Neoadjuvant Chemoradiation Combined with Regional Hyperthermia in Locally Advanced or Recurrent Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
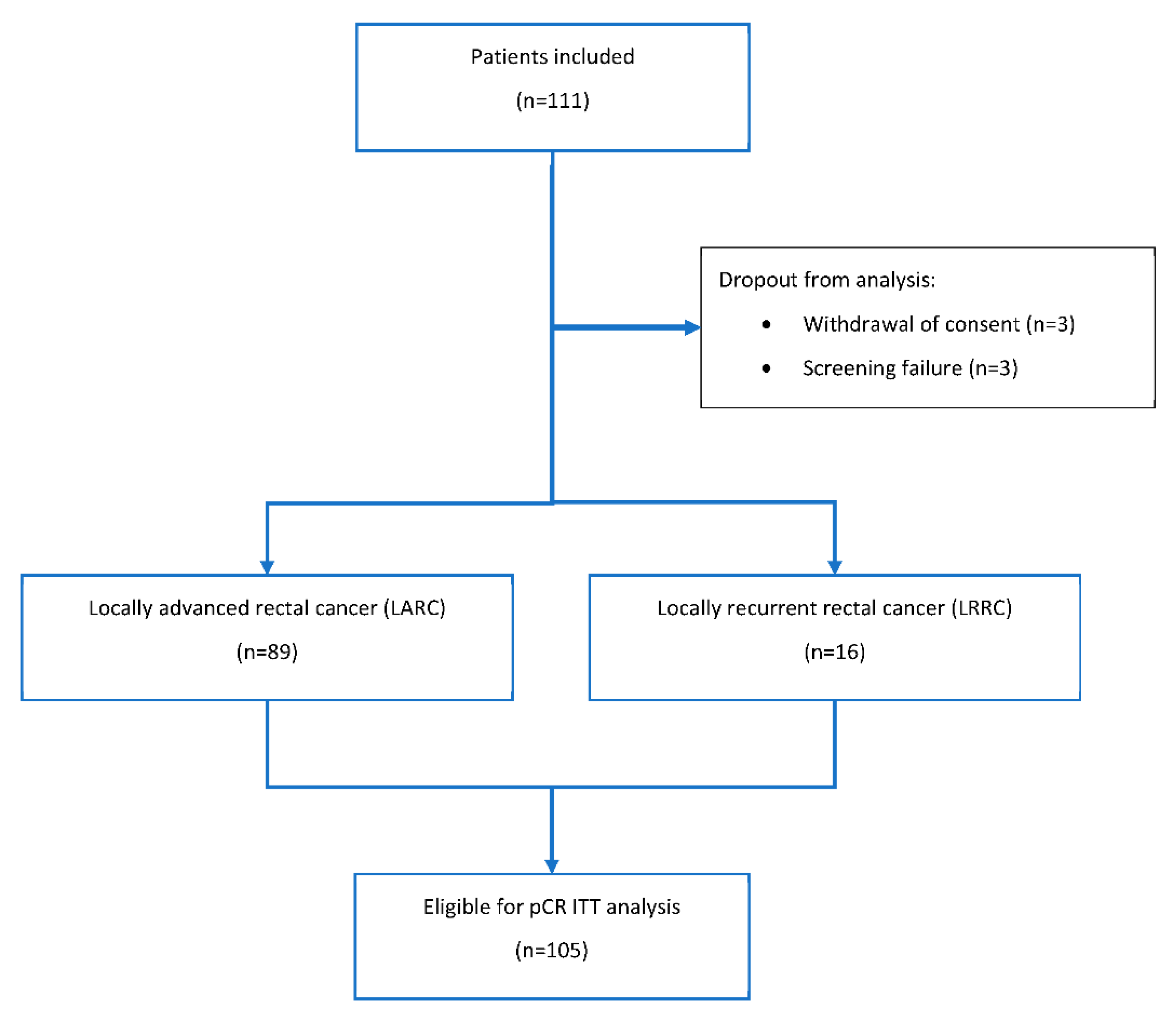
2. Patients and Methods
3. Results
3.1. Protocol Treatment Adherence
3.2. Stage 1 Feasibility Analysis
3.3. Stage 2 Feasibility Analysis
3.4. Primary Efficacy Endpoint pCR Rate und Tumor Regression Grading
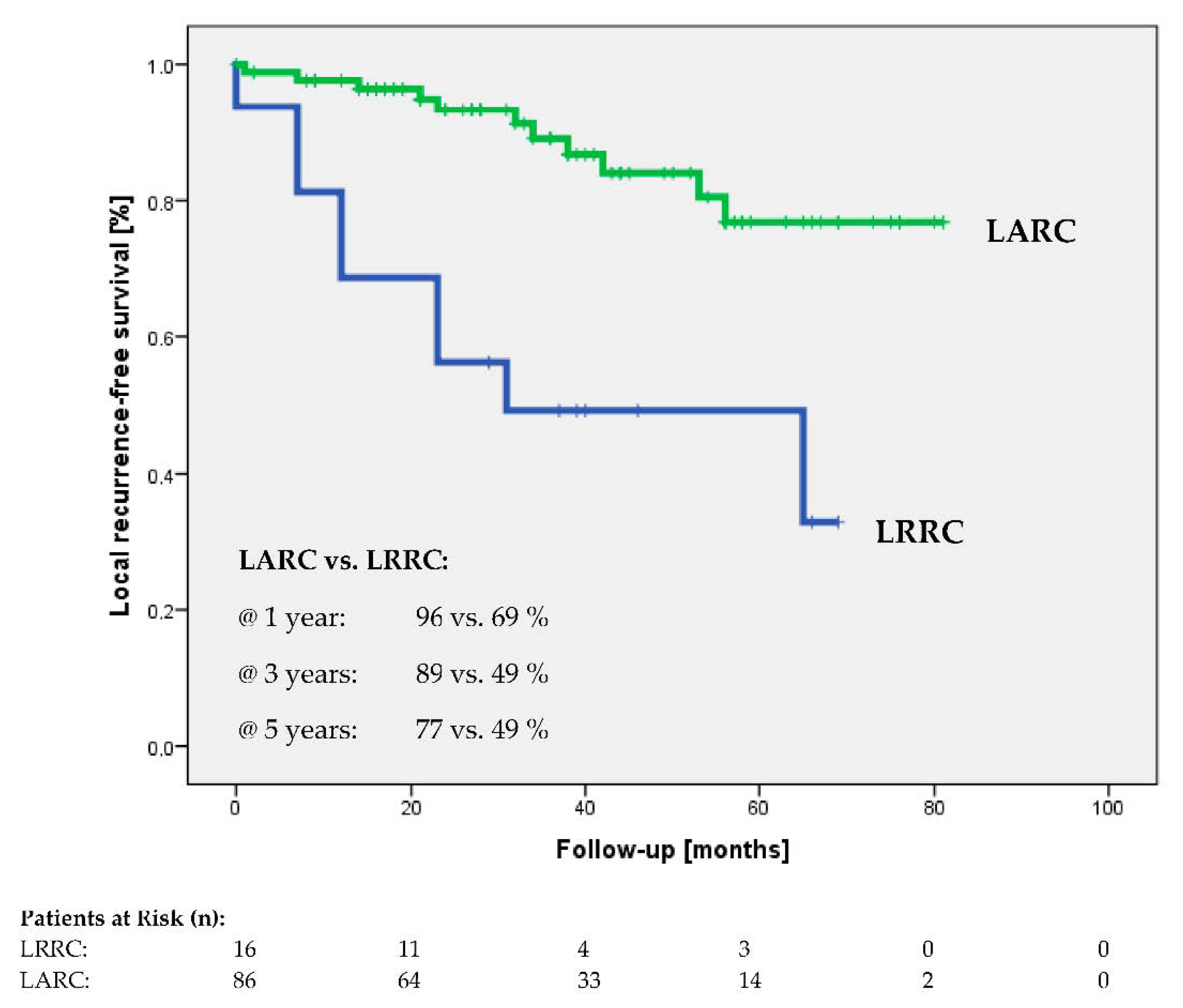
3.5. Secondary Endpoints
3.6. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanis, P.J.; Doeksen, A.; van Lanschot, J.J. Intentionally curative treatment of locally recurrent rectal cancer: A systematic review. Can. J. Surg. 2013, 56, 135–144. [Google Scholar] [CrossRef]
- Berdov, B.A.; Menteshashvili, G.Z. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int. J. Hyperth. 1990, 6, 881–890. [Google Scholar] [CrossRef]
- De Haas-Kock, D.F.; Buijsen, J.; Pijls-Johannesma, M.; Lutgens, L.; Lammering, G.; van Mastrigt, G.A.; De Ruysscher, D.K.; Lambin, P.; van der Zee, J. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst. Rev. 2009, 3, CD006269. [Google Scholar] [CrossRef]
- Van der Zee, J.; González González, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Gani, C.; Bonomo, P.; Zwirner, K.; Schroeder, C.; Menegakis, A.; Rödel, C.; Zips, D. Organ preservation in rectal cancer—Challenges and future strategies. Clin. Transl. Radiat. Oncol. 2017, 3, 9–15. [Google Scholar] [CrossRef][Green Version]
- Huang, A.; Xiao, Y.; Peng, C.; Liu, T.; Lin, Z.; Yang, Q.; Zhang, T.; Liu, J.; Ma, H. 53BP1 expression and immunoscore are associated with the efficacy of neoadjuvant chemoradiotherapy for rectal cancer. Strahlenther. Onkol. 2020, 196, 465–473. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Chavaudra, J.; Bridier, A. Definition of volumes in external radiotherapy: ICRU reports 50 and 62. Cancer Radiother. 2001, 5, 472–478. [Google Scholar] [CrossRef]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther. Onkol. 2011, 187, 605–610. [Google Scholar] [CrossRef]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: Quality management in regional deep hyperthermia. Strahlenther. Onkol. 2012, 188 (Suppl. 2), 198–211. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Grabenbauer, G.G.; Papadopoulos, T.; Hohenberger, W.; Schmoll, H.J.; Sauer, R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J. Clin. Oncol. 2003, 21, 3098–3104. [Google Scholar] [CrossRef]
