Association between Antiretroviral Therapy and Cancers among Children Living with HIV in Sub-Saharan Africa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Cases
2.3. Controls
2.4. Study Variables and Definition
2.5. Statistical Analysis
3. Results
3.1. Incidence of Cancer among CLWH Receiving ART
3.2. Subtypes of Cancer
3.3. Risk Factors for Cancer in Children with HIV Infection
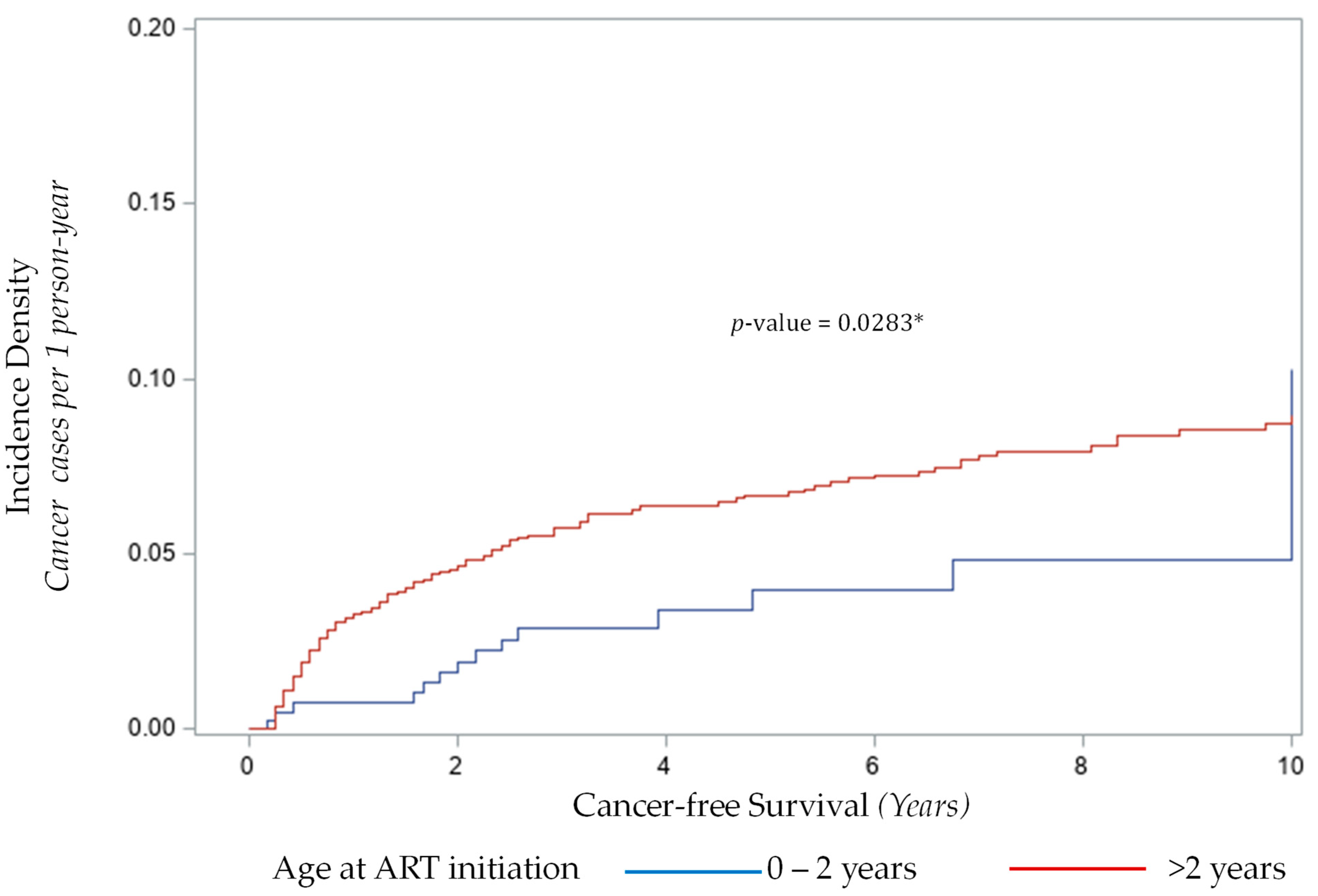
3.3.1. Age at Initiation of ART and Cancer Risk
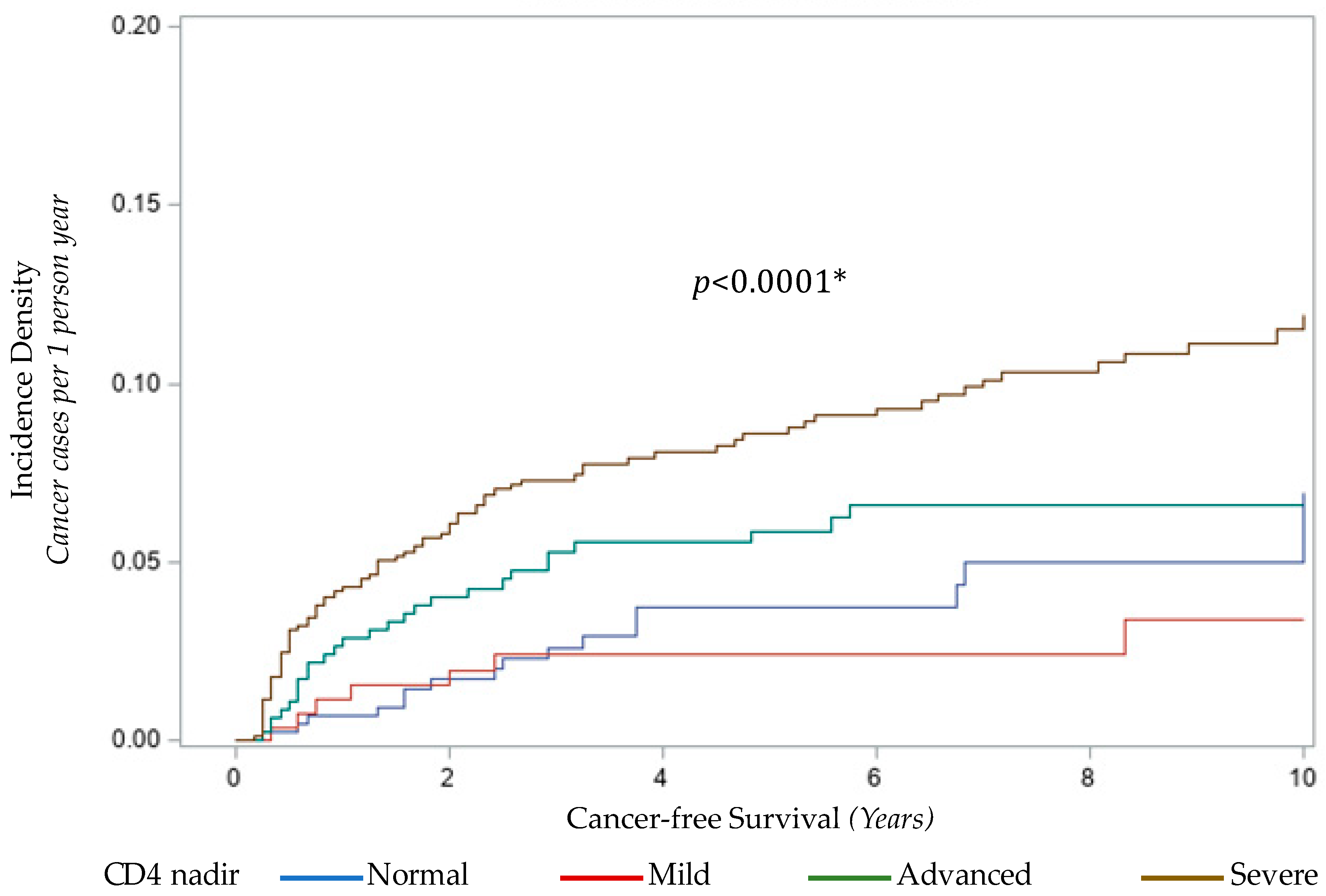
3.3.2. CD4 Nadir and Cancer Risk
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—2019 Fact Sheet. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 10 October 2020).
- Biggar, R.J.; Frisch, M.; Goedert, J.J. Risk of cancer in children with AIDS. AIDS-Cancer Match Registry Study Group. JAMA 2000, 284, 205–209. [Google Scholar] [CrossRef]
- Chiappini, E.; Berti, E.; Gianesin, K.; Petrara, M.R.; Galli, L.; Giaquinto, C.; de Martino, M.; De Rossi, A. Pediatric human immunodeficiency virus infection and cancer in the highly active antiretroviral treatment (HAART) era. Cancer Lett. 2014, 347, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Simard, E.P.; Shiels, M.S.; Bhatia, K.; Engels, E.A. Long-term cancer risk among people diagnosed with AIDS during childhood. Cancer Epidemiol. Biomark. Prev. 2012, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Alvaro-Meca, A.; Micheloud, D.; Jensen, J.; Diaz, A.; Garcia-Alvarez, M.; Resino, S. Epidemiologic trends of cancer diagnoses among HIV-infected children in Spain from 1997 to 2008. Pediatr. Infect. Dis. J. 2011, 30, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Pfeiffer, R.M.; Hall, H.I.; Li, J.; Goedert, J.J.; Morton, L.M.; Hartge, P.; Engels, E.A. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA 2011, 305, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- The Pediatric AIDS-Defining Cancer Project Working Group for IeDEA Southern Africa, TApHOD, and COHERE in EuroCoord; Rohner, E.; Schmidlin, K.; Zwahlen, M.; Chakraborty, R.; Clifford, G.; Obel, N.; Grabar, S.; Verbon, A.; Noguera-Julian, A.; et al. Kaposi Sarcoma Risk in HIV-Infected Children and Adolescents on Combination Antiretroviral Therapy From Sub-Saharan Africa, Europe, and Asia. Clin. Infect. Dis. 2016, 63, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Biggar, R.J.; Hall, H.I.; Cross, H.; Crutchfield, A.; Finch, J.L.; Grigg, R.; Hylton, T.; Pawlish, K.S.; McNeel, T.S.; et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int. J. Cancer 2008, 123, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Sinclair, M.D.; Biggar, R.J.; Whitby, D.; Ebbesen, P.; Goedert, J.J.; Gastwirth, J.L. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-saharan Africa and Malta. Int. J. Cancer 2000, 88, 1003–1008. [Google Scholar] [CrossRef]
- Zoufaly, A.; Stellbrink, H.J.; Heiden, M.A.; Kollan, C.; Hoffmann, C.; van Lunzen, J.; Hamouda, O.; ClinSurv Study Group. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J. Infect. Dis. 2009, 200, 79–87. [Google Scholar] [CrossRef]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T.; Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Landgren, O.; Moore, R.D. Immunologic and virologic predictors of AIDS-related non-hodgkin lymphoma in the highly active antiretroviral therapy era. J. Acquir. Immune Defic. Syndr. 2010, 54, 78–84. [Google Scholar] [CrossRef]
- Franceschi, S.; Lise, M.; Clifford, G.M.; Rickenbach, M.; Levi, F.; Maspoli, M.; Bouchardy, C.; Dehler, S.; Jundt, G.; Ess, S.; et al. Changing patterns of cancer incidence in the early- and late-HAART periods: The Swiss HIV Cohort Study. Br. J. Cancer 2010, 103, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Cobucci, R.N.; Lima, P.H.; de Souza, P.C.; Costa, V.V.; Cornetta Mda, C.; Fernandes, J.V.; Goncalves, A.K. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: A systematic review. J. Infect. Public Health 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Bohlius, J.; Schmidlin, K.; Costagliola, D.; Fatkenheuer, G.; May, M.; Caro-Murillo, A.M.; Mocroft, A.; Bonnet, F.; Clifford, G.; Karafoulidou, A.; et al. Incidence and risk factors of HIV-related non-Hodgkin’s lymphoma in the era of combination antiretroviral therapy: A European multicohort study. Antivir. Ther. 2009, 14, 1065–1074. [Google Scholar] [CrossRef]
- Yanik, E.L.; Achenbach, C.J.; Gopal, S.; Coghill, A.E.; Cole, S.R.; Eron, J.J.; Moore, R.D.; Mathews, W.C.; Drozd, D.R.; Hamdan, A.; et al. Changes in Clinical Context for Kaposi’s Sarcoma and Non-Hodgkin Lymphoma Among People With HIV Infection in the United States. J. Clin. Oncol. 2016, 34, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Lanoy, E.; Rosenberg, P.S.; Fily, F.; Lascaux, A.S.; Martinez, V.; Partisani, M.; Poizot-Martin, I.; Rouveix, E.; Engels, E.A.; Costagliola, D.; et al. HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood 2011, 118, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, H.W.; De Stavola, B.L.; Carpenter, L.M.; Porter, K.; Cox, D.R.; Collaboration, C. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS 2011, 25, 1395–1403. [Google Scholar] [CrossRef]
