Population-Based Estimates of the Age-Specific Cumulative Risk of Breast Cancer for Pathogenic Variants in CHEK2: Findings from the Australian Breast Cancer Family Registry
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Pathogenic CHEK2 Variants Identified in the Australian Breast Cancer Family Registry
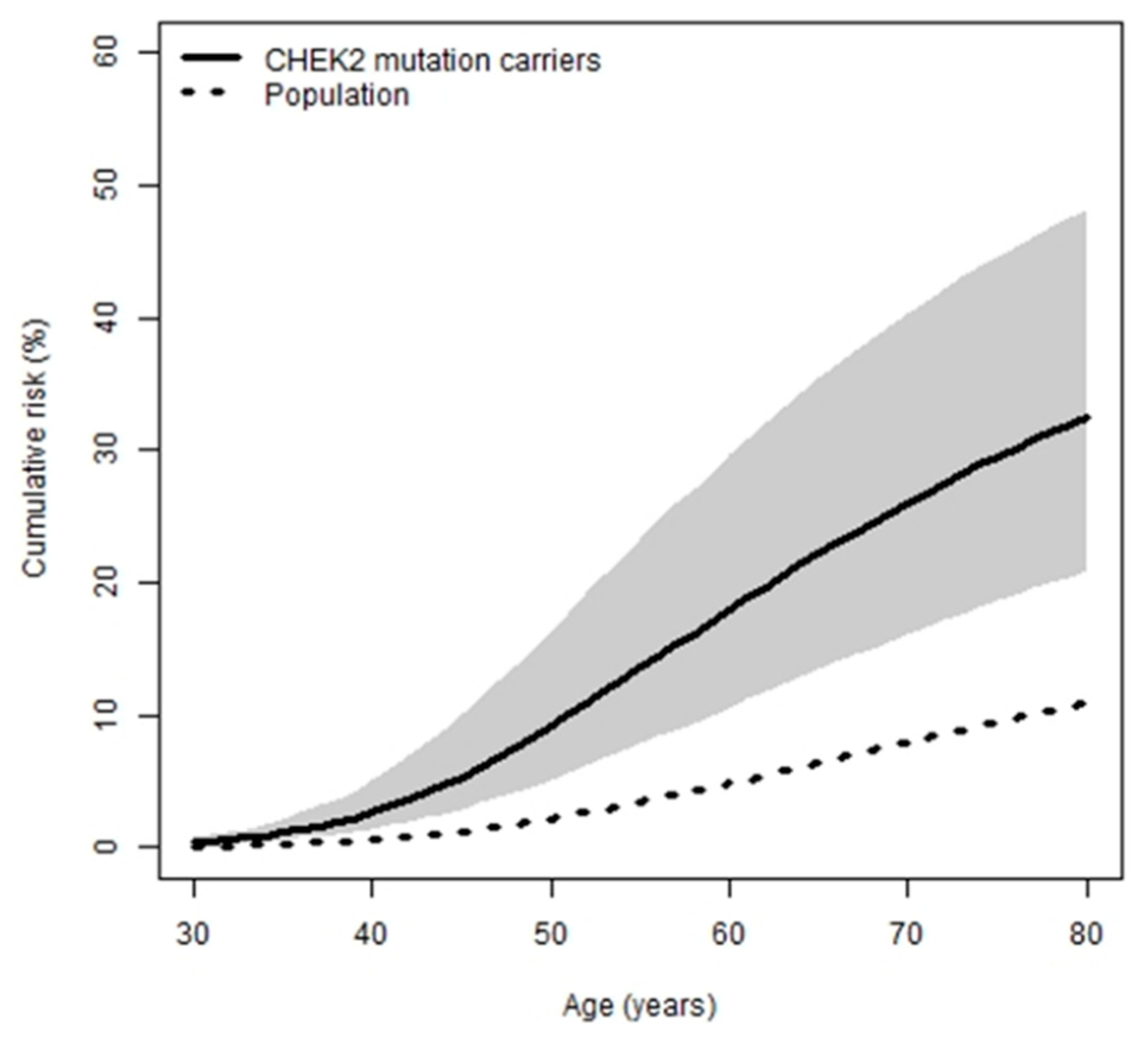
2.2. Risk Estimates
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Targeted-Sequencing in Proband Subjects
4.3. Sequencing Data Processing and Variant Selection
4.4. Targeted-Sequencing in Family Members
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Shieh, S.Y.; Ahn, J.; Tamai, K.; Taya, Y.; Prives, C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000, 14, 289–300. [Google Scholar]
- CHEK2 Breast Cancer Case-Control Consortium. CHEK2*1100delC and susceptibility to breast cancer: A collaborative analysis involving 10,860 breast cancer cases and 9065 controls from 10 studies. Am. J. Hum. Genet 2004, 74, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Schutte, M.; Seal, S.; Barfoot, R.; Meijers-Heijboer, H.; Wasielewski, M.; Evans, D.G.; Eccles, D.; Meijers, C.; Lohman, F.; Klijn, J.; et al. Variants in CHEK2 other than 1100delC do not make a major contribution to breast cancer susceptibility. Am. J. Hum. Genet 2003, 72, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, C.; Gorski, B.; Huzarski, T.; Byrski, T.; Gronwald, J.; Debniak, T.; Wokolorczyk, D.; Jakubowska, A.; Kowalska, E.; Oszurek, O.; et al. CHEK2-positive breast cancers in young Polish women. Clin. Cancer Res. 2006, 12, 4832–4835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weischer, M.; Bojesen, S.E.; Ellervik, C.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: Meta-analyses of 26,000 patient cases and 27,000 controls. J. Clin. Oncol. 2008, 26, 542–548. [Google Scholar] [CrossRef]
- Zhang, S.; Phelan, C.M.; Zhang, P.; Rousseau, F.; Ghadirian, P.; Robidoux, A.; Foulkes, W.; Hamel, N.; McCready, D.; Trudeau, M.; et al. Frequency of the CHEK2 1100delC mutation among women with breast cancer: An international study. Cancer Res. 2008, 68, 2154–2157. [Google Scholar] [CrossRef]
- Schmidt, M.K.; Tollenaar, R.A.; de Kemp, S.R.; Broeks, A.; Cornelisse, C.J.; Smit, V.T.; Peterse, J.L.; van Leeuwen, F.E.; Van’t Veer, L.J. Breast cancer survival and tumor characteristics in premenopausal women carrying the CHEK2*1100delC germline mutation. J. Clin. Oncol. 2007, 25, 64–69. [Google Scholar] [CrossRef]
- Weischer, M.; Nordestgaard, B.G.; Pharoah, P.; Bolla, M.K.; Nevanlinna, H.; Van’t Veer, L.J.; Garcia-Closas, M.; Hopper, J.L.; Hall, P.; Andrulis, I.L.; et al. CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer. J. Clin. Oncol. 2012, 30, 4308–4316. [Google Scholar] [CrossRef]
- de Bock, G.H.; Schutte, M.; Krol-Warmerdam, E.M.; Seynaeve, C.; Blom, J.; Brekelmans, C.T.; Meijers-Heijboer, H.; van Asperen, C.J.; Cornelisse, C.J.; Devilee, P.; et al. Tumour characteristics and prognosis of breast cancer patients carrying the germline CHEK2*1100delC variant. J. Med. Genet. 2004, 41, 731–735. [Google Scholar] [CrossRef]
