Salvage Radiation for Pelvic Relapse after Surgically Treated Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
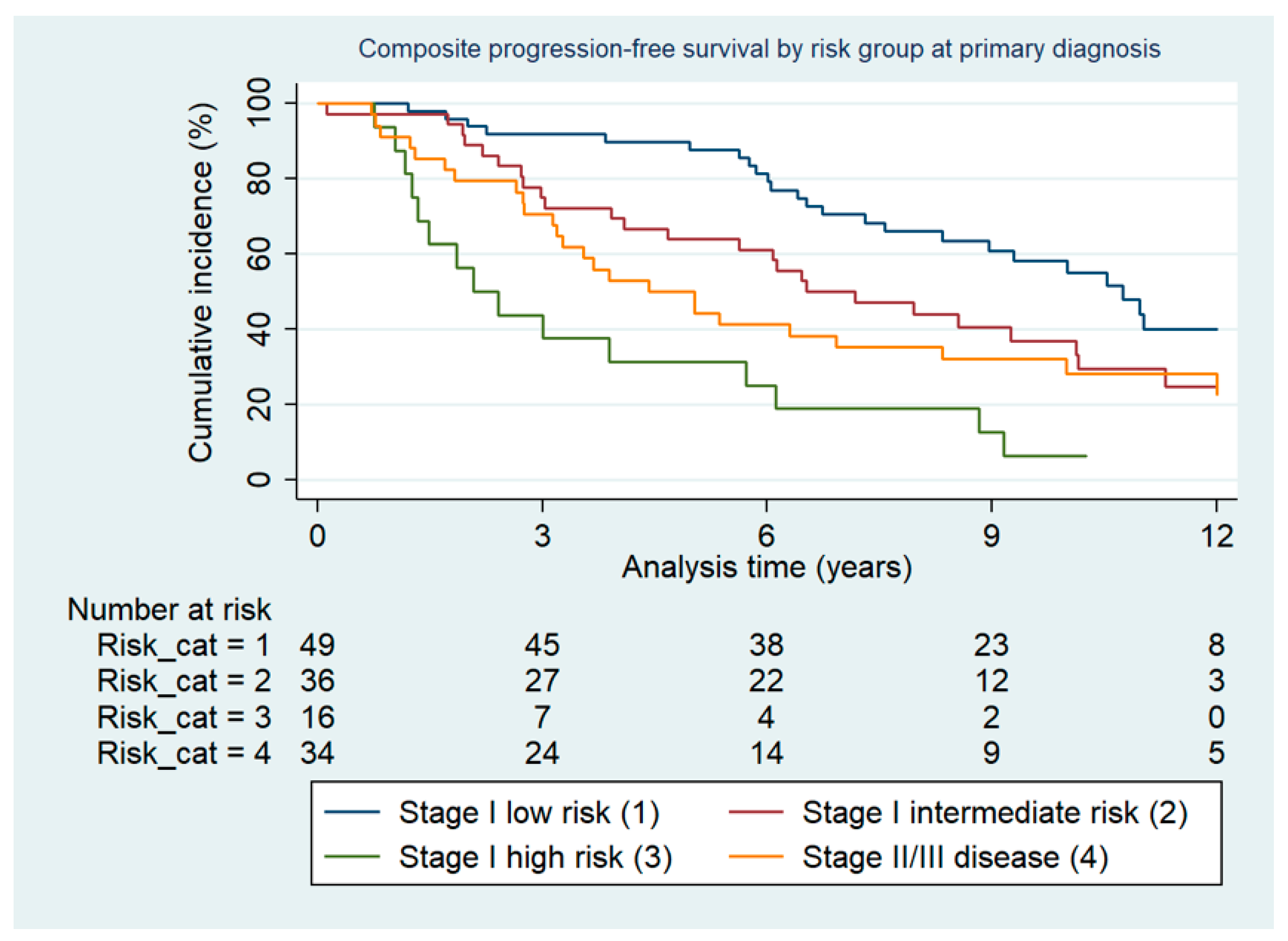
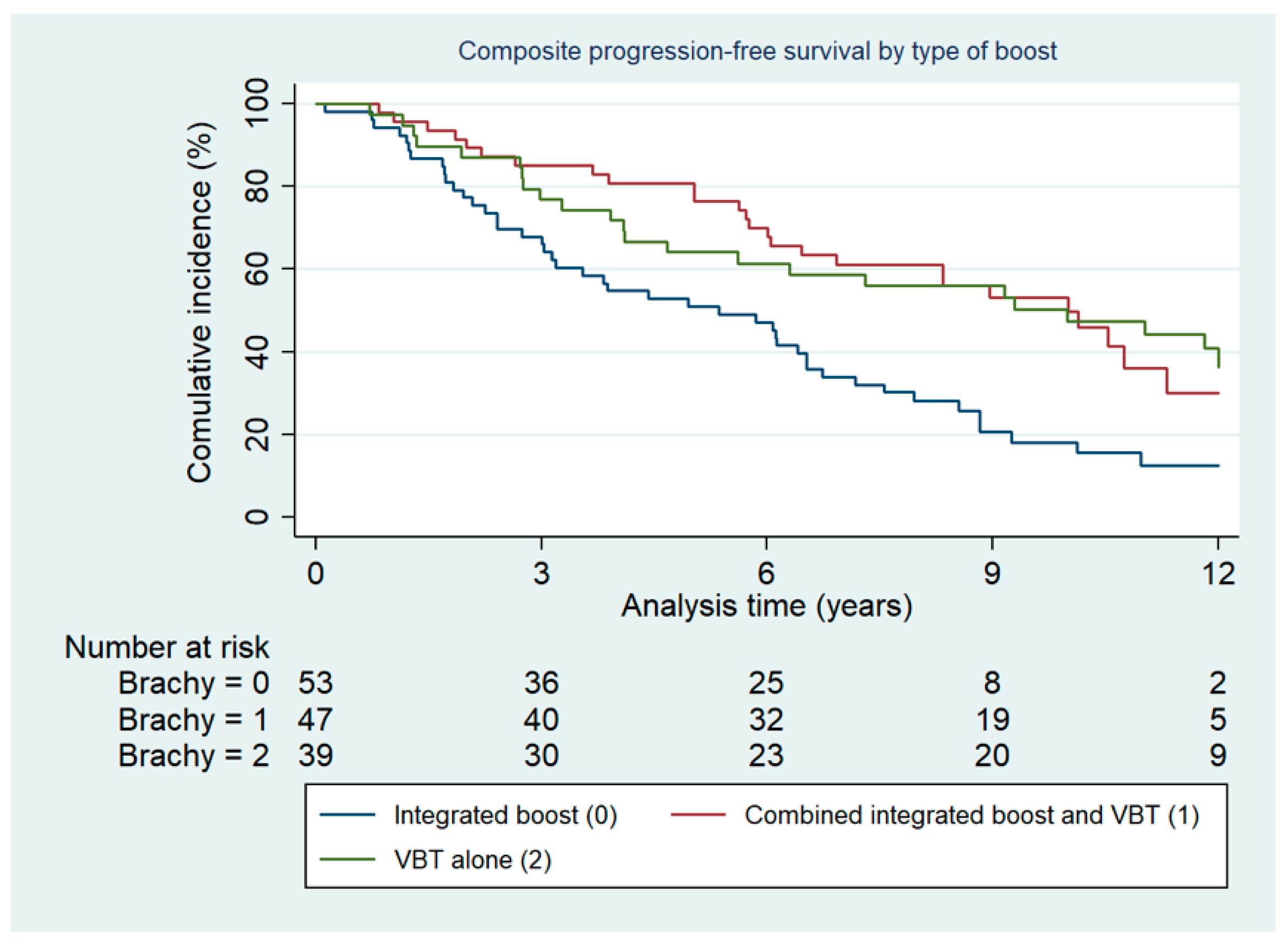
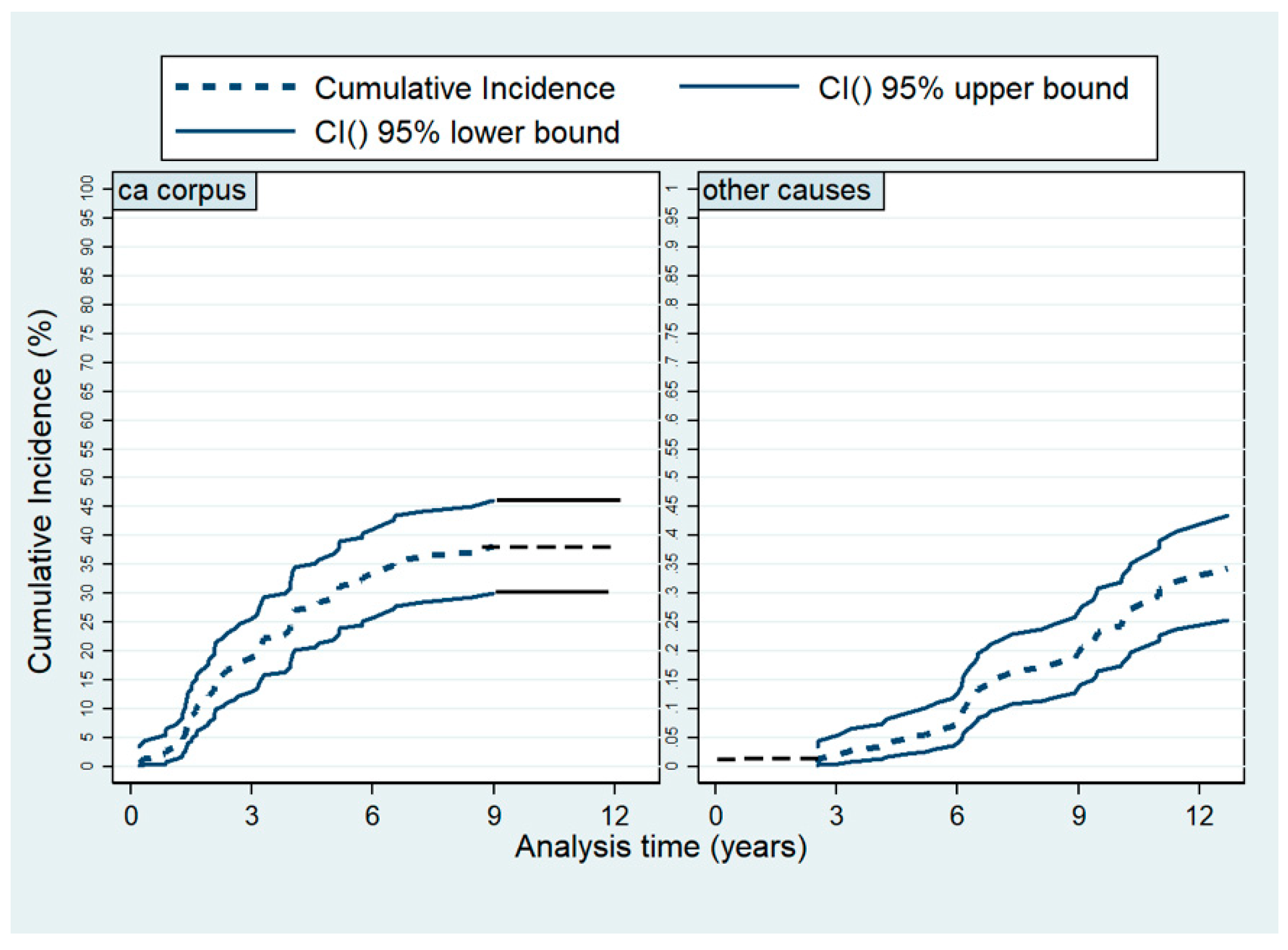
2.1. Survival
2.2. Predictive Factors for Survival: Relative Risk of Second Relapse
2.3. Predictive Factors for Survival: Overall Mortality Risk
3. Discussion
4. Materials and Methods
4.1. Patients and Follow-Up
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. J. Int. Du Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. Esmo 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Jeppesen, M.M.; Jensen, P.T.; Gilsa Hansen, D.; Iachina, M.; Mogensen, O. The nature of early-stage endometrial cancer recurrence-A national cohort study. Eur J Cancer 2016, 69, 51–60. [Google Scholar] [CrossRef]
- Smogeli, E.; Davidson, B.; Cvancarova, M.; Holth, A.; Katz, B.; Risberg, B.; Kristensen, G.; Lindemann, K. L1CAM as a prognostic marker in stage I endometrial cancer: A validation study. BMC Cancer 2016, 16, 596. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Aalders, J.; Abeler, V.; Kolstad, P.; Onsrud, M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet. Gynecol. 1980, 56, 419–427. [Google Scholar] [PubMed]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Swart, A.M.; Orton, J.; Kitchener, H.; Whelan, T.; Lukka, H.; Eisenhauer, E.; Bacon, M.; Tu, D.; Parmar, M.K.; et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009, 373, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; van de Poll-Franse, L.V.