Complement in Tumourigenesis and the Response to Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. The Complement System
2.1. Complement Activation Pathways
2.2. Regulation of Complement Activation
2.3. Functions of Complement
3. The Complement System and Cancer
3.1. Activation of the Complement System in Cancer
3.2. Tumour Expression of Complement Regulators
3.3. Complement in Tumour Growth
3.3.1. Murine Studies
| Immune Cell | Model | Component | Observation | Mechanism | Ref |
|---|---|---|---|---|---|
| MDSC CD8+ T cell | Ovarian cancer, syngeneic (TC-1 cells) | C5a, C5aR | Tumour growth is impaired in C5aR−/− mice Pharmacological blockade of C5aR reduces tumour growth | Recruitment of PMN MDSCs to tumours and production of ROS/RNS by MO MDSCs, suppresses CD8+ T cell responses | [16] |
| CD8+ T cell | Melanoma, syngeneic (B16 cells) Breast cancer, syngeneic (E0771) | C3, C3aR C5aR | Tumour growth is impaired in C3−/− mice C3aR and C5aR antagonism reduces tumour growth. | Complement signalling inhibits IL-10 expression by CD8+ tumour-infiltrating lymphocytes (TILs), hindering the anti-tumour response | [136] |
| CD8+ T cell | Breast cancer, syngeneic (4T1 and 4TI-GFP cells) | C5a, C5aR | Reduced lung and liver metastases in C5aR−/− mice C5aR antagonism reduces lung metastases | Recruitment of MDSCs, and induction of TGFB and IL-10 production, leads to suppression of CD8+ T cell function by Treg cells | [89] |
| CD8+ T cell | Lung cancer, syngeneic KrasLSL-G12D/+ mice (393P cells) | C5aR | Decreased tumour volume in C5aR−/− mice Pharmacological blockade of C5a and PD-1 impairs tumour growth | Fewer MDSCs accompanied by an increase in CD8+ T cells, which had lower levels of exhaustion markers | [137] |
| CD8+ T cell | Colon cancer, syngeneic (MC38) | C3 | Complement (C3) depletion using cobra venom factor (CVF) impairs tumour growth | C3 contributes to the generation of an immunosuppressive environment (Increased MDSCs, fewer CD8+ T cells, lower expression of CCL5, CXCL10 and CXCL11) | [138] |
| MDSC CD8+ T cell | Colitis-associated colorectal cancer (Induced by azoxymethane and dextran sulfate sodium) | C5aR | Tumour growth is impaired in C5aR−/− mice C5aR antagonism reduces tumour growth | C5a recruits MDSCs to CRC tissue, inhibiting CD8+ T cell responses | [139] |
| CD4+ T cell CD8+ T cell | Lymphoma, syngeneic (RMA-3CF4 and RMA-1474 cells) | C5a | Tumour growth is impaired in mice with lymphoma cells producing low C5a levels | Increase in effector (IFN-y producing) CD4 and CD8+ T cells | [140] |
| CD4+ T cell | Lung cancer, syngeneic and orthotopic (LLC-luc, CMT-luc and EML4-ALK cells) | C3, C3aR, C5aR | Tumour growth is impaired and metastases are reduced in C3−/− mice C3aR or C5aR antagonism reduces tumour growth. | Signalling of C3 prevents cytokine production by CD4+ T cells | [141] |
| MDSC | Lung cancer, syngeneic (3LL cells) | C5a, C5aR | C5aR antagonism reduces tumour growth | C5a contributes to the generation of an immunosuppressive microenvironment | [91] |
| MDSC | Hepatocellular carcinoma, syngenic (H22 cells) | C3 | Tumour growth is impaired in mice with C3−/− hepatic stellate cells | Hepatic stellate cells produce C3 leading to MDSC accumulation and immunosuppression | [142] |
| Neutrophil | Small intestine tumorigenesis (APCMin/+ mice) | C3aR | Tumour growth is impaired in C3aR−/− mice | Engagement of C3aR on neutrophils drives NETosis and coagulation pathways to induce pro-tumorigenic low density neutrophils | [143] |
| Neutrophil | Colitis-associated colorectal cancer (Induced by azoxymethane and dextran sulfate sodium) | C3, C5, C5aR | Tumour growth is impaired in C3−/−, C5−/− and C5aR−/− mice | C5a induces neutrophil infiltration and IL-1B expression which drives IL-17A production | [144] |
| Neutrophil | Melanoma, syngeneic (B16F10) | C3aR | Tumour growth is impaired in C3aR−/− mice C3aR antagonism arrests growth of established tumours | C3aR signalling reduces infiltrating neutrophils and CD4+ T cell populations | [145] |
| Macrophage | Melanoma, syngeneic (B16F10) | C3a, C3aR | C3a neutralization impairs tumour growth | C3a recruits macrophages which suppress the CD8+ T cell response | [146] |
| Macrophage | Sarcoma (Induced by 3-methylcholanthrane) | PTX3, C5a | PTX3 controls complement activation by recruiting Factor H. Ptx3−/− mice are more susceptible to carcinogenesis | In the absence of PTX3, C5a generation is uninterrupted. An increase in CCL2 skews macrophages to an M2 phenotype | [147] |
| Macrophage | Colon cancer (metastatic), syngeneic (SL4 cells) Colon cancer xenograft (HCT116 and SW116 cells) | C5a, C5aR | Growth of hepatic metastases is impaired in C5aR−/− mice or when C5 is downregulated or targeted via pharmacological blockade | C5a induces MCP-1 production by macrophages via the Akt pathway and promotes an immunosuppressive microenvironment | [148] |
| Macrophage | Pancreatic neuroendocrine tumours, transgenic (BT2B6) | C5aR | C5aR antagonism reduces tumour growth. | Increased infiltration of macrophages | [149] |
| Macrophage | Colon cancer, syngeneic (SL4-luc) | C5aR | Growth of hepatic metastases is impaired in C5aR−/− mice | C5a polarises tumour associated macrophages (TAMs) to an M2 phenotype via NF-kB signalling | [150] |
| Macrophage and Mast cells | Squamous cell carcinoma, transgenic (K14-HPV16) | C5aR | Tumour growth is impaired in C5aR−/− mice | C5aR signalling activates macrophages and mast cells, promoting a pro-tumour microenvironment and limiting CD8+ T cell responses | [151] |
| Natural Killer cell | Melanoma, syngeneic (B16gp33 cells) | C3 | Complement (C3) depletion using CVF impairs tumour growth | Complement limits natural killer (NK) cell-mediation of the CD8+ T cell anti-tumour immune response | [152] |
| Natural Killer cell | Melanoma, syngeneic (B16-luc cells) | CR3 | Metastases were reduced in CD11b−/− (CR3 deficient) mice and mice with CR3 deficient NK cells | Interaction of iC3b with CR3 suppresses NK cells by activating SHIP and JNK pathways | [153] |
3.3.2. Human Studies
4. Role of the Complement System in the Response to Cytotoxic Therapy
4.1. Complement and the Response to Radiotherapy
4.2. Complement and the Response to Chemotherapy
4.3. Potential Mechanisms Underlying Complement-Mediated Resistance to Cytotoxic Therapy
4.3.1. Hypoxia
4.3.2. DNA Repair
4.3.3. Metabolism
4.3.4. PI3K/Akt Signalling
4.3.5. Exosomes
4.4. Complement as A Biomarker of Response to Cytotoxic Therapy
5. The Implications of the Complement System and Immunotherapy
5.1. Immune Checkpoint Inhibition
Combination Inhibition of Complement and PD-1 Signalling
5.2. Implications of CRPs for Immunotherapy