- Fokas, E.; Ströbel, P.; Fietkau, R.; Ghadimi, M.; Liersch, T.; Grabenbauer, G.; Hartmann, A.; Kaufmann, M.; Sauer, R.; Graeven, U.; et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J. Natl. Cancer Inst. 2017, 109, djx095. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Colorectal Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Simon, R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials 1989, 10, 1–10. [Google Scholar] [CrossRef]
- Schroeder, C.; Gani, C.; Lamprecht, U.; von Weyhern, C.H.; Weinmann, M.; Bamberg, M.; Berger, B. Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int. J. Hyperth. 2012, 28, 707–714. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Lee, J.; Kim, C.Y.; Koom, W.S.; Rim, C.H. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: A meta-analysis and systematic review. Radiother. Oncol. 2019, 140, 10–19. [Google Scholar] [CrossRef]
- Gani, C.; Schroeder, C.; Heinrich, V.; Spillner, P.; Lamprecht, U.; Berger, B.; Zips, D. Long-term local control and survival after preoperative radiochemotherapy in combination with deep regional hyperthermia in locally advanced rectal cancer. Int. J. Hyperth. 2016, 32, 187–192. [Google Scholar] [CrossRef]
- De-Colle, C.; Weidner, N.; Heinrich, V.; Brucker, S.; Hahn, M.; MacMillan, K.; Lamprecht, U.; Gaupp, S.; Voigt, O.; Zips, D. Hyperthermic chest wall re-irradiation in recurrent breast cancer: A prospective observational study. Strahlenther. Onkol. 2019, 195, 318–326. [Google Scholar] [CrossRef]
- Guren, M.G.; Undseth, C.; Rekstad, B.L.; Brændengen, M.; Dueland, S.; Garm Spindler, K.L.; Glynne-Jones, R.; Tveit, K.M. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother. Oncol. 2014, 113, 151–157. [Google Scholar] [CrossRef]
- Alberda, W.J.; Verhoef, C.; Nuyttens, J.J.; Rothbarth, J.; van Meerten, E.; de Wilt, J.H.; Burger, J.W. Outcome in patients with resectable locally recurrent rectal cancer after total mesorectal excision with and without previous neoadjuvant radiotherapy for the primary rectal tumor. Ann. Surg. Oncol. 2014, 21, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Fucini, C.; Pellino, G.; Sciaudone, G.; Maretto, I.; Mondi, I.; Bartolini, N.; Caminati, F.; Pucciarelli, S. Outcome and prognostic factors of local recurrent rectal cancer: A pooled analysis of 150 patients. Tech. Coloproctol. 2015, 19, 135–144. [Google Scholar] [CrossRef]
- Gambacorta, M.A.; Masciocchi, C.; Chiloiro, G.; Meldolesi, E.; Macchia, G.; van Soest, J.; Peters, F.; Collette, L.; Gérard, J.P.; Ngan, S.; et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: A pooled analysis of 3085 patients from 7 randomized trials. Radiother. Oncol. 2020, 154, 154–160. [Google Scholar] [CrossRef]
- Rödel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.R.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef]
- Rödel, C.; Liersch, T.; Hermann, R.M.; Arnold, D.; Reese, T.; Hipp, M.; Fürst, A.; Schwella, N.; Bieker, M.; Hellmich, G.; et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J. Clin. Oncol. 2007, 25, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Gani, N.; Zschaeck, S.; Eberle, F.; Schaeffeler, N.; Hehr, T.; Berger, B.; Fischer, S.G.; Claßen, J.; Zipfel, S.; et al. Organ Preservation in Rectal Cancer: The Patients’ Perspective. Front. Oncol. 2019, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Beart, R.W.; Wozniak, T.F.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; Arora, A.; et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J. Natl. Cancer Inst. 2015, 107, djv248. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Stein, A.; Van Cutsem, E.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Pre-and Postoperative Capecitabine Without or With Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J. Clin. Oncol. 2021, 39, 17–29. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.; Putter, H.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.; Nagtegaal, I.D.; Beets-Tan, R.G.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trialShort-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar]
| Characteristics | Locally Advanced Rectal Cancer | Locally Recurrent Rectal Cancer (LRRC, n = 16) |
|---|---|---|
| (LARC, n = 89) | ||
| Initial clinical T-category (n/N [%]) | ||
| T2/rT2 | 6/89 (7) | 1/16 (6) |
| T3/rT3 | 63/89 (71) | 7/16 (44) |
| T4/rT4 | 19/89 (21) | 8/16 (50) |
| n.a. | 1/89 (1) | - |
| Initial clinical N-category (n/N [%]) | ||
| N0/rN0 | 6/89 (7) | 11/16 (69) |
| N1/rN0 | 46/89 (52) | 2/16 (12) |
| N2/rN2 | 36/89 (40) | 3/16 (19) |
| n.a. | 1/89 (1) | - |
| Initial M-category (n/N [%]) | ||
| M0 | 84/89 (94) | 11/16 (69) |
| M1 | 4/89 (5) | 5/16 (31) |
| n.a. | 1/89 (1) | - |
| Previous oncological treatments (n/N [%]) | ||
| surgery | 1/89 (1) | 15/16 (94) |
| radiotherapy | - | 10/16 (63) |
| chemotherapy | - | 12/16 (75) |
| External beam radiotherapy | ||
| median total dose [Gy] | 50.4 (45.0–50.4) | 45.0 (45.0–50.4) |
| median single dose [Gy] | 1.80 (-) | 1.80 (-) |
| radiotherapy delay (n/N [%]) * | 5/88 (6) | 3/16 (19) |
| median duration [days] | 2 (2–6) | 1 (-) |
| total dose reduction (n/N [%]) * | 1/88 (1) | - |
| premature termination * | 1/89 (1) | - |
| Chemotherapy (n/N [%]) | ||
| schedule | ||
| 5-FU and oxaliplatin | 81/89 (91) | 12/16 (75) |
| capecitabine and oxaliplatin | 7/89 (8) | 3/16 (19) |
| 5-FU/capecitabine and oxaliplatin | 1/89 (1) | 1/16 (6) |
| 5-FU protocol adherence | ||
| administered per protocol | 56/81 (69) | 11/12 (92) |
| interval prolongation * | 14/81 (17) | 1/12 (8) |
| dose reduction * | 11/81 (14) | 1/12 (8) |
| premature termination * | 2/81 (2) | - |
| n.a. | 2/81 (2) | - |
| capecitabine protocol adherence | ||
| administered per protocol | 3/7 (43) | 2/3 (67) |
| interval prolongation * | 3/7 (43) | 1/3 (33) |
| dose reduction * | 3/7 (43) | 1/3 (33) |
| premature termination * | 3/7 (43) | - |
| oxaliplatin protocol adherence | ||
| administered per protocol | 58/89 (87) | 13/16 (81) |
| interval prolongation* | 17/89 (19) | 2/16 (13) |
| dose reduction * | 9/89 (10) | 1/16 (6) |
| premature termination * | 6/89 (7) | 1/16 (6) |
| n.a. | 2/89 (2) | - |
| Regional hyperthermia (RHT) | ||
| median number of fractions [n] | 10 (1–11) | 10 (4–10) |
| ≥7 fractions (n/N [%]) | 80/88 (91) | 14/16 (88) |
| mean total treatment time [min] | 837 ± 142 | 831 ± 170 |
| mean CEM43 °C [min] | 6.4 ± 5.2 | ±4.9 |
| Cases | Allergic Reaction | Proctitis | Pain | Radiodermatitis | Nausea | Others | Discontinuation Radiotherapy | Discontinuation 5-FU | Discontinuation Oxaliplatin | Discontinuation Hyperthermia |
|---|---|---|---|---|---|---|---|---|---|---|
| 01010 | x | x | x | x | x * | |||||
| 02001 | x | |||||||||
| 01014 | x | x | ||||||||
| 01019 | x | x | ||||||||
| 01020 | x | |||||||||
| 01023 | x | |||||||||
| 01026 | x | x | ||||||||
| 01028 | x | x | ||||||||
| 01030 | x | |||||||||
| 01031 | x | |||||||||
| 01035 | x | x | x | |||||||
| 01034 | x | |||||||||
| 01036 | x * | |||||||||
| 01037 | x | |||||||||
| 01039 | x | x | x | x | ||||||
| 01041 | x |
| TRG score | All Patients (n = 94) | LARC (n = 84) | LRRC (n = 10) |
|---|---|---|---|
| Dworak 1 | 7/94 (7) | 5/84 (6) | 2/10 (20) |
| Dworak 2 | 16/94 (17) | 14/84 (17) | 2/10 (20) |
| Dworak 3 | 28/94 (30) | 27/84 (32) | 1/10 (10) |
| Dworak 4 | 21/94 (22) | 18/84 (21) | 3/10 (30) |
| n.a. * | 22/94 (23) | 20/84 (24) | 2/10 (20) |
| Adverse Event | Grade 1–2 n/N (%) | Grade 3 n/N (%) | N.a. n/N (%) |
|---|---|---|---|
| Anemia | 71/105 (68) | 1/105 (1) | 6/105 (6) |
| Leucopenia | 44/105 (42) | 1/105 (1) | 6/105 (6) |
| Neutropenia | 6/105 (6) | - | 6/105 (6) |
| Neutropenic fever | - | 1/105 (1) | 6/105 (6) |
| Thrombocytopenia | 38/105 (36) | - | 6/105 (6) |
| Elevated creatinine | 15/105 (14) | - | 6/105 (6) |
| Elevated bilirubine | 12/105 (11) | - | 6/105 (6) |
| Elevated transaminases (AST/ALT) | 41/105 (39) | 2/105 (2) | 6/105 (6) |
| Elevated alkaline phosphatase | 19/105 (18) | - | 6/105 (6) |
| Aconuresis | 4/105 (4) | - | 7/105 (7) |
| Allergic reaction | 11/105 (10) | 2/105 (2) | 7/105 (7) |
| Alopecia | 5/105 (5) | - | 7/105 (7) |
| Anal incontinence | 29/105 (28) | 1/105 (1) | 7/105 (7) |
| Diarrhea | 74/105 (70) | 10/105 (10) | 7/105 (7) |
| Dyspnea | 9/105 (9) | - | 7/105 (7) |
| Emesis | 15/105 (14) | - | 7/105 (7) |
| Erectile dysfunction | 9/82 (11) | 1/82 (1) | 5/82 (6) |
| Fatigue | 67/105 (64) | - | 7/105 (7) |
| Fever | 15/105 (14) | - | 7/105 (7) |
| Hand-foot-syndrome | 7/105 (7) | - | 7/105 (7) |
| Heart disorder | 5/105 (5) | - | 7/105 (7) |
| Hemorrhage | 50/105 (48) | 1/105 (1) | 7/105 (7) |
| Mucositis | 18/105 (17) | 1/105 (1) | 7/105 (7) |
| Nausea | 39/105 (37) | 2/105 (2) | 7/105 (7) |
| Non-infectious cystitis | 53/105 (50) | 2/105 (2) | 7/105 (7) |
| Obstipation | 39/105 (37) | - | 7/105 (7) |
| Pain | 56/105 (53) | 4/105 (4) | 7/105 (7) |
| Peripheral motoric neuropathy | 8/105 (8) | - | 7/105 (7) |
| Peripheral sensoric neuropathy | 64/105 (61) | - | 7/105 (7) |
| Proctitis | 62/105 (59) | 3/105 (3) | 7/105 (7) |
| Radiodermatitis | 71/105 (68) | 7/105 (7) | 7/105 (7) |
| Urge to urinate | 40/105 (38) | - | 7/105 (7) |
| Vaginal stenosis | 3/23 (13) | - | - |
| Weight loss | 23/105 (22) | - | 7/105 (7) |
| Other non-hematological AEs | 20/105 (19) | 7/105 (7) | 6/105 (6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ott, O.J.; Gani, C.; Lindner, L.H.; Schmidt, M.; Lamprecht, U.; Abdel-Rahman, S.; Hinke, A.; Weissmann, T.; Hartmann, A.; Issels, R.D.; et al. Neoadjuvant Chemoradiation Combined with Regional Hyperthermia in Locally Advanced or Recurrent Rectal Cancer. Cancers 2021, 13, 1279. https://doi.org/10.3390/cancers13061279
Ott OJ, Gani C, Lindner LH, Schmidt M, Lamprecht U, Abdel-Rahman S, Hinke A, Weissmann T, Hartmann A, Issels RD, et al. Neoadjuvant Chemoradiation Combined with Regional Hyperthermia in Locally Advanced or Recurrent Rectal Cancer. Cancers. 2021; 13(6):1279. https://doi.org/10.3390/cancers13061279
Chicago/Turabian StyleOtt, Oliver J., Cihan Gani, Lars H. Lindner, Manfred Schmidt, Ulf Lamprecht, Sultan Abdel-Rahman, Axel Hinke, Thomas Weissmann, Arndt Hartmann, Rolf D. Issels, and et al. 2021. "Neoadjuvant Chemoradiation Combined with Regional Hyperthermia in Locally Advanced or Recurrent Rectal Cancer" Cancers 13, no. 6: 1279. https://doi.org/10.3390/cancers13061279
APA StyleOtt, O. J., Gani, C., Lindner, L. H., Schmidt, M., Lamprecht, U., Abdel-Rahman, S., Hinke, A., Weissmann, T., Hartmann, A., Issels, R. D., Zips, D., Belka, C., Grützmann, R., & Fietkau, R. (2021). Neoadjuvant Chemoradiation Combined with Regional Hyperthermia in Locally Advanced or Recurrent Rectal Cancer. Cancers, 13(6), 1279. https://doi.org/10.3390/cancers13061279