- Nagel, J.H.; Peeters, J.K.; Smid, M.; Sieuwerts, A.M.; Wasielewski, M.; de Weerd, V.; Trapman-Jansen, A.M.; van den Ouweland, A.; Bruggenwirth, H.; van IJcken, W.F.; et al. Gene expression profiling assigns CHEK2 1100delC breast cancers to the luminal intrinsic subtypes. Breast Cancer Res. Treat. 2012, 132, 439–448. [Google Scholar] [CrossRef]
- Fletcher, O.; Johnson, N.; Dos Santos Silva, I.; Kilpivaara, O.; Aittomaki, K.; Blomqvist, C.; Nevanlinna, H.; Wasielewski, M.; Meijers-Heijerboer, H.; Broeks, A.; et al. Family history, genetic testing, and clinical risk prediction: Pooled analysis of CHEK2 1100delC in 1,828 bilateral breast cancers and 7030 controls. Cancer Epidemiol. Biomark. Prev. 2009, 18, 230–234. [Google Scholar] [CrossRef]
- Meyer, A.; Dork, T.; Sohn, C.; Karstens, J.H.; Bremer, M. Breast cancer in patients carrying a germ-line CHEK2 mutation: Outcome after breast conserving surgery and adjuvant radiotherapy. Radiother. Oncol. 2007, 82, 349–353. [Google Scholar] [CrossRef]
- Schmidt, M.K.; Hogervorst, F.; van Hien, R.; Cornelissen, S.; Broeks, A.; Adank, M.A.; Meijers, H.; Waisfisz, Q.; Hollestelle, A.; Schutte, M.; et al. Age- and Tumor Subtype-Specific Breast Cancer Risk Estimates for CHEK2*1100delC Carriers. J. Clin. Oncol. 2016, 34, 2750–2760. [Google Scholar] [CrossRef]
- Le Calvez-Kelm, F.; Lesueur, F.; Damiola, F.; Vallée, M.; Voegele, C.; Babikyan, D.; Durand, G.; Forey, N.; McKay-Chopin, S.; Robinot, N. Rare, evolutionarily unlikely missense substitutions in CHEK2 contribute to breast cancer susceptibility: Results from a breast cancer family registry case-control mutation-screening study. Breast Cancer Res. 2011, 13, R6. [Google Scholar] [CrossRef]
- Young, E.L.; Feng, B.J.; Stark, A.W.; Damiola, F.; Durand, G.; Forey, N.; Francy, T.C.; Gammon, A.; Kohlmann, W.K.; Kaphingst, K.A.; et al. Multigene testing of moderate-risk genes: Be mindful of the missense. J. Med. Genet. 2016, 53, 366–376. [Google Scholar] [CrossRef]
- Darabi, H.; Beesley, J.; Droit, A.; Kar, S.; Nord, S.; Moradi Marjaneh, M.; Soucy, P.; Michailidou, K.; Ghoussaini, M.; Fues Wahl, H.; et al. Fine scale mapping of the 17q22 breast cancer locus using dense SNPs, genotyped within the Collaborative Oncological Gene-Environment Study (COGs). Sci. Rep. 2016, 6, 32512. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, K.; Hall, P.; Gonzalez-Neira, A.; Ghoussaini, M.; Dennis, J.; Milne, R.L.; Schmidt, M.K.; Chang-Claude, J.; Bojesen, S.E.; Bolla, M.K.; et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet. 2013, 45, 353–361. [Google Scholar] [CrossRef]
