1386. https://doi.org/10.3390/cancers13061386
Chicago/Turabian StyleChen, Jenny Ling-Yu, Chao-Yuan Huang, Yu-Sen Huang, Che-Yu Hsu, Keng-Hsueh Lan, I-Lun Shih, Wen-Fang Cheng, Chi-An Chen, Bor-Ching Sheu, and Sung-Hsin Kuo. 2021. "Real-World Evaluation of Modern Adjuvant Radiotherapy in Women with Stage IB Endometrial Cancer" Cancers 13, no. 6: 1386. https://doi.org/10.3390/cancers13061386
APA StyleChen, J. L.-Y., Huang, C.-Y., Huang, Y.-S., Hsu, C.-Y., Lan, K.-H., Shih, I.-L., Cheng, W.-F., Chen, C.-A., Sheu, B.-C., & Kuo, S.-H. (2021). Real-World Evaluation of Modern Adjuvant Radiotherapy in Women with Stage IB Endometrial Cancer. Cancers, 13(6), 1386. https://doi.org/10.3390/cancers13061386