- Bower, M.; Nelson, M.; Young, A.M.; Thirlwell, C.; Newsom-Davis, T.; Mandalia, S.; Dhillon, T.; Holmes, P.; Gazzard, B.G.; Stebbing, J. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J. Clin. Oncol. 2005, 23, 5224–5228. [Google Scholar] [CrossRef]
- Yanik, E.L.; Napravnik, S.; Cole, S.R.; Achenbach, C.J.; Gopal, S.; Olshan, A.; Dittmer, D.P.; Kitahata, M.M.; Mugavero, M.J.; Saag, M.; et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin. Infect. Dis. 2013, 57, 756–764. [Google Scholar] [CrossRef]
- Maksimovic-Ivanic, D.; Fagone, P.; McCubrey, J.; Bendtzen, K.; Mijatovic, S.; Nicoletti, F. HIV-protease inhibitors for the treatment of cancer: Repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives? Int. J. Cancer 2017, 140, 1713–1726. [Google Scholar] [CrossRef]
- Sussman, H.E.; Olivero, O.A.; Meng, Q.; Pietras, S.M.; Poirier, M.C.; O’Neill, J.P.; Finette, B.A.; Bauer, M.J.; Walker, V.E. Genotoxicity of 3′-azido-3′-deoxythymidine in the human lymphoblastoid cell line, TK6: Relationships between DNA incorporation, mutant frequency, and spectrum of deletion mutations in HPRT. Mutat. Res. 1999, 429, 249–259. [Google Scholar] [CrossRef]
- Damonti, J.; Doykos, P.; Wanless, R.S.; Kline, M. HIV/AIDS in African children: The Bristol-Myers Squibb Foundation and Baylor response. Health Aff. (Millwood) 2012, 31, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunologic Classification of HIV-Related Disease in Adults and Children. Available online: https://apps.who.int/iris/handle/10665/43699 (accessed on 10 October 2020).
- Segi, M.; Fujisaku, S.; Kurihara, M.; Narai, Y.; Sasajima, K. The age-adjusted death rates for malignant neoplasms in some selected sites in 23 countries in 1954-1955 and their geographical correlation. Tohoku J. Exp. Med. 1960, 72, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Contributors, I. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Ward, Z.J.; Yeh, J.M.; Bhakta, N.; Frazier, A.L.; Atun, R. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019, 20, 483–493. [Google Scholar] [CrossRef]
- Stefan, C.; Bray, F.; Ferlay, J.; Liu, B.; Maxwell Parkin, D. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience 2017, 11, 755. [Google Scholar] [CrossRef]
- Lubega, J. Endemic Burkitt lymphoma is not associated with HIV infection: What is the carcinogenesis of the tumor? In Proceedings of the 27th Annual Meeting of the International Association of Cancer Registries: Cancer in Low-Resource Populations, Entebbe, Uganda, 13–15 September 2005. [Google Scholar]
- Lubega, J. T-helper 1 versus T-helper 2 lymphocyte immunodysregulation is the central factor in genesis of Burkitt lymphoma: Hypothesis. Infect. Agents Cancer 2007, 2, e10. [Google Scholar] [CrossRef]
- Brady, M.T.; Oleske, J.M.; Williams, P.L.; Elgie, C.; Mofenson, L.M.; Dankner, W.M.; Van Dyke, R.B.; Pediatric AIDS Clinical Trials Group219/219C Team. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J. Acquir. Immune Defic. Syndr. 2010, 53, 86–94. [Google Scholar] [CrossRef]
- Vuppula, S.; Tyungu, D.; Kaul, A.; Chandwani, S.; Rigaud, M.; Borkowsky, W. Thirty-year Perspective of the Long-term Survival, CD4 Percentage and Social Achievements of Perinatally HIV-infected Children as a Function of Their Birth Era. Pediatr. Infect. Dis. J. 2017, 36, 198–201. [Google Scholar] [CrossRef]
- Montagnani, C.; Chiappini, E.; Bonsignori, F.; Galli, L.; de Martino, M. Long-term effect of highly active antiretroviral therapy on immunologic features in children. Pediatr. Infect. Dis. J. 2015, 34, S3–S6. [Google Scholar] [CrossRef]
- Dolcetti, R.; Gloghini, A.; Caruso, A.; Carbone, A. A lymphomagenic role for HIV beyond immune suppression? Blood 2016, 127, 1403–1409. [Google Scholar] [CrossRef]
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Available online: https://www.who.int/hiv/pub/guidelines/arv2013/art/statartchildren/en/ (accessed on 2 October 2020).
- McCollum, E.D.; Preidis, G.A.; Golitko, C.L.; Siwande, L.D.; Mwansambo, C.; Kazembe, P.N.; Hoffman, I.; Hosseinipour, M.C.; Schutze, G.E.; Kline, M.W. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr. Infect. Dis. J. 2011, 30, e75–e81. [Google Scholar] [CrossRef]
- Bohlius, J.; Maxwell, N.; Spoerri, A.; Wainwright, R.; Sawry, S.; Poole, J.; Eley, B.; Prozesky, H.; Rabie, H.; Garone, D.; et al. Incidence of AIDS-defining and Other Cancers in HIV-positive Children in South Africa: Record Linkage Study. Pediatr. Infect. Dis. J. 2016, 35, e164–e170. [Google Scholar] [CrossRef] [PubMed]
- Amerson, E.; Woodruff, C.M.; Forrestel, A.; Wenger, M.; McCalmont, T.; LeBoit, P.; Maurer, T.; Laker-Oketta, M.; Muyindike, W.; Bwana, M.; et al. Accuracy of Clinical Suspicion and Pathologic Diagnosis of Kaposi Sarcoma in East Africa. J. Acquir. Immune Defic. Syndr. 2016, 71, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ogwang, M.D.; Zhao, W.; Ayers, L.W.; Mbulaiteye, S.M. Accuracy of Burkitt lymphoma diagnosis in constrained pathology settings: Importance to epidemiology. Arch. Pathol. Lab. Med. 2011, 135, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Orem, J.; Sandin, S.; Weibull, C.E.; Odida, M.; Wabinga, H.; Mbidde, E.; Wabwire-Mangen, F.; Meijer, C.J.; Middeldorp, J.M.; Weiderpass, E. Agreement between diagnoses of childhood lymphoma assigned in Uganda and by an international reference laboratory. Clin. Epidemiol. 2012, 4, 339–347. [Google Scholar] [CrossRef][Green Version]
- Pantanowitz, L.; Grayson, W.; Simonart, T.; Dezube, B.J. Pathology of Kaposi’s sarcoma. J. HIV Ther. 2009, 14, 41–47. [Google Scholar]
- Little, R.F.; Dunleavy, K. Update on the treatment of HIV-associated hematologic malignancies. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 382–388. [Google Scholar] [CrossRef]
- Pipkin, S.; Scheer, S.; Okeigwe, I.; Schwarcz, S.; Harris, D.H.; Hessol, N.A. The effect of HAART and calendar period on Kaposi’s sarcoma and non-Hodgkin lymphoma: Results of a match between an AIDS and cancer registry. AIDS 2011, 25, 463–471. [Google Scholar] [CrossRef]
| Incident Cases (n = 117) | Controls (n = 1488) | |
|---|---|---|
| Follow-up time (Years) (median, mean) | 1.5, 2.28 | 1.50, 2.34 |
| Age at enrollment (Years) (median, mean) | 5.6, 6.9 | 4.9, 6.3 |
| Sex | ||
| Male (n, %) | 70 (59.83%) | 915 (61.49%) |
| Female (n, %) | 47 (40.17%) | 573 (38.51%) |
| Country | ||
| Botswana (n, %) | 8 (6.84%) | 31 (2.08%) |
| Malawi (n, %) | 45 (38.46%) | 651 (43.75%) |
| Tanzania (n, %) | 7 (5.98%) | 0 (0.00%) |
| Uganda (n, %) | 57 (48.72%) | 806 (54.17%) |
| Period of registration in HIV care | ||
| 2004–2008 (n, %) | 43 (36.75%) | 486 (32.66%) |
| 2008–2014 (n, %) | 74 (63.25%) | 1002 (67.34%) |
| Cancer Subtype | Prevalent Cases n = 310 | Incident Cases n = 117 | Crude Incidence Density 1 (95% CI) | Adjusted 2 Incidence Density 1 (95% CI) |
|---|---|---|---|---|
| Kaposi Sarcoma | 226 (72.90%) | 90 (76.92%) | 91.5 (74.4, 112.5) | 36.6 (26.5, 50.5) |
| Non-Hodgkin Lymphoma | 18 (5.80%) | 17 (14.53%) | 17.3 (10.7, 27.8) | 8.9 (4.7, 17.0) |
| Hodgkin Lymphoma | 2 (0.65%) | 5 (4.27%) | 5.1 (2.1, 12.2) | |
| Leukemia | 1 (0.32%) | 1 (0.85%) | 1.0 (0.2, 5.7) | 0.4 (0.0, 4.6) |
| Solid Tumors 3 | 5 (1.61%) | 4 (3.42%) | 4.1 (1.5, 10.8) | N/A |
| Risk Variable | HIV and Cancer n = 117 | HIV No Cancer n = 1488 | Odds Ratio (95% CI) | Adjusted * Odds Ratio (95% CI) |
|---|---|---|---|---|
| Age at ART initiation | ||||
| 0–2 years | 13 (11.11%) | 377 (25.34%) | - | - |
| >2 years | 104 (88.89%) | 1111 (74.66%) | 2.71 (1.51, 4.89) ** | 2.84 (1.57, 5.13) ** |
| CD4 nadir | ||||
| Normal | 15 (12.82%) | 223 (14.99%) | - | - |
| Mild | 7 (5.98%) | 203 (13.64%) | 0.51 (0.19, 1.24) | 0.52 (0.21, 1.31) |
| Advanced | 23 (19.66%) | 306 (20.56%) | 1.12 (0.58, 2.23) | 1.12 (0.57, 2.19) |
| Severe | 72 (61.54%) | 756 (50.81%) | 1.42 (0.82, 2.61) | 1.44 (0.81, 2.57) |
| WHO Stage at enrollment *** | N/A | |||
| I–III | 56 (73.68%) | 912 (84.92%) | - | |
| IV | 20 (26.32%) | 162 (15.08%) | 2.01 (1.18, 3.44) ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, H.; Elyanu, P.; Bulsara, S.; Bacha, J.M.; Campbell, L.R.; El-Mallawany, N.K.; Keating, E.M.; Kisitu, G.P.; Mehta, P.S.; Rees, C.A.; et al. Association between Antiretroviral Therapy and Cancers among Children Living with HIV in Sub-Saharan Africa. Cancers 2021, 13, 1379. https://doi.org/10.3390/cancers13061379
Haq H, Elyanu P, Bulsara S, Bacha JM, Campbell LR, El-Mallawany NK, Keating EM, Kisitu GP, Mehta PS, Rees CA, et al. Association between Antiretroviral Therapy and Cancers among Children Living with HIV in Sub-Saharan Africa. Cancers. 2021; 13(6):1379. https://doi.org/10.3390/cancers13061379
Chicago/Turabian StyleHaq, Heather, Peter Elyanu, Shaun Bulsara, Jason M. Bacha, Liane R. Campbell, Nader K. El-Mallawany, Elizabeth M. Keating, Grace P. Kisitu, Parth S. Mehta, Chris A. Rees, and et al. 2021. "Association between Antiretroviral Therapy and Cancers among Children Living with HIV in Sub-Saharan Africa" Cancers 13, no. 6: 1379. https://doi.org/10.3390/cancers13061379
APA StyleHaq, H., Elyanu, P., Bulsara, S., Bacha, J. M., Campbell, L. R., El-Mallawany, N. K., Keating, E. M., Kisitu, G. P., Mehta, P. S., Rees, C. A., Slone, J. S., Kekitiinwa, A. R., Matshaba, M., Mizwa, M. B., Mwita, L., Schutze, G. E., Wanless, S. R., Scheurer, M. E., & Lubega, J. (2021). Association between Antiretroviral Therapy and Cancers among Children Living with HIV in Sub-Saharan Africa. Cancers, 13(6), 1379. https://doi.org/10.3390/cancers13061379