- Southey, M.C.; Goldgar, D.E.; Winqvist, R.; Pylkas, K.; Couch, F.; Tischkowitz, M.; Foulkes, W.D.; Dennis, J.; Michailidou, K.; van Rensburg, E.J.; et al. PALB2, CHEK2 and ATM rare variants and cancer risk: Data from COGS. J. Med. Genet. 2016, 53, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.F.; Pharoah, P.D.; Antoniou, A.C.; Tischkowitz, M.; Tavtigian, S.V.; Nathanson, K.L.; Devilee, P.; Meindl, A.; Couch, F.J.; Southey, M.; et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 2015, 372, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- LaDuca, H.; Polley, E.C.; Yussuf, A.; Hoang, L.; Gutierrez, S.; Hart, S.N.; Yadav, S.; Hu, C.; Na, J.; Goldgar, D.E.; et al. A clinical guide to hereditary cancer panel testing: Evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet. Med. 2020, 22, 407–415. [Google Scholar] [CrossRef]
- Kurian, A.W.; Ward, K.C.; Howlader, N.; Deapen, D.; Hamilton, A.S.; Mariotto, A.; Miller, D.; Penberthy, L.S.; Katz, S.J. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J. Clin. Oncol. 2019, 37, 1305–1315. [Google Scholar] [CrossRef]
- Couch, F.J.; Shimelis, H.; Hu, C.; Hart, S.N.; Polley, E.C.; Na, J.; Hallberg, E.; Moore, R.; Thomas, A.; Lilyquist, J.; et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017, 3, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.M.; Li, S.; Black, M.H.; Lee, S.; Hoiness, R.; Wu, S.; Mu, W.; Huether, R.; Chen, J.; Sridhar, S.; et al. Association of Breast and Ovarian Cancers With Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol. 2019, 5, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hauke, J.; Horvath, J.; Gross, E.; Gehrig, A.; Honisch, E.; Hackmann, K.; Schmidt, G.; Arnold, N.; Faust, U.; Sutter, C.; et al. Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: Results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancer Med. 2018, 7, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Fostira, F.; Kostantopoulou, I.; Apostolou, P.; Papamentzelopoulou, M.S.; Papadimitriou, C.; Faliakou, E.; Christodoulou, C.; Boukovinas, I.; Razis, E.; Tryfonopoulos, D.; et al. One in three highly selected Greek patients with breast cancer carries a loss-of-function variant in a cancer susceptibility gene. J. Med. Genet. 2020, 57, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.P.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Hopper, J.L.; Southey, M.C.; Dite, G.S.; Jolley, D.J.; Giles, G.G.; McCredie, M.R.; Easton, D.F.; Venter, D.J. Population-based estimate of the average age-specific cumulative risk of breast cancer for a defined set of protein-truncating mutations in BRCA1 and BRCA2. Australian Breast Cancer Family Study. Cancer Epidemiol. Biomark. Prev. 1999, 8, 741–747. [Google Scholar]
- Southey, M.C.; Teo, Z.L.; Dowty, J.G.; Odefrey, F.A.; Park, D.J.; Tischkowitz, M.; Sabbaghian, N.; Apicella, C.; Byrnes, G.B.; Winship, I.; et al. A PALB2 mutation associated with high risk of breast cancer. Breast Cancer Res. 2010, 12, R109. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Hannon, N.; Whittemore, A.S. Estimating gene penetrance from family data. Genet. Epidemiol. 2010, 34, 373–381. [Google Scholar] [CrossRef]
- Smith, L.D.; Tesoriero, A.A.; Ramus, S.J.; Dite, G.; Royce, S.G.; Giles, G.G.; McCredie, M.R.; Hopper, J.L.; Southey, M.C. BRCA1 promoter deletions in young women with breast cancer and a strong family history: A population-based study. Eur. J. Cancer 2007, 43, 823–827. [Google Scholar] [CrossRef][Green Version]
- Smith, L.D.; Tesoriero, A.A.; Wong, E.M.; Ramus, S.J.; O’Malley, F.P.; Mulligan, A.M.; Terry, M.B.; Senie, R.T.; Santella, R.M.; John, E.M.; et al. Contribution of large genomic BRCA1 alterations to early-onset breast cancer selected for family history and tumour morphology: A report from The Breast Cancer Family Registry. Breast Cancer Res. 2011, 13, R14. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.; Hughes, E.; Wagner, S.; Tshiaba, P.; Rosenthal, E.; Roa, B.B.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Lancaster, J.; et al. Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw. Open 2020, 3, e208501. [Google Scholar] [CrossRef]
- De Silva, D.L.; Winship, I. Is CHEK2 a moderate-risk breast cancer gene or the younger sister of Li-Fraumeni? BMJ Case Rep. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Brandao, A.; Paulo, P.; Maia, S.; Pinheiro, M.; Peixoto, A.; Cardoso, M.; Silva, M.P.; Santos, C.; Eeles, R.A.; Kote-Jarai, Z.; et al. The CHEK2 Variant C.349A>G Is Associated with Prostate Cancer Risk and Carriers Share a Common Ancestor. Cancers 2020, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- McCredie, M.R.; Dite, G.S.; Giles, G.G.; Hopper, J.L. Breast cancer in Australian women under the age of 40. Cancer Causes Control 1998, 9, 189–198. [Google Scholar] [CrossRef]
- John, E.M.; Hopper, J.L.; Beck, J.C.; Knight, J.A.; Neuhausen, S.L.; Senie, R.T.; Ziogas, A.; Andrulis, I.L.; Anton-Culver, H.; Boyd, N.; et al. The Breast Cancer Family Registry: An infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004, 6, R375–R389. [Google Scholar] [CrossRef]
- Dite, G.S.; Jenkins, M.A.; Southey, M.C.; Hocking, J.S.; Giles, G.G.; McCredie, M.R.E.; Venter, D.J.; Hopper, J.L. Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J. Natl. Cancer Inst. 2003, 95, 448–457. [Google Scholar] [CrossRef]
- Hammet, F.; Mahmood, K.; Green, T.R.; Nguyen-Dumont, T.; Southey, M.C.; Buchanan, D.D.; Lonie, A.; Nathanson, K.L.; Couch, F.J.; Pope, B.J.; et al. Hi-Plex2: A simple and robust approach to targeted sequencing-based genetic screening. Biotechniques 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Lai, Z.; Markovets, A.; Ahdesmaki, M.; Chapman, B.; Hofmann, O.; McEwen, R.; Johnson, J.; Dougherty, B.; Barrett, J.C.; Dry, J.R. VarDict: A novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016, 44, e108. [Google Scholar] [CrossRef]
- Antoniou, A.C.; Pharoah, P.D.; McMullan, G.; Day, N.E.; Ponder, B.A.; Easton, D. Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet. Epidemiol. 2001, 21, 1–18. [Google Scholar] [CrossRef]
- Lange, K. Mathematical and Statistical Methods for Genetic Analysis; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Lange, K.; Weeks, D.; Boehnke, M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet. Epidemiol. 1988, 5, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Thomas, D.C. Bias and efficiency in family-based gene-characterization studies: Conditional, prospective, retrospective, and joint likelihoods. Am. J. Hum. Genet. 2000, 66, 1119–1131. [Google Scholar] [CrossRef]
- Dowty, J.G.; Win, A.K.; Buchanan, D.D.; Lindor, N.M.; Macrae, F.A.; Clendenning, M.; Antill, Y.C.; Thibodeau, S.N.; Casey, G.; Gallinger, S.; et al. Cancer risks for MLH1 and MSH2 mutation carriers. Hum. Mutat. 2013, 34, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Forman, D. Cancer Incidence in Five Continents, CI5plus: IARC Cancer Base No. 9; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Jekimovs, C.R.; Chen, X.; Arnold, J.; Gatei, M.; Richard, D.J.; Spurdle, A.B.; Khanna, K.K.; Chenevix-Trench, G.; kConFab, I. Low frequency of CHEK2 1100delC allele in Australian multiple-case breast cancer families: Functional analysis in heterozygous individuals. Br. J. Cancer 2005, 92, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, D.M.; Malone, K.E.; Doody, D.R.; Daling, J.R.; Ostrander, E.A. Frequency of CHEK2 mutations in a population based, case-control study of breast cancer in young women. Breast Cancer Res. 2004, 6, R629–R635. [Google Scholar] [CrossRef]
- Bell, D.W.; Kim, S.H.; Godwin, A.K.; Schiripo, T.A.; Harris, P.L.; Haserlat, S.M.; Wahrer, D.C.; Haiman, C.A.; Daly, M.B.; Niendorf, K.B.; et al. Genetic and functional analysis of CHEK2 (CHK2) variants in multiethnic cohorts. Int. J. Cancer 2007, 121, 2661–2667. [Google Scholar] [CrossRef]
- Cannings, C.; Thompson, E.; Skolnick, M. Probability functions on complex pedigrees. Adv. Appl. Probab. 1978, 10, 26–61. [Google Scholar] [CrossRef]
- Pharoah, P.D.; Antoniou, A.; Bobrow, M.; Zimmern, R.L.; Easton, D.F.; Ponder, B.A. Polygenic susceptibility to breast cancer and implications for prevention. Nat. Genet. 2002, 31, 33–36. [Google Scholar] [CrossRef] [PubMed]
| Variant Type | HGVS.c b | HGVS.p b | Number of Relatives Who Are Carriers/Tested/Total | Number of Relatives with Breast Cancer Who Are Carriers/Tested/Total | |
|---|---|---|---|---|---|
| Case proband | Nonsense | NM_007194.3:c.1528C>T | NP_009125.1:p.Gln510Ter | 3/5/34 | 1/1/3 |
| NM_007194.3:c.823G>T | NP_009125.1:p.Glu275Ter | 1/1/97 | 0/0/5 | ||
| Frameshift | NM_007194.3:c.1263delT | NP_009125.1:p.Ser422Valfs *15 | 0/0/40 | 0/0/1 | |
| NM_007194.3:c.1263delT | NP_009125.1:p.Ser422Valfs *15 | 3/5/78 | 2/2/3 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 2/6/46 | 1/2/4 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 2/3/20 | 0/0/0 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 0/5/16 | 0/0/1 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 4/17/67 | 2/5/10 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 2/3/41 | 0/0/0 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 1/4/75 | 0/1/1 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 1/1/19 | 0/0/0 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 2/2/33 | 0/0/0 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 0/0/19 | 0/0/0 | ||
| NM_007194.3:c.920dupG | NP_009125.1:p.Glu308Argfs *4 | 1/1/17 | 0/0/0 | ||
| NM_007194.3:c.405delA | NP_009125.1:p.Lys135Asnfs *26 | 0/0/47 | 0/0/2 | ||
| Splice donor | NM_007194.3:c.444+1G>A | 0/0/18 | 0/0/0 | ||
| NM_007194.3:c.319+2T>A c | 2/2/23 | 0/0/0 | |||
| Missense | NM_007194.3:c.349A>G | NP_009125.1:p.Arg117Gly | 3/4/32 | 2/2/2 | |
| NM_007194.3:c.349A>G | NP_009125.1:p.Arg117Gly | 2/12/190 | 1/1/6 | ||
| NM_007194.3:c.349A>G | NP_009125.1:p.Arg117Gly | 1/3/24 | 0/0/1 | ||
| Control proband | Frameshift | NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 0/0/41 | 0/0/2 |
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 0/0/19 | 0/0/0 | ||
| NM_007194.3:c.1100delC | NP_009125.1:p.Thr367Metfs *15 | 0/0/32 | 0/0/0 | ||
| NM_007194.3:c.591delA | NP_009125.1:p.Val198Phefs *7 | 0/0/23 | 0/0/1 | ||
| Missense | NM_007194.3:c.349A>G | NP_009125.1:p.Arg117Gly | 0/0/21 | 0/0/1 | |
| NM_007194.3:c.349A>G | NP_009125.1:p.Arg117Gly | 0/0/13 | 0/0/0 | ||
| NM_007194.3:c.349A>G | NP_009125.1:p.Arg117Gly | 0/0/9 | 0/0/1 |
| Age (Years) | Cumulative Risk (%) to Each Given Age, with 95% Confidence Intervals in Parentheses |
|---|---|
| 30 | 0.4 (0.2–0.7) |
| 40 | 2.6 (1.4–5.1) |
| 50 | 9.1 (5.1–16) |
| 60 | 18 (11–30) |
| 70 | 26 (16–40) |
| 80 | 33 (21–48) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Dumont, T.; Dowty, J.G.; Steen, J.A.; Renault, A.-L.; Hammet, F.; Mahmoodi, M.; Theys, D.; Rewse, A.; Tsimiklis, H.; Winship, I.M.; et al. Population-Based Estimates of the Age-Specific Cumulative Risk of Breast Cancer for Pathogenic Variants in CHEK2: Findings from the Australian Breast Cancer Family Registry. Cancers 2021, 13, 1378. https://doi.org/10.3390/cancers13061378
Nguyen-Dumont T, Dowty JG, Steen JA, Renault A-L, Hammet F, Mahmoodi M, Theys D, Rewse A, Tsimiklis H, Winship IM, et al. Population-Based Estimates of the Age-Specific Cumulative Risk of Breast Cancer for Pathogenic Variants in CHEK2: Findings from the Australian Breast Cancer Family Registry. Cancers. 2021; 13(6):1378. https://doi.org/10.3390/cancers13061378
Chicago/Turabian StyleNguyen-Dumont, Tú, James G. Dowty, Jason A. Steen, Anne-Laure Renault, Fleur Hammet, Maryam Mahmoodi, Derrick Theys, Amanda Rewse, Helen Tsimiklis, Ingrid M. Winship, and et al. 2021. "Population-Based Estimates of the Age-Specific Cumulative Risk of Breast Cancer for Pathogenic Variants in CHEK2: Findings from the Australian Breast Cancer Family Registry" Cancers 13, no. 6: 1378. https://doi.org/10.3390/cancers13061378
APA StyleNguyen-Dumont, T., Dowty, J. G., Steen, J. A., Renault, A.-L., Hammet, F., Mahmoodi, M., Theys, D., Rewse, A., Tsimiklis, H., Winship, I. M., Giles, G. G., Milne, R. L., Hopper, J. L., & Southey, M. C. (2021). Population-Based Estimates of the Age-Specific Cumulative Risk of Breast Cancer for Pathogenic Variants in CHEK2: Findings from the Australian Breast Cancer Family Registry. Cancers, 13(6), 1378. https://doi.org/10.3390/cancers13061378






