Anti-Tumor Efficacy of PD-L1 Targeted Alpha-Particle Therapy in a Human Melanoma Xenograft Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Mouse Xenograft Model
2.3. Histology and Immunohistochemistry Staining
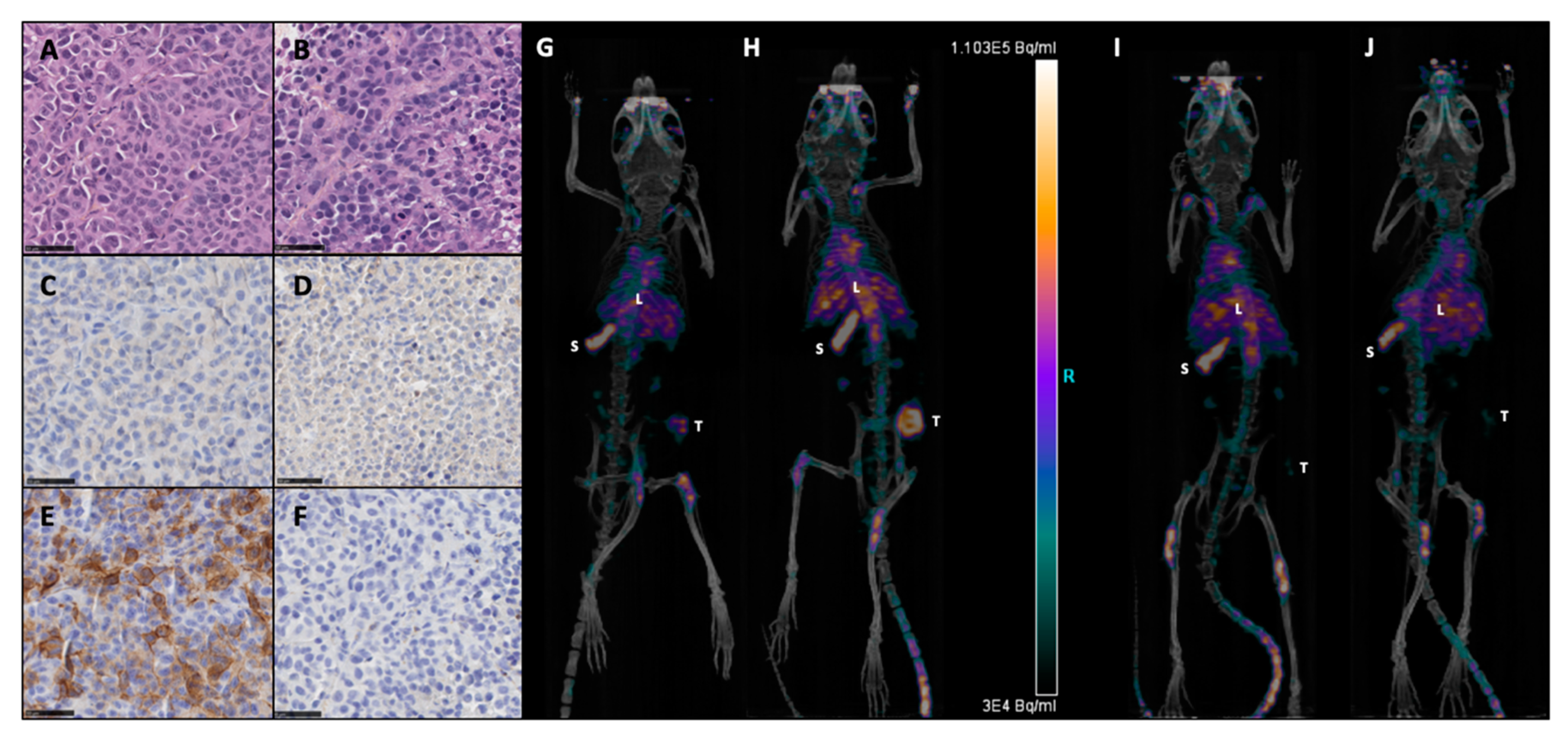
2.4. Immuno-PET Imaging
2.5. Dose-Escalation Study
2.6. Therapy Studies
2.7. Toxicity Study
2.8. Statistical Analysis
3. Results
3.1. PD-L1 Expression on M113PD-L1+ and M113WT Xenograft Tumors
3.2. Dose Escalation Study of 213Bi-anti-hPD-L1 mAb
3.3. Assessment of TAT Efficacy Using 213Bi-anti-hPD-L1 mAb in M113PD-L1+ Melanoma Xenograft Model
3.4. Toxicity after TAT Using 213Bi-anti-hPD-L1 mAb in M113PD-L1+ Melanoma Xenograft Model
3.5. Assessment of 213Bi-anti-hPD-L1 mAb Efficacy in M113WT Melanoma Xenograft Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a Third Member of the B7 Family, Co-Stimulates T-Cell Proliferation and Interleukin-10 Secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of Programmed Death 1 Ligands by Murine T Cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 Regulates the Development, Maintenance, and Function of Induced Regulatory T Cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Nguyen, P.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Esandrio, J.; Xu, H.; Ogurtsova, A.; Bleich, K.B.; Cornish, T.C.; et al. PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin. Cancer Res. 2017, 23, 4938–4944. [Google Scholar] [CrossRef]
- Pawelczyk, K.; Piotrowska, A.; Ciesielska, U.; Jablonska, K.; Gletzel-Plucinska, N.; Grzegrzolka, J.; Podhorska-Okolow, M.; Dziegiel, P.; Nowinska, K. Role of PD-L1 Expression in Non-Small Cell Lung Cancer and Their Prognostic Significance According to Clinicopathological Factors and Diagnostic Markers. Int. J. Mol. Sci. 2019, 20, 824. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 Is a Novel Direct Target of HIF-1α, and Its Blockade under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Barach, Y.S.; Lee, J.S.; Zang, X. T Cell Coinhibition in Prostate Cancer: New Immune Evasion Pathways and Emerging Therapeutics. Trends Mol. Med. 2011, 17, 47–55. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and T(Regs) in the Melanoma Tumor Microenvironment Is Driven by CD8(+) T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of Inflammatory Response with B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci. Transl. Med. 2012, 4, 127ra37. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Gajewski, T.F.; Mackensen, A. Interaction of PD-L1 on Tumor Cells with PD-1 on Tumor-Specific T Cells as a Mechanism of Immune Evasion: Implications for Tumor Immunotherapy. Cancer Immunol. Immunother. 2005, 54, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.-Y.; Huang, J.-A.; Chen, Y.; Chen, C.; Zhang, X.-G. High Expression of PD-L1 in Lung Cancer May Contribute to Poor Prognosis and Tumor Cells Immune Escape through Suppressing Tumor Infiltrating Dendritic Cells Maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Baker, M.; Cordes, L.; Brownell, I. Avelumab: A New Standard for Treating Metastatic Merkel Cell Carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 319–326. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients with Advanced Melanoma Receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 Expression in Cancer Patients Receiving Anti PD-1/PD-L1 Antibodies: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hemat. 2016, 100, 88–98. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined Nivolumab and Ipilimumab versus Ipilimumab Alone in Patients with Advanced Melanoma: 2-Year Overall Survival Outcomes in a Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Rehman, J.A.; Han, G.; Carvajal-Hausdorf, D.E.; Wasserman, B.E.; Pelekanou, V.; Mani, N.L.; McLaughlin, J.; Schalper, K.A.; Rimm, D.L. Quantitative and Pathologist-Read Comparison of the Heterogeneity of Programmed Death-Ligand 1 (PD-L1) Expression in Non-Small Cell Lung Cancer. Mod. Pathol. 2017, 30, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Zito, C.R.; Turcu, G.; Baine, M.K.; Zhang, H.; Adeniran, A.; Sznol, M.; Rimm, D.L.; Kluger, Y.; Chen, L.; et al. PD-L1 Studies Across Tumor Types, Its Differential Expression and Predictive Value in Patients Treated with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2017, 23, 4270–4279. [Google Scholar] [CrossRef]
- Verhoeff, S.R.; van den Heuvel, M.M.; van Herpen, C.M.L.; Piet, B.; Aarntzen, E.H.J.G.; Heskamp, S. Programmed Cell Death-1/Ligand-1 PET Imaging: A Novel Tool to Optimize Immunotherapy? PET Clin. 2020, 15, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Nedrow, J.R.; Park, S.; Banerjee, S.R.; Rittenbach, A.; Jammes, F.; Tsui, B.; Sgouros, G. Imaging, Biodistribution, and Dosimetry of Radionuclide-Labeled PD-L1 Antibody in an Immunocompetent Mouse Model of Breast Cancer. Cancer Res. 2016, 76, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Brill, A.B.; Song, H.; Akabani, G.; Committee, S.M.; Bolch, W.E.; et al. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of Alpha-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular Pathways: Targeted α-Particle Radiation Therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.H.; Jiao, R.; Malo, M.E.; Frank, C.; Fisher, D.R.; Rickles, D.; Dadachova, E. Comparative Radioimmunotherapy of Experimental Melanoma with Novel Humanized Antibody to Melanin Labeled with 213Bismuth and 177Lutetium. Pharmaceutics 2019, 11, 348. [Google Scholar] [CrossRef]
- Allen, B.J.; Raja, C.; Rizvi, S.; Li, Y.; Tsui, W.; Graham, P.; Thompson, J.F.; Reisfeld, R.A.; Kearsley, J. Intralesional Targeted Alpha Therapy for Metastatic Melanoma. Cancer Biol. Ther. 2005, 4, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.A.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of Patient Survival in a Phase I Trial of Systemic Targeted α-Therapy for Metastatic Melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Raja, C.; Graham, P.; Rizvi, S.M.A.; Song, E.; Goldsmith, H.; Thompson, J.; Bosserhoff, A.; Morgenstern, A.; Apostolidis, C.; Kearsley, J.; et al. Interim Analysis of Toxicity and Response in Phase 1 Trial of Systemic Targeted Alpha Therapy for Metastatic Melanoma. Cancer Biol. Ther. 2007, 6, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hino, R.; Kabashima, K.; Kato, Y.; Yagi, H.; Nakamura, M.; Honjo, T.; Okazaki, T.; Tokura, Y. Tumor Cell Expression of Programmed Cell Death-1 Ligand 1 Is a Prognostic Factor for Malignant Melanoma. Cancer 2010, 116, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Molinero, L.; Bolen, C.R.; Sosman, J.A.; Muñoz-Couselo, E.; Kluger, H.M.; McDermott, D.F.; Powderly, J.D.; Sarkar, I.; Ballinger, M.; et al. Safety, Clinical Activity, and Biological Correlates of Response in Patients with Metastatic Melanoma: Results from a Phase I Trial of Atezolizumab. Clin. Cancer Res. 2019, 25, 6061–6072. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma (KEYNOTE-006): Post-Hoc 5-Year Results from an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Chérel, M.; Gouard, S.; Gaschet, J.; Saï-Maurel, C.; Bruchertseifer, F.; Morgenstern, A.; Bourgeois, M.; Gestin, J.-F.; Bodéré, F.K.; Barbet, J.; et al. 213Bi Radioimmunotherapy with an Anti-MCD138 Monoclonal Antibody in a Murine Model of Multiple Myeloma. J. Nucl. Med. 2013, 54, 1597–1604. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Bruchertseifer, F. Development of Targeted Alpha Therapy from Bench to Bedside. J. Med. Imaging Radiat. Sci. 2019, 50, S18–S20. [Google Scholar] [CrossRef]
- Marcu, L.; Bezak, E.; Allen, B.J. Global Comparison of Targeted Alpha vs Targeted Beta Therapy for Cancer: In Vitro, in Vivo and Clinical Trials. Crit. Rev. Oncol. Hemat. 2018, 123, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Marotte, L.; Simon, S.; Vignard, V.; Dupre, E.; Gantier, M.; Cruard, J.; Alberge, J.-B.; Hussong, M.; Deleine, C.; Heslan, J.-M.; et al. Increased Antitumor Efficacy of PD-1-Deficient Melanoma-Specific Human Lymphocytes. J. Immunother. Cancer 2020, 8, e000311. [Google Scholar] [CrossRef]
- Madore, J.; Vilain, R.E.; Menzies, A.M.; Kakavand, H.; Wilmott, J.S.; Hyman, J.; Yearley, J.H.; Kefford, R.F.; Thompson, J.F.; Long, G.V.; et al. PD-L1 Expression in Melanoma Shows Marked Heterogeneity within and between Patients: Implications for Anti-PD-1/PD-L1 Clinical Trials. Pigment. Cell Melanoma Res. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Bodet-Milin, C.; Ferrer, L.; Pallardy, A.; Eugène, T.; Rauscher, A.; Faivre-Chauvet, A.; Barbet, J.; Kraeber-Bodéré, F. Radioimmunotherapy of B-Cell Non-Hodgkin’s Lymphoma. Front. Oncol. 2013, 3, 177. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Chen, W.-C.; Chang, Y.-H.; Lin, W.-Y.; Chen, M.-F. The Role of PD-L1 in the Radiation Response and Clinical Outcome for Bladder Cancer. Sci. Rep. 2016, 6, 19740–19749. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Clump, D.A.; Srivastava, R.M.; Sun, L.; Zeng, D.; Diaz-Perez, J.A.; Anderson, C.J.; Edwards, W.B.; Ferris, R.L. Preclinical ImmunoPET/CT Imaging Using Zr-89-Labeled Anti-PD-L1 Monoclonal Antibody for Assessing Radiation-Induced PD-L1 Upregulation in Head and Neck Cancer and Melanoma. Oncoimmunology 2017, 6, e1329071. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA Double-Strand Break Repair Pathway Regulates PD-L1 Expression in Cancer Cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef] [PubMed]

 ), 165 (
), 165 ( ), 205 (
), 205 ( ), 335 (
), 335 ( ), 395 (
), 395 ( ) kBq/g 213Bi-anti-hPD-L1 mAb. (A) Kaplan–Meier survival analysis. (B) Mean weight variation in each group, as expressed as percent of initial weight. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced acute toxicity as demonstrated by weight loss > 20% of initial weight and resulting in mouse sacrifice. Animals surviving acute toxicity were followed for a 100-day period before euthanasia. (C) Platelet, (D) RBC counts, as well as (E) plasma FLT3-Ligand and (F) urea concentrations were monitored for each animal before injection of radiolabeled anti-hPD-L1 mAb (T0) and at end point. Each sample was assessed in duplicate. Bar represents the median. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced significant toxicity on platelets (* p = 0.0297, ** p = 0.0023, *** p = 0.0001, **** p < 0.0001), bone marrow (* p = 0.0163, **** p < 0.0001), and kidneys (* p = 0.0297, ** p = 0.0045). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test.
) kBq/g 213Bi-anti-hPD-L1 mAb. (A) Kaplan–Meier survival analysis. (B) Mean weight variation in each group, as expressed as percent of initial weight. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced acute toxicity as demonstrated by weight loss > 20% of initial weight and resulting in mouse sacrifice. Animals surviving acute toxicity were followed for a 100-day period before euthanasia. (C) Platelet, (D) RBC counts, as well as (E) plasma FLT3-Ligand and (F) urea concentrations were monitored for each animal before injection of radiolabeled anti-hPD-L1 mAb (T0) and at end point. Each sample was assessed in duplicate. Bar represents the median. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced significant toxicity on platelets (* p = 0.0297, ** p = 0.0023, *** p = 0.0001, **** p < 0.0001), bone marrow (* p = 0.0163, **** p < 0.0001), and kidneys (* p = 0.0297, ** p = 0.0045). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test.
 ), 165 (
), 165 ( ), 205 (
), 205 ( ), 335 (
), 335 ( ), 395 (
), 395 ( ) kBq/g 213Bi-anti-hPD-L1 mAb. (A) Kaplan–Meier survival analysis. (B) Mean weight variation in each group, as expressed as percent of initial weight. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced acute toxicity as demonstrated by weight loss > 20% of initial weight and resulting in mouse sacrifice. Animals surviving acute toxicity were followed for a 100-day period before euthanasia. (C) Platelet, (D) RBC counts, as well as (E) plasma FLT3-Ligand and (F) urea concentrations were monitored for each animal before injection of radiolabeled anti-hPD-L1 mAb (T0) and at end point. Each sample was assessed in duplicate. Bar represents the median. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced significant toxicity on platelets (* p = 0.0297, ** p = 0.0023, *** p = 0.0001, **** p < 0.0001), bone marrow (* p = 0.0163, **** p < 0.0001), and kidneys (* p = 0.0297, ** p = 0.0045). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test.
) kBq/g 213Bi-anti-hPD-L1 mAb. (A) Kaplan–Meier survival analysis. (B) Mean weight variation in each group, as expressed as percent of initial weight. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced acute toxicity as demonstrated by weight loss > 20% of initial weight and resulting in mouse sacrifice. Animals surviving acute toxicity were followed for a 100-day period before euthanasia. (C) Platelet, (D) RBC counts, as well as (E) plasma FLT3-Ligand and (F) urea concentrations were monitored for each animal before injection of radiolabeled anti-hPD-L1 mAb (T0) and at end point. Each sample was assessed in duplicate. Bar represents the median. Activities ranging from 205 to 395 kBq/g 213Bi-anti-hPD-L1 mAb induced significant toxicity on platelets (* p = 0.0297, ** p = 0.0023, *** p = 0.0001, **** p < 0.0001), bone marrow (* p = 0.0163, **** p < 0.0001), and kidneys (* p = 0.0297, ** p = 0.0045). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test.
 , n = 17), 165 kBq/g 213Bi-Anti-hPD-L1 mAb (
, n = 17), 165 kBq/g 213Bi-Anti-hPD-L1 mAb ( , n = 10), 125 kBq/g 213Bi IgG2b isotype control (
, n = 10), 125 kBq/g 213Bi IgG2b isotype control ( , n = 10), 165 kBq/g 213Bi IgG2b isotype control (
, n = 10), 165 kBq/g 213Bi IgG2b isotype control ( , n = 10), or PBS for control animals (◆, n = 20). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. Compared to PBS and isotype control groups, TAT significantly delayed tumor growth in mice treated with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 165 kBq/g 213Bi-Anti-hPD-L1 mAb (* p = 0.0313, *** p = 0.0007, **** p < 0.0001). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test. (B) Kaplan–Meier survival analysis. TAT with 125 kBq/g and 165 kBq/g 213Bi-Anti-hPD-L1 mAb significantly increased survival (MS = 64 and 67 days, respectively) compared to PBS control group (MS = 47.5 days, **** p < 0.0001). Survival was not significantly different in both isotype control groups compared to PBS control group. The p-values were determined by log-rank test. (C) Mean weight variation in each group is expressed as percent of initial weight.
, n = 10), or PBS for control animals (◆, n = 20). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. Compared to PBS and isotype control groups, TAT significantly delayed tumor growth in mice treated with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 165 kBq/g 213Bi-Anti-hPD-L1 mAb (* p = 0.0313, *** p = 0.0007, **** p < 0.0001). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test. (B) Kaplan–Meier survival analysis. TAT with 125 kBq/g and 165 kBq/g 213Bi-Anti-hPD-L1 mAb significantly increased survival (MS = 64 and 67 days, respectively) compared to PBS control group (MS = 47.5 days, **** p < 0.0001). Survival was not significantly different in both isotype control groups compared to PBS control group. The p-values were determined by log-rank test. (C) Mean weight variation in each group is expressed as percent of initial weight.
 , n = 17), 165 kBq/g 213Bi-Anti-hPD-L1 mAb (
, n = 17), 165 kBq/g 213Bi-Anti-hPD-L1 mAb ( , n = 10), 125 kBq/g 213Bi IgG2b isotype control (
, n = 10), 125 kBq/g 213Bi IgG2b isotype control ( , n = 10), 165 kBq/g 213Bi IgG2b isotype control (
, n = 10), 165 kBq/g 213Bi IgG2b isotype control ( , n = 10), or PBS for control animals (◆, n = 20). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. Compared to PBS and isotype control groups, TAT significantly delayed tumor growth in mice treated with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 165 kBq/g 213Bi-Anti-hPD-L1 mAb (* p = 0.0313, *** p = 0.0007, **** p < 0.0001). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test. (B) Kaplan–Meier survival analysis. TAT with 125 kBq/g and 165 kBq/g 213Bi-Anti-hPD-L1 mAb significantly increased survival (MS = 64 and 67 days, respectively) compared to PBS control group (MS = 47.5 days, **** p < 0.0001). Survival was not significantly different in both isotype control groups compared to PBS control group. The p-values were determined by log-rank test. (C) Mean weight variation in each group is expressed as percent of initial weight.
, n = 10), or PBS for control animals (◆, n = 20). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. Compared to PBS and isotype control groups, TAT significantly delayed tumor growth in mice treated with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 165 kBq/g 213Bi-Anti-hPD-L1 mAb (* p = 0.0313, *** p = 0.0007, **** p < 0.0001). Statistical analysis was performed with two-way ANOVA followed by Sidak’s multiple comparison test. (B) Kaplan–Meier survival analysis. TAT with 125 kBq/g and 165 kBq/g 213Bi-Anti-hPD-L1 mAb significantly increased survival (MS = 64 and 67 days, respectively) compared to PBS control group (MS = 47.5 days, **** p < 0.0001). Survival was not significantly different in both isotype control groups compared to PBS control group. The p-values were determined by log-rank test. (C) Mean weight variation in each group is expressed as percent of initial weight.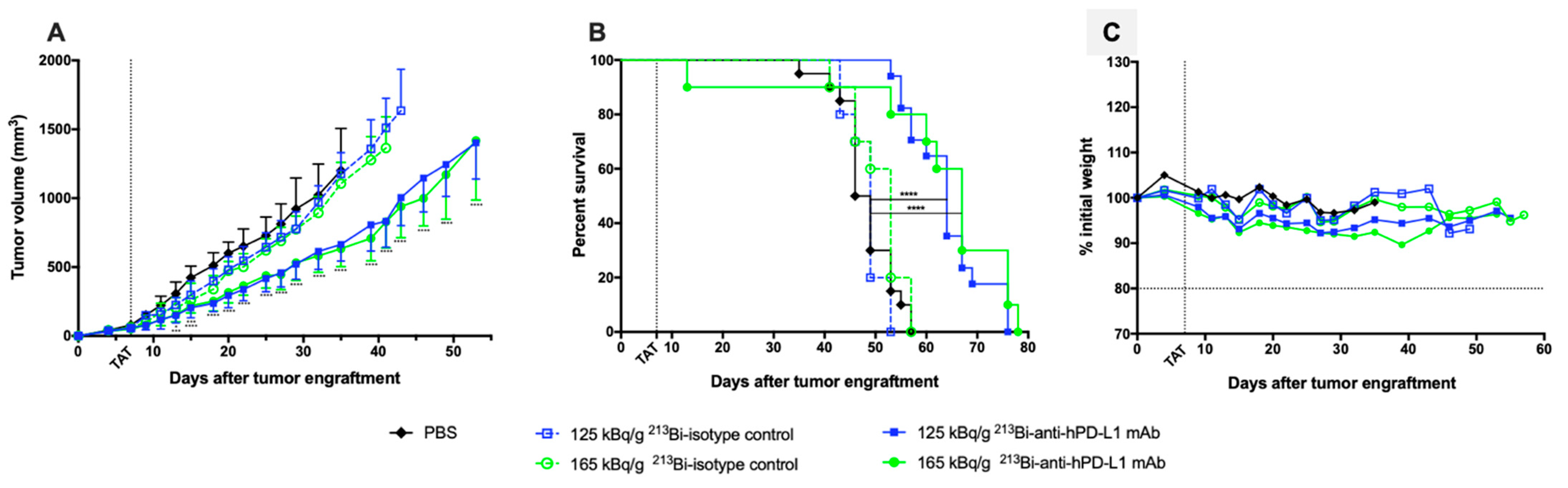
 ), 165 kBq/g 213Bi-Anti-hPD-L1 mAb (
), 165 kBq/g 213Bi-Anti-hPD-L1 mAb ( ) or receiving PBS (☐). CBC were performed before TAT (T0), 20 to 28 days after TAT (Intermediate), and at end point. Other toxicity parameters were assessed in duplicate before TAT (T0) and at end point. Box extends from the 25th to 75th percentiles, line represents the median and the whiskers go down to the smallest value and up to the largest. Statistical analysis was performed with two-way ANOVA followed by Tukey’s multiple comparison test (** p = 0.0023, **** p < 0.0001).
) or receiving PBS (☐). CBC were performed before TAT (T0), 20 to 28 days after TAT (Intermediate), and at end point. Other toxicity parameters were assessed in duplicate before TAT (T0) and at end point. Box extends from the 25th to 75th percentiles, line represents the median and the whiskers go down to the smallest value and up to the largest. Statistical analysis was performed with two-way ANOVA followed by Tukey’s multiple comparison test (** p = 0.0023, **** p < 0.0001).
 ), 165 kBq/g 213Bi-Anti-hPD-L1 mAb (
), 165 kBq/g 213Bi-Anti-hPD-L1 mAb ( ) or receiving PBS (☐). CBC were performed before TAT (T0), 20 to 28 days after TAT (Intermediate), and at end point. Other toxicity parameters were assessed in duplicate before TAT (T0) and at end point. Box extends from the 25th to 75th percentiles, line represents the median and the whiskers go down to the smallest value and up to the largest. Statistical analysis was performed with two-way ANOVA followed by Tukey’s multiple comparison test (** p = 0.0023, **** p < 0.0001).
) or receiving PBS (☐). CBC were performed before TAT (T0), 20 to 28 days after TAT (Intermediate), and at end point. Other toxicity parameters were assessed in duplicate before TAT (T0) and at end point. Box extends from the 25th to 75th percentiles, line represents the median and the whiskers go down to the smallest value and up to the largest. Statistical analysis was performed with two-way ANOVA followed by Tukey’s multiple comparison test (** p = 0.0023, **** p < 0.0001).
 , n = 10), 125 kBq/g 213Bi IgG2b isotype control (
, n = 10), 125 kBq/g 213Bi IgG2b isotype control ( , n = 10), or PBS for control animals (◇, n = 14). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. No difference was observed in tumor growth between the different groups. (B) Kaplan–Meier survival analysis. Treatment with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 213Bi IgG2b isotype control had no impact on survival (MS = 43 and 42 days, respectively) compared to PBS control group (MS = 41 days).
, n = 10), or PBS for control animals (◇, n = 14). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. No difference was observed in tumor growth between the different groups. (B) Kaplan–Meier survival analysis. Treatment with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 213Bi IgG2b isotype control had no impact on survival (MS = 43 and 42 days, respectively) compared to PBS control group (MS = 41 days).
 , n = 10), 125 kBq/g 213Bi IgG2b isotype control (
, n = 10), 125 kBq/g 213Bi IgG2b isotype control ( , n = 10), or PBS for control animals (◇, n = 14). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. No difference was observed in tumor growth between the different groups. (B) Kaplan–Meier survival analysis. Treatment with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 213Bi IgG2b isotype control had no impact on survival (MS = 43 and 42 days, respectively) compared to PBS control group (MS = 41 days).
, n = 10), or PBS for control animals (◇, n = 14). (A) Tumor volume, represented by mean and SD, was determined sequentially from engraftment until signs of tumor necrosis or volume reached 2000 mm3 and animals were sacrificed. No difference was observed in tumor growth between the different groups. (B) Kaplan–Meier survival analysis. Treatment with 125 kBq/g 213Bi-Anti-hPD-L1 mAb or 213Bi IgG2b isotype control had no impact on survival (MS = 43 and 42 days, respectively) compared to PBS control group (MS = 41 days).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capitao, M.; Perrin, J.; Simon, S.; Gouard, S.; Chouin, N.; Bruchertseifer, F.; Morgenstern, A.; Rbah-Vidal, L.; Chérel, M.; Scotet, E.; et al. Anti-Tumor Efficacy of PD-L1 Targeted Alpha-Particle Therapy in a Human Melanoma Xenograft Model. Cancers 2021, 13, 1256. https://doi.org/10.3390/cancers13061256
Capitao M, Perrin J, Simon S, Gouard S, Chouin N, Bruchertseifer F, Morgenstern A, Rbah-Vidal L, Chérel M, Scotet E, et al. Anti-Tumor Efficacy of PD-L1 Targeted Alpha-Particle Therapy in a Human Melanoma Xenograft Model. Cancers. 2021; 13(6):1256. https://doi.org/10.3390/cancers13061256
Chicago/Turabian StyleCapitao, Marisa, Justine Perrin, Sylvain Simon, Sébastien Gouard, Nicolas Chouin, Frank Bruchertseifer, Alfred Morgenstern, Latifa Rbah-Vidal, Michel Chérel, Emmanuel Scotet, and et al. 2021. "Anti-Tumor Efficacy of PD-L1 Targeted Alpha-Particle Therapy in a Human Melanoma Xenograft Model" Cancers 13, no. 6: 1256. https://doi.org/10.3390/cancers13061256
APA StyleCapitao, M., Perrin, J., Simon, S., Gouard, S., Chouin, N., Bruchertseifer, F., Morgenstern, A., Rbah-Vidal, L., Chérel, M., Scotet, E., Labarrière, N., Guilloux, Y., & Gaschet, J. (2021). Anti-Tumor Efficacy of PD-L1 Targeted Alpha-Particle Therapy in a Human Melanoma Xenograft Model. Cancers, 13(6), 1256. https://doi.org/10.3390/cancers13061256