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, B.; van Putten, W.L.; Creutzberg, C.L. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1692–1700. [Google Scholar] [CrossRef]
- Onsrud, M.; Cvancarova, M.; Hellebust, T.P.; Trope, C.G.; Kristensen, G.B.; Lindemann, K. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Stenfert Kroese, M.C.; van Bunningen, B.N.; et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: First results of the randomized PORTEC-2 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3547–3556. [Google Scholar] [CrossRef]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van der Steen-Banasik, E.; et al. Survival after relapse in patients with endometrial cancer: Results from a randomized trial. Gynecol. Oncol. 2003, 89, 201–209. [Google Scholar] [CrossRef]
- Bertelsen, K.; Ortoft, G.; Hansen, E.S. Survival of Danish patients with endometrial cancer in the intermediate-risk group not given postoperative radiotherapy: The Danish Endometrial Cancer Study (DEMCA). Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Stelloo, E.; Bosse, T.; Nout, R.A.; MacKay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 2015, 28, 836–844. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. Esmo 2018, 29, 1180–1188. [Google Scholar] [CrossRef]
- Norwegian Directorate of Health. Nasjonalt Handlingsprogram Med Retningslinjer for Gynekologisk Kreft. Available online: https://www.helsedirektoratet.no/ (accessed on 10 March 2021).