5.3. Cancer Vaccines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2012, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Reis, E.S.; Mastellos, D.C.; Ricklin, D.; Mantovani, A.; Lambris, J.D. Complement in cancer: Untangling an intricate relationship. Nat. Rev. Immunol. 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Carroll, M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004, 5, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Schifferli, J.A.; Ng, Y.C.; Peters, D.K. The role of complement and its receptor in the elimination of immune complexes. N. Engl. J. Med. 1986, 315, 488–495. [Google Scholar] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Pio, R.; Corrales, L.; Lambris, J.D. The Role of Complement in Tumour Growth. Adv. Exp. Med. Biol. 2014, 772, 229–262. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–647. [Google Scholar] [CrossRef]
- Ytting, H.; Christensen, I.J.; Steffensen, R.; Alsner, J.; Thiel, S.; Jensenius, J.C.; Hansen, U.; Nielsen, H.J. Mannan-Binding Lectin (MBL) and MBL-Associated Serine Protease 2 (MASP-2) Genotypes in Colorectal Cancer. Scand. J. Immunol. 2011, 73, 122–127. [Google Scholar] [CrossRef]
- Storm, L.; Christensen, I.J.; Jensenius, J.C.; Nielsen, H.J.; Thiel, S. Evaluation of complement proteins as screening markers for colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 41–50. [Google Scholar] [CrossRef]
- Kesselring, R.; Thiel, A.; Pries, R.; Fichtner-Feigl, S.; Brunner, S.; Seidel, P.; Bruchhage, K.L.; Wollenberg, B. The complement receptors CD46, CD55 and CD59 are regulated by the tumour microenvironment of head and neck cancer to facilitate escape of complement attack. Eur. J. Cancer 2014, 50, 2152–2161. [Google Scholar] [CrossRef]
- Imamura, T.; Yamamoto-Ibusuki, M.; Sueta, A.; Kubo, T.; Irie, A.; Kikuchi, K.; Kariu, T.; Iwase, H. Influence of the C5a–C5a receptor system on breast cancer progression and patient prognosis. Breast Cancer 2016, 23, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Roumenina, L.T.; Daugan, M.V.; Noé, R.; Petitprez, F.; Vano, Y.A.; Sanchez-Salas, R.; Becht, E.; Meilleroux, J.; Clec’h, B.L.; Giraldo, N.A.; et al. Tumor Cells Hijack Macrophage-Produced Complement C1q to Promote Tumor Growth. Cancer Immunol. Res. 2019, 7, 1091–1106. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Deangelis, R.; Benencia, F.; Ricklin-, S.K.; Koutoulaki, A.; Gerard, C.; Coukos, G.; Lambris, J.D. Modulation of the anti-tumor immune response by complement. Nat. Immunol. 2008, 9, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Elvington, M.; Scheiber, M.; Yang, X.; Lyons, K.; Jacqmin, D.; Wadsworth, C.; Marshall, D.; Vanek, K.; Tomlinson, S. Complement dependent modulation of anti-tumor immunity following radiation therapy. Cell Rep. 2014, 8, 818–830. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Rafail, S. The dual role of complement in cancer and its implication in anti-tumor therapy. Ann. Transl. Med. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef]
- Bordet, J.; Gengou, O. Sur l’existence de substances sensibilisatrices dans la plu- part des serum antimicrobiens. Ann. Inst. Pasteur Paris 1901, 15, 289–302. [Google Scholar]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The Complement System. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ricklin, D.; Lambris, J.D. Recent Developments in Low Molecular Weight Complement Inhibitors. Mol. Immunol. 2009, 47, 185–195. [Google Scholar] [CrossRef]
- Klos, A.; Tenner, A.J.; Johswich, K.O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef]
- Kishore, U.; Ghai, R.; Greenhough, T.J.; Shrive, A.K.; Bonifati, D.M.; Gadjeva, M.G.; Waters, P.; Kojouharova, M.S.; Chakraborty, T.; Agrawal, A. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol. Lett. 2004, 95, 113–128. [Google Scholar] [CrossRef]
- Szalai, A.J.; Agrawal, A.; Greenhough, T.J.; Volanakis, J.E. C-Reactive Protein: Structural Biology and Host Defense Function. Clin. Chem. Lab. Med. 1999, 37, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Volanakis, J.E.; Kaplan, M.H. Interaction of C-Reactive Protein Complexes with the Complement system.; I.I. Consumption of Guinea Pig Complement by CRP Complexes: Requirement for Human C1q. J. Immunol. 1974, 113, 9–17. [Google Scholar] [PubMed]
- Kang, Y.S.; Do, Y.; Lee, H.K.; Park, S.H.; Cheong, C.; Lynch, R.M.; Loeffler, J.M.; Steinman, R.M.; Park, C.G. A Dominant Complement Fixation Pathway for Pneumococcal Polysaccharides Initiated by SIGN-R1 Interacting with C1q. Cell 2006, 125, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Ebenbichler, C.F. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J. Exp. Med. 1991, 174, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Spear, G.T.; Jiang, H.X.; Sullivan, B.L.; Gewurz, H.; Landay, A.L.; Lint, T.F. Direct Binding of Complement Component C1q to Human Immunodeficiency Virus (HIV) and Human T Lymphotrophic Virus-I (HTLV-I) Coinfected Cells. AIDS Res. Hum. Retrovir. 1991, 7, 579–585. [Google Scholar] [CrossRef]
- Thielens, N.M.; Tacnet-Delorme, P.; Arlaud, G.J. Interaction of C1q and Mannan-binding lectin with viruses. Immunobiology 2002, 205, 563–574. [Google Scholar] [CrossRef]
- Holmskov, U.; Thiel, S.; Jensenius, J.C. Collectins and ficolins: Humoral Lectins of the Innate Immune Defense. Annu. Rev. Immunol. 2003, 21, 547–578. [Google Scholar] [CrossRef] [PubMed]
- Thiel, S.; Vorup-Jensen, T.; Stover, C.M.; Schwaeble, W.; Laursen, S.B.; Poulsen, K.; Willis, A.C.; Eggleton, P.; Hansen, S.; Holmskov, U.; et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature 1997, 386, 506–510. [Google Scholar] [CrossRef]
- Megyeri, M.; Harmat, V.; Major, B.; Végh, Á.; Balczer, J.; Héja, D.; Szilágyi, K.; Datz, D.; Pál, G.; Závodszky, P.; et al. Quantitative characterization of the activation steps of mannan-binding lectin (MBL)-associated serine proteases (MASPs) points to the central role of MASP-1 in the initiation of the complement lectin pathway. J. Biol. Chem. 2013, 288, 8922–8934. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part I—Molecular mechanisms of activation and regulation. Front. Immunol. 2015, 6, 1–30. [Google Scholar] [CrossRef]
- Pangburn, M.K.; Schreiber, R.D.; Müller-Eberhard, H.J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J. Exp. Med. 1981, 154, 856–867. [Google Scholar] [CrossRef]
- Bexborn, F.; Andersson, P.O.; Chen, H.; Nilsson, B.; Ekdahl, K.N. The Tick-Over Theory Revisited: Formation and Regulation of the soluble Alternative Complement C3 Convertase (C3(H2O)Bb). Mol. Immunol. 2008, 45, 2370–2379. [Google Scholar] [CrossRef]
- Reid, K.B.M.; Porter, R.R. The Proteolytic Activation Systems of Complement. Annu. Rev. Biochem. 1981, 50, 434–464. [Google Scholar] [CrossRef] [PubMed]
- Ganter, M.T.; Brohi, K.; Cohen, M.J.; Shaffer, L.A.; Walsh, M.C.; Stahl, G.L.; Pittet, J.F. Role of the alternative pathway in the early complement activation following major trauma. Shock 2007, 28, 29–34. [Google Scholar] [CrossRef]
- Spitzer, D.; Mitchell, L.M.; Atkinson, J.P.; Hourcade, D.E. Properdin Can Initiate Complement Activation by Binding Specific Target Surfaces and Providing a Platform for De Novo Convertase Assembly. J. Immunol. 2007, 179, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Hourcade, D.E. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J. Biol. Chem. 2006, 281, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.; Mollnes, T.E. The alternative complement pathway revisited. J. Cell Mol. Med. 2008, 12, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Walport, M.J. Complement. First of Two Parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Ehrnthaller, C.; Ignatius, A.; Gebhard, F.; Huber-lang, M. New Insights of an Old Defense System: Structure, Function and Clinical Relevance of the Complement System. Mol. Med. 2011, 17, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Koski, C.L.; Ramm, L.E.; Hammer, C.H.; Mayer, M.M.; Shin, M.L. Cytolysis of nucleated cells by complement: Cell death displays multi-hit characteristics. Proc. Natl. Acad. Sci. USA 1983, 80, 3816–3820. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.P. Complement membrane attack on nucleated cells: Resistance, recovery and non-lethal effects. Biochem. J. 1989, 264, 1–14. [Google Scholar] [CrossRef]
- Tegla, C.A.; Cudrici, C.; Patel, S.; Trippe, R.; Rus, V.; Niculescu, F.; Rus, H. Membrane attack by complement: The assembly and biology of terminal complement complexes. Immunol. Res. 2011, 51, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Song, W. Membrane complement regulatory proteins. Clin. Immunol. 2006, 118, 127–136. [Google Scholar] [CrossRef]
- Schmidt, C.Q.; Lambris, J.D.; Ricklin, D. Protection of host cells by complement regulators. Immunol. Rev. 2016, 274, 152–171. [Google Scholar] [CrossRef]
- Fearon, D.T.; Austen, K.F. Properdin: Binding to C3b and stabilization of the C3b dependent C3 convertase. J. Exp. Med. 1975, 142, 856–863. [Google Scholar] [CrossRef]
- Fearon, D.T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc. Natl. Acad. Sci. USA 1979, 76, 5867–5871. [Google Scholar] [CrossRef]
- Fearon, D.T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J. Exp. Med. 1980, 152, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Nussenzweig, V. Complement receptor is an inhibitor of the complement cascade. J. Exp. Med. 1981, 153, 1138–1150. [Google Scholar] [CrossRef]
- Medicus, R.G.; Melamed, J.; Arnaout, M.A. Role of human factor I and C3b receptor in the cleavage of surface-bound C3bi molecules. Eur. J. Immunol. 1983, 13, 465–470. [Google Scholar] [CrossRef]
- Edward Medof, M.; Iida, K.; Mold, C.; Nussenzweig, V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J. Exp. Med. 1982, 156, 1739–1754. [Google Scholar] [CrossRef]
- Seya, T.; Turner, J.R.; Atkinson, J.P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3B and C4B. J. Exp. Med. 1986, 163, 837–855. [Google Scholar] [CrossRef]
- Seya, T.; Ballard, L.L.; Bora, N.S.; Kumar, V.; Cui, W.; Atkinson, J.P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur. J. Immunol. 1988, 18, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Post, T.W.; Atkinson, J.P. Membrane cofactor protein (MCP or CD46): Newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 1991, 9, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Nicholson-Weller, A.; Burge, J.; Fearon, D.T.; Weller, P.F.; Austen, K.F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J. Immunol. 1982, 129, 184–189. [Google Scholar]
- Medof, B.Y.M.E.; Kinoshita, T.; Nussenzweig, V. Inhibition of Complement Activation on the Surface of Cells After of Incorporation of Decay-Accelerating Factor (DAF) Into Their Membranes. J. Exp. Med. 1984, 160, 1558–1578. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Medof, M.E.; Nussenzweig, V. Endogenous association of decay-accelerating factor (DAF) with C4b and C3b on cell membranes. J. Immunol. 1986, 136, 3390–3395. [Google Scholar]
- Meri, S.; Morgan, B.P.; Davies, A.; Daniels, R.H.; Olavesen, M.G.; Waldmann, H.; Lachmann, P.J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 1990, 71, 1–9. [Google Scholar]
- Ninomiya, H.; Sims, P.J. The human complement regulatory protein CD59 binds to the α-chain of C8 and to the “b” domain of C9. J. Biol. Chem. 1992, 267, 13675–13680. [Google Scholar] [CrossRef]
- Ames, R.S.; Li, Y.; Sarau, H.M.; Nuthulaganti, P.; Foley, J.J.; Ellis, C.; Zeng, Z.; Su, K.; Jurewicz, A.J.; Hertzberg, R.P.; et al. Molecular cloning and characterization of the human anaphylatoxin C3a receptor. J. Biol. Chem. 1996, 271, 20231–20234. [Google Scholar] [CrossRef]
- Gerard, N.P.; Gerard, C. The chemotactic receptor for human C5a anaphylatoxin. Nature 1991, 354, 614–617. [Google Scholar] [CrossRef]
- Ohno, M.; Hirata, T.; Enomoto, M.; Araki, T.; Ishimaru, H.; Takahashi, T.A. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol. Immunol. 2000, 37, 407–412. [Google Scholar] [CrossRef]
- Okinaga, S.; Slattery, D.; Humbles, A.; Zsengeller, Z.; Morteau, O.; Kinrade, M.B.; Brodbeck, R.M.; Krause, J.E.; Choe, H.-R.; Gerard, N.P.; et al. C5L2, a nonsignaling C5A binding protein. Biochemistry 2003, 42, 9406–9415. [Google Scholar] [CrossRef] [PubMed]
- Van Lith, L.H.C.; Oosterom, J.; Van Elsas, A.; Zaman, G.J.R. C5a-Stimulated Recruitment of ß-Arrestin2 to the Nonsignaling 7-Transmembrane Decoy Receptor C5L2. J. Biomol. Screen. 2009, 14, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Croker, D.E.; Halai, R.; Kaeslin, G.; Wende, E.; Fehlhaber, B.; Klos, A.; Monk, P.N.; Cooper, M.A. C5a2 can modulate ERK1/2 signaling in macrophages via heteromer formation with C5a1 and β-arrestin recruitment. Immunol. Cell Biol. 2014, 92, 631–639. [Google Scholar] [CrossRef]
- Hartmann, K.; Henz, B.M.; Krüger-Krasagakes, S.; Köhl, J.; Burger, R.; Gurtl, S.; Haase, I.; Lippert, U.; Zuberbier, T. C3a and C5a stimulate chemotaxis of human mast cells. Blood 1997, 89, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Johnell, M.; Hammer, C.H.; Tiffany, H.L.; Metcalfe, D.D.; Siegbahn, A.; Murphyt, P.M. C3a and C5a Are Chemotaxins for Human Mast Cells and Act Through Distinct Receptors via a Pertussis Toxin-Sensitive Signal Transduction Pathway. J. Immunol. 1996, 157, 1693–1998. [Google Scholar]
- Daffern, B.P.J.; Pfeifer, P.H.; Ember, J.A.; Hugli, T.E. C3a is a Chemotaxin for Human Eosinophils but Not for Neutrophils. I. C3a Stimulation of Neutrophils Is Secondary to Eosinophil Activation. J. Exp. Med. 1995, 181, 2119–2127. [Google Scholar] [CrossRef]
- Aksamit, R.R.; Falk, W.; Leonard, E.J. Chemotaxis by mouse macrophage cell lines. J. Immunol. 1981, 126, 2194–2199. [Google Scholar]
- Yancey, K.B.; Lawley, T.J.; Dersookian, M.; Harvath, L. Analysis of the Interaction of Human C5a and C5a des Arg with Human Monocytes and Neutrophils: Flow Cytometric and Chemotaxis Studies. J. Investig. Dermatol. 1989, 92, 184–189. [Google Scholar] [CrossRef]
- Ehrengruber, M.U.; Geiser, T.; Deranleau, D.A. Activation of human neutrophils by C3a and C5a. FEBS Lett. 1994, 346, 181–184. [Google Scholar]
- Lett-Brown, M.A.; Leonard, E.J. Histamine-Induced Inhibition of Normal Human Basophil Chemotaxis to C5a. J. Immunol. 1977, 118, 815–818. [Google Scholar]
- Nataf, S.; Davoust, N.; Ames, R.S.; Barnum, S.R. Human T Cells Express the C5a Receptor and Are Chemoattracted to C5a. J. Immunol. 1999, 162, 4018–4023. [Google Scholar]
- Ottonello, L.; Corcione, A.; Tortolina, G.; Albesiano, E.; Favre, A.; Agostino, R.D.; Malavasi, F.; Pistoia, V.; Dallegri, F.; Favre, A.; et al. rC5a Directs the In Vitro Migration of Human Memory and Naive Tonsillar B Lymphocytes: Implications for B Cell Trafficking in Secondary Lymphoid Tissues. J. Immunol. 1999, 162, 6510–6517. [Google Scholar]
- Van Lookeren Campagne, M.; Wiesmann, C.; Brown, E.J. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 2007, 9, 2095–2102. [Google Scholar] [CrossRef]
- Karsten, C.M.; Köhl, J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology 2012, 217, 1067–1079. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, C.; Fu, Y.X.; Holers, V.M.; Molina, H. Expression of Complement Receptors 1 and 2 on Follicular Dendritic Cells Is Necessary for the Generation of a Strong Antigen-Specific IgG Response. J. Immunol. 1998, 160, 5273–5279. [Google Scholar]
- Carter, R.H.; Fearon, D.T. CD19: Lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992, 256, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, A.; Cheng, P.C.; Pierce, S.K. The Role of the CD19/CD21 Complex in B Cell Processing and Presentation of Complement-Tagged Antigens. J. Immunol. 2001, 167, 163–172. [Google Scholar] [CrossRef]
- Matsumoto, A.K.; Kopicky-Burd, J.; Carter, R.H.; Tuveson, D.A.; Tedder, T.F.; Fearon, D.T. Intersection of the Complement and Immune Systems: A Signal Transduction Complex of the B Lymphocyte-containing Complement Receptor Type 2 and CD19. J. Exp. Med. 1991, 173, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Strainic, M.G.; Liu, J.; Huang, D.; An, F.; Lalli, P.N.; Muqim, N.; Shapiro, V.S.; Dubyak, G.R.; Heeger, P.S.; Medof, M.E. Locally Produced Complement Fragments C5a and C3a Provide Both Costimulatory and Survival Sinals to naive CD4+ T Cells. Immunity 2008, 28, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lalli, P.N.; Strainic, M.G.; Yang, M.; Lin, F.; Medof, M.E.; Heeger, P.S. Locally produced C5a binds to T cell expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 2008, 112, 1759–1766. [Google Scholar] [CrossRef]
- Strainic, M.G.; Shevach, E.M.; An, F.; Lin, F.; Medof, M.E. Absent C3a and C5a receptor signaling into CD4+T cells enables auto-inductive TGF-β1 signaling and induction of Foxp3+T regulatory cells. Nat. Immunol. 2013, 14, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Lalli, P.N.; Strainic, M.G.; Lin, F.; Medof, M.E.; Heeger, P.S. Decay Accelerating Factor Can Control T Cell Differentiation into IFN-γ-Producing Effector Cells via Regulating Local C5a-Induced IL-12 Production. J. Immunol. 2007, 179, 5793–5802. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, S.K.; Chintala, N.K.; Sharma, S.K.; Sharma, P.; Cleