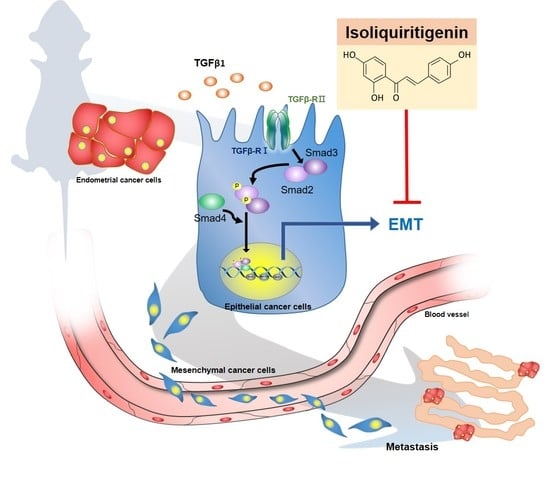
Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. The Effect of Various Concentrations of ISL on Human Endometrial Cancer Cells and Human Endometrial Stromal Cells
2.2. ISL Inhibits TGF-β1-Induced Migration of Human Endometrial Cancer Cells
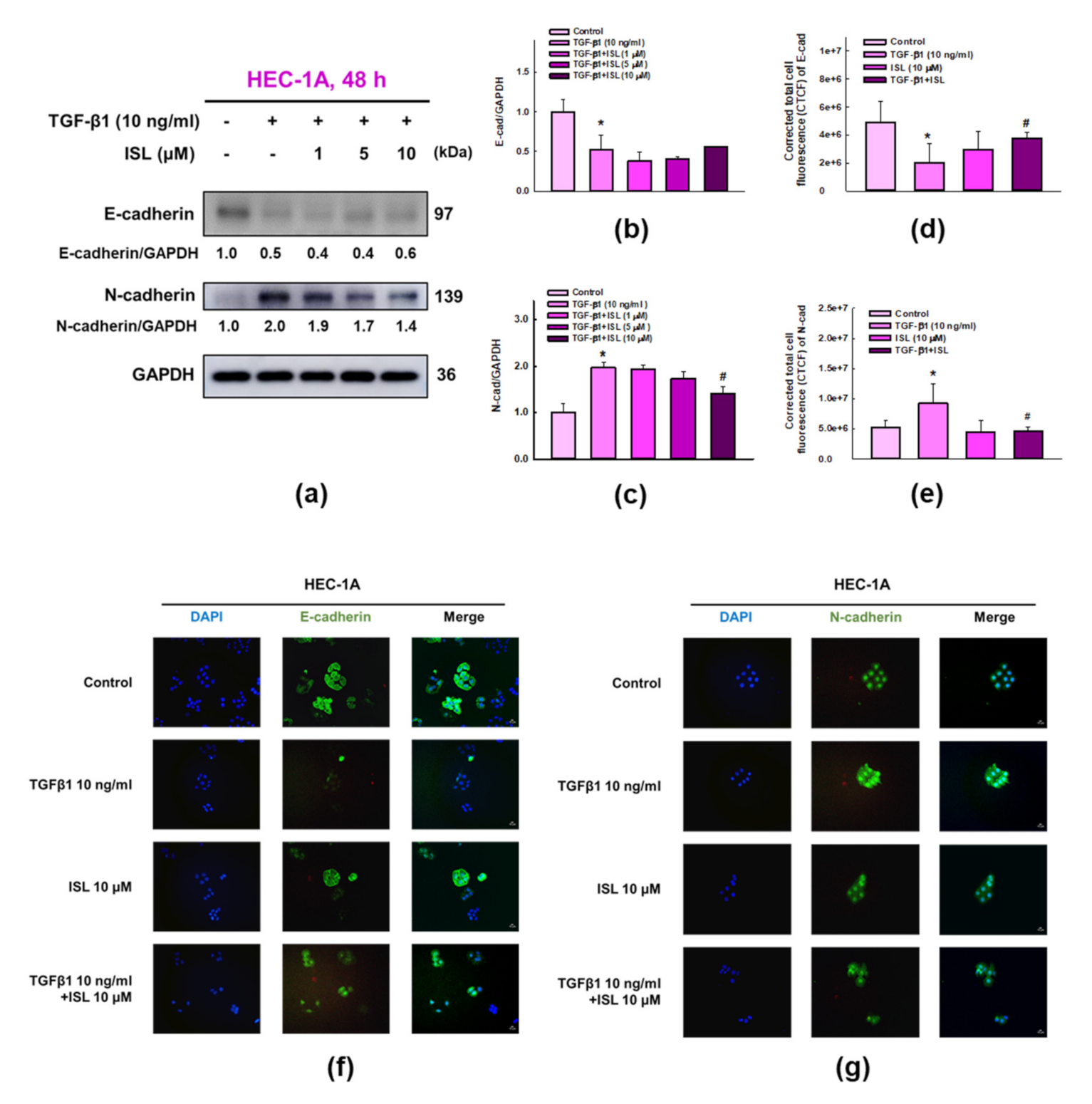
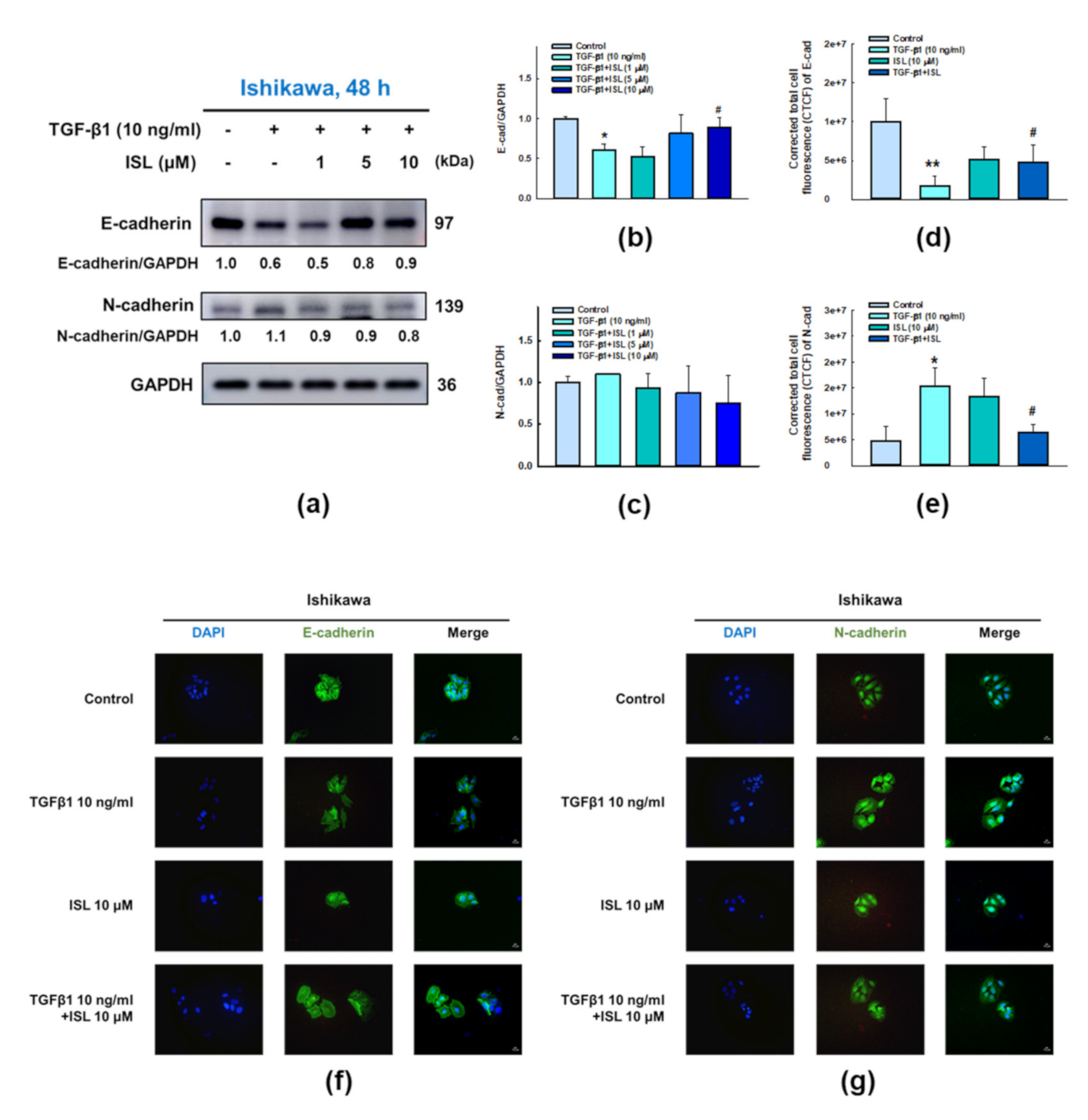
2.3. ISL Inhibits TGF-β1-Induced Expression of EMT Markers on Human Endometrial Cancer Cells
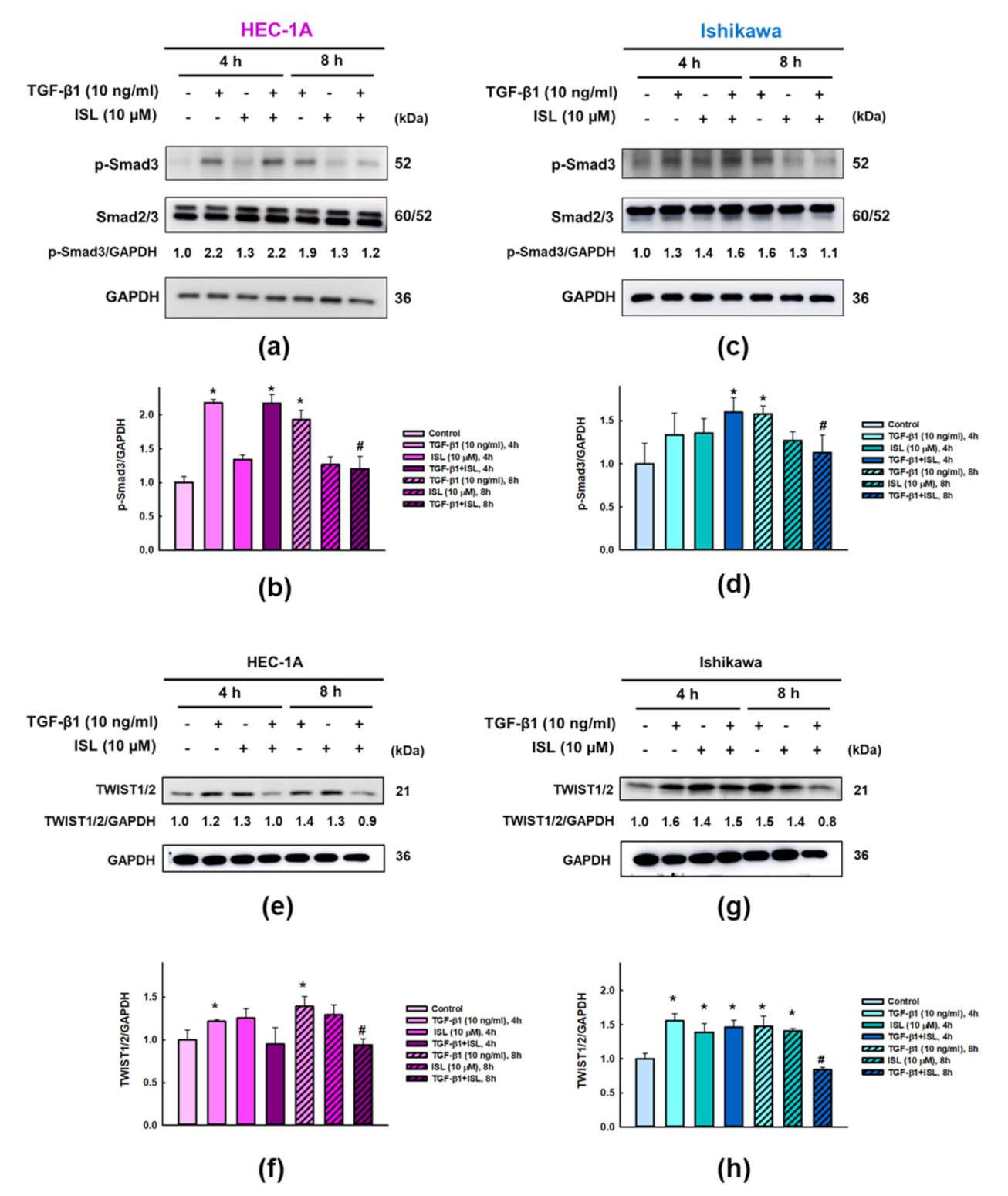
2.4. Effect of ISL on Expression of TGFβ/Smad Signaling Pathway Related Markers in Human Endometrial Cancer Cells
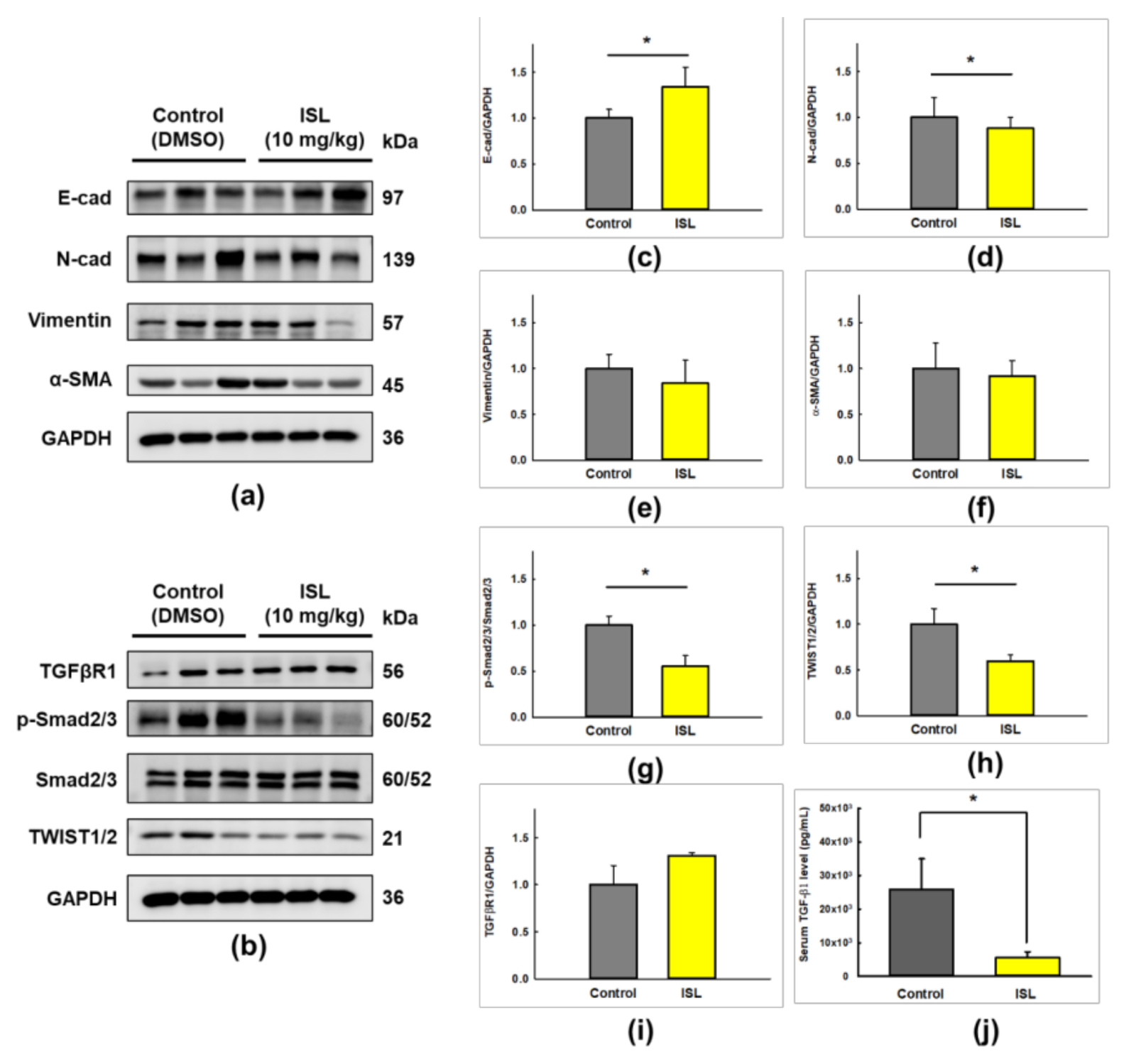
2.5. ISL Suppresses Peritoneal Dissemination In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Isoliquiritigenin and Transforming Growth Factor-β1
4.3. Cell Viability Assay
4.4. Wound-Healing Assay
4.5. Transwell Migration Assay
4.6. Immunofluorescence Analysis
4.7. Western Blot Analysis
4.8. RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR (qPCR) Analysis
4.9. Tumor Xenograft in Nude Mice
4.10. Enzyme-Linked Immunosorbent Assay (ELISA).
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinton, L.A.; Berman, M.L.; Mortel, R.; Twiggs, L.B.; Barrett, R.J.; Wilbanks, G.D.; Lannom, L.; Hoover, R.N. Reproductive, menstrual, and medical risk factors for endometrial cancer: Results from a case-control study. Am. J. Obstet. Gynecol. 1992, 167, 1317–1325. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: An update. Endocr Relat Cancer 2016, 23, R85–R111. [Google Scholar] [CrossRef] [PubMed]
- Dijkhuizen, F.P.H.L.J.; Mol, B.W.J.; Brlmann, H.A.M.; Heintz, A.P.M. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia. Cancer 2000, 89, 1765–1772. [Google Scholar] [CrossRef]
- Terry, P.; Vainio, H.; Wolk, A.; Weiderpass, E. Dietary Factors in Relation to Endometrial Cancer: A Nationwide Case-Control Study in Sweden. Nutr. Cancer 2002, 42, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Terai, Y.; Kawaguchi, H.; Fujiwara, S.; Yoo, S.; Tsunetoh, S.; Takai, M.; Kanemura, M.; Tanabe, A.; Ohmichi, M. Prognostic impact of EMT (epithelial-mesenchymal-transition)-related protein expression in endometrial cancer. Cancer Biol. Ther. 2013, 14, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 2005, 24, 7443–7454. [Google Scholar] [CrossRef]
- Kemler, R. From cadherins to catenins: Cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993, 9, 317–321. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, Y.; ten Dijke, P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012, 31, 553–568. [Google Scholar] [CrossRef]
- Smith, A.L.; Robin, T.P.; Ford, H.L. Molecular pathways: Targeting the TGF-beta pathway for cancer therapy. Clin. Cancer Res. 2012, 18, 4514–4521. [Google Scholar] [CrossRef]
- Zavadil, J.; Bottinger, E.P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef]
- Levy, L.; Hill, C.S. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006, 17, 41–58. [Google Scholar] [CrossRef]
- Valdes, F.; Alvarez, A.M.; Locascio, A.; Vega, S.; Herrera, B.; Fernandez, M.; Benito, M.; Nieto, M.A.; Fabregat, I. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol. Cancer Res. MCR 2002, 1, 68–78. [Google Scholar]
- Hawinkels, L.J.; Ten Dijke, P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 2011, 29, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, L.M.; Roberts, A.B. TGF-β signaling: Positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002, 12, 22–29. [Google Scholar] [CrossRef]
- Gold, L.I.; Saxena, B.; Mittal, K.R.; Marmor, M.; Goswami, S.; Nactigal, L.; Korc, M.; Demopoulos, R.I. Increased expression of transforming growth factor β Isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: Evidence for paracrine and autocrine action. Cancer Res. 1994, 54, 2347–2358. [Google Scholar]
- Massague, J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Iyer, N.R. Cancer chemoprevention: Current state of the art. Cancer Growth Metastasis 2014, 7, 19–25. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers (Basel) 2020, 12, 1498. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Ji, C.; Ye, J. Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection. J. Chromatogr. A 2004, 1042, 203–209. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Kwon, H.-M.; Choi, Y.-J.; Choi, J.-S.; Kang, S.-W.; Bae, J.-Y.; Kang, I.-J.; Jun, J.-G.; Lee, S.-S.; Lim, S.S.; Kang, Y.-H. Blockade of Cytokine-Induced Endothelial Cell Adhesion Molecule Expression by Licorice Isoliquiritigenin Through NF-κB Signal Disruption. Exp. Biol. Med. 2007, 232, 235–245. [Google Scholar] [CrossRef]
- Yamamoto, S.; Aizu, E.; Jiang, H.; Nakadate, T.; Kiyoto, I.; Wang, J.C.; Kato, R. The potent anti-tumor-promoting agent isoliquiritigenin. Carcinogenesis 1991, 12, 317–323. [Google Scholar] [CrossRef]
- Wang, K.L.; Yu, Y.C.; Hsia, S.M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers (Basel) 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chen, H.Y.; Wang, C.W.; Shieh, T.M.; Huang, T.C.; Lin, L.C.; Wang, K.L.; Hsia, S.M. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 2016, 7, 73432–73447. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, T.C.; Shieh, T.M.; Wu, C.H.; Lin, L.C.; Hsia, S.M. Isoliquiritigenin Induces Autophagy and Inhibits Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2017, 18, 2025. [Google Scholar] [CrossRef]
- Lin, P.-H.; Kung, H.-L.; Chen, H.-Y.; Huang, K.-C.; Hsia, S.-M. Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation. Cancers (Basel) 2019, 11, 1131. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef]
- Montserrat, N.; Mozos, A.; Llobet, D.; Dolcet, X.; Pons, C.; de Herreros, A.G.; Matias-Guiu, X.; Prat, J. Epithelial to mesenchymal transition in early stage endometrioid endometrial carcinoma. Hum. Pathol. 2012, 43, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Du, X.L.; Jiang, T. Prognostic significance of reduced immunohistochemical expression of E-cadherin in endometrial cancer-results of a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 18689–18696. [Google Scholar]
- Lei, X.; Wang, L.; Yang, J.; Sun, L.Z. TGFbeta signaling supports survival and metastasis of endometrial cancer cells. Cancer Manag. Res. 2009, 2009, 15–24. [Google Scholar]
- Muinelo-Romay, L.; Colas, E.; Barbazan, J.; Alonso-Alconada, L.; Alonso-Nocelo, M.; Bouso, M.; Curiel, T.; Cueva, J.; Anido, U.; Forteza, J.; et al. High-risk endometrial carcinoma profiling identifies TGF-beta1 as a key factor in the initiation of tumor invasion. Mol. Cancer Ther. 2011, 10, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Grunert, S.; Jechlinger, M.; Beug, H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Chen, Q.L.; Zhao, M.; Su, S.B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Cheng, J.-C.; Klausen, C.; Zhao, J.; Leung, P.C.K. TGF-β1 stimulates migration of type II endometrial cancer cells by down-regulating PTEN via activation of SMAD and ERK1/2 signaling pathways. Oncotarget 2016, 7, 61262–61272. [Google Scholar] [CrossRef]
- Feng, H.-T.; Zhao, W.-W.; Lu, J.-J.; Wang, Y.-T.; Chen, X.-P. Hypaconitine inhibits TGF- β 1-induced epithelial–mesenchymal transition and suppresses adhesion, migration, and invasion of lung cancer A549 cells. Chin. J. Nat. Med. 2017, 15, 427–435. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef]
- Wang, K.L.; Hsia, S.M.; Chan, C.J.; Chang, F.Y.; Huang, C.Y.; Bau, D.T.; Wang, P.S. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin. Ther. Targets 2013, 17, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS ONE 2013, 8, e68566. [Google Scholar] [CrossRef]
- Zheng, H.; Li, Y.; Wang, Y.; Zhao, H.; Zhang, J.; Chai, H.; Tang, T.; Yue, J.; Guo, A.M.; Yang, J. Downregulation of COX-2 and CYP 4A signaling by isoliquiritigenin inhibits human breast cancer metastasis through preventing anoikis resistance, migration and invasion. Toxicol. Appl. Pharmacol. 2014, 280, 10–20. [Google Scholar] [CrossRef]
- Li, J.; Kang, S.W.; Kim, J.L.; Sung, H.Y.; Kwun, I.S.; Kang, Y.H. Isoliquiritigenin entails blockade of TGF-beta1-SMAD signaling for retarding high glucose-induced mesangial matrix accumulation. J. Agric. Food Chem. 2010, 58, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, S.; Chen, C.L.; Su, S.B.; Fang, D.D. Isoliquiritigenin Inhibits Ovarian Cancer Metastasis by Reversing Epithelial-to-Mesenchymal Transition. Molecules 2019, 24, 3725. [Google Scholar] [CrossRef]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef]
- Nakamura, M.; Ono, Y.J.; Kanemura, M.; Tanaka, T.; Hayashi, M.; Terai, Y.; Ohmichi, M. Hepatocyte growth factor secreted by ovarian cancer cells stimulates peritoneal implantation via the mesothelial-mesenchymal transition of the peritoneum. Gynecol. Oncol. 2015, 139, 345–354. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| VIM | AGTCCACTGAGTACCGGAGAC | CATTTCACGCATCTGGCGTTC |
| ACTA2 | GCTTGTCCAGGAGTTCCGCTCC | GATGCGAAGTGCTGACCCCGC |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Chiang, Y.-F.; Huang, J.-S.; Huang, T.-C.; Shih, Y.-H.; Wang, K.-L.; Ali, M.; Hong, Y.-H.; Shieh, T.-M.; Hsia, S.-M. Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer. Cancers 2021, 13, 1236. https://doi.org/10.3390/cancers13061236
Chen H-Y, Chiang Y-F, Huang J-S, Huang T-C, Shih Y-H, Wang K-L, Ali M, Hong Y-H, Shieh T-M, Hsia S-M. Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer. Cancers. 2021; 13(6):1236. https://doi.org/10.3390/cancers13061236
Chicago/Turabian StyleChen, Hsin-Yuan, Yi-Fen Chiang, Jia-Syuan Huang, Tsui-Chin Huang, Yin-Hwa Shih, Kai-Lee Wang, Mohamed Ali, Yong-Han Hong, Tzong-Ming Shieh, and Shih-Min Hsia. 2021. "Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer" Cancers 13, no. 6: 1236. https://doi.org/10.3390/cancers13061236
APA StyleChen, H.-Y., Chiang, Y.-F., Huang, J.-S., Huang, T.-C., Shih, Y.-H., Wang, K.-L., Ali, M., Hong, Y.-H., Shieh, T.-M., & Hsia, S.-M. (2021). Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer. Cancers, 13(6), 1236. https://doi.org/10.3390/cancers13061236