- Smogeli, E.; Cvancarova, M.; Wang, Y.; Davidson, B.; Kristensen, G.; Lindemann, K. Clinical Outcome of Patients With High-Risk Endometrial Carcinoma After Treatment With Chemotherapy Only. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.K.; Straughn, J.M., Jr.; Mariani, A.; Podratz, K.C.; Havrilesky, L.J.; Alvarez-Secord, A.; Gold, M.A.; McMeekin, D.S.; Modesitt, S.; Cooper, A.L.; et al. Salvage of isolated vaginal recurrences in women with surgical stage I endometrial cancer: A multiinstitutional experience. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2007, 17, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Isohashi, F.; Yamaguchi, H.; Mabuchi, S.; Yoshida, K.; Kotsuma, T.; Yamazaki, H.; Tanaka, E.; Sumida, I.; Tamari, K.; et al. Salvage high-dose-rate brachytherapy for isolated vaginal recurrence of endometrial cancer. Brachytherapy 2016, 15, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.H.; Maghsoudi, K.; Littell, R.D.; Chen, L.M.; Hsu, I.C. Salvage high-dose-rate brachytherapy and external beam radiotherapy for isolated vaginal recurrences of endometrial cancer with no prior adjuvant therapy. Brachytherapy 2017, 16, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Jhingran, A.; Burke, T.W.; Eifel, P.J. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1366–1372. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.; Badzio, A.; Jassem, J. Recurrent endometrial cancer after surgery alone: Results of salvage radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 405–413. [Google Scholar] [CrossRef]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Final results of the international randomized PORTEC-3 trial of adjuvant chemotherapy and radiation therapy (RT) versus RT alone for women with high-risk endometrial cancer. J. Clin. Oncol. 2017, 35, 5502. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value | Value |
|---|---|---|
| Age (mean/range) | 71.3 years | 43.9 to 88.8 years |
| Stage at initial diagnosis | n | % |
| IA | 71 | 51.08% |
| IB | 34 | 24.46% |
| II | 16 | 11.51% |
| III | 18 | 12.95% |
| Histology at primary diagnosis | ||
| Endometrioid adenocarcinoma | 111 | 79.86% |
| Serous/clear cell adenocarcinoma | 20 | 14.39% |
| Other * | 6 | 4.32% |
| Risk group at primary diagnosis for patients with stage I disease ** | ||
| Stage I low-risk | 49 | 36.30% |
| Stage I intermediate-risk | 36 | 26.67% |
| Stage I high-risk | 16 | 11.85% |
| Previous treatment | ||
| Hysterectomy/BSOE | 139 | 100% |
| Pelvic lymphadenectomy | 81 | 58.27% |
| Adjuvant chemotherapy | 28 | 20.14% |
| Variable | Association with Risk of Relapse | Association with Risk of Death | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | * | * | * | 1.03 | 1.00–1.05 | 0.04 |
| Stage I low risk (reference) | 1.0 | 1.0 | ||||
| Stage I intermediate risk | 1.93 | 0.86–4.35 | 0.112 | 1.27 | 0.69–2.36 | 0.44 |
| Stage I high risk | 5.60 | 2.36–13.27 | <0.001 | 3.68 | 1.83–7.42 | <0.01 |
| Stage II–III | 3.31 | 1.52–7.18 | 0.002 | 2.34 | 1.30–4.21 | 0.01 |
| External boost only (reference) | 1.0 | 1.0 | ||||
| Combined boost | 0.41 | 0.21–0.79 | 0.008 | 0.50 | 0.29–0.86 | 0.01 |
| VBT | 0.48 | 0.25–0.93 | 0.030 | 0.45 | 0.25–0.80 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindemann, K.; Smogeli, E.; Småstuen, M.C.; Bruheim, K.; Trovik, J.; Nordberg, T.; Kristensen, G.B.; Werner, H.M.J.; Nakken, E. Salvage Radiation for Pelvic Relapse after Surgically Treated Endometrial Cancer. Cancers 2021, 13, 1367. https://doi.org/10.3390/cancers13061367
Lindemann K, Smogeli E, Småstuen MC, Bruheim K, Trovik J, Nordberg T, Kristensen GB, Werner HMJ, Nakken E. Salvage Radiation for Pelvic Relapse after Surgically Treated Endometrial Cancer. Cancers. 2021; 13(6):1367. https://doi.org/10.3390/cancers13061367
Chicago/Turabian StyleLindemann, Kristina, Elisabeth Smogeli, Milada Cvancarova Småstuen, Kjersti Bruheim, Jone Trovik, Terje Nordberg, Gunnar B. Kristensen, Henrica M. J. Werner, and Esten Nakken. 2021. "Salvage Radiation for Pelvic Relapse after Surgically Treated Endometrial Cancer" Cancers 13, no. 6: 1367. https://doi.org/10.3390/cancers13061367
APA StyleLindemann, K., Smogeli, E., Småstuen, M. C., Bruheim, K., Trovik, J., Nordberg, T., Kristensen, G. B., Werner, H. M. J., & Nakken, E. (2021). Salvage Radiation for Pelvic Relapse after Surgically Treated Endometrial Cancer. Cancers, 13(6), 1367. https://doi.org/10.3390/cancers13061367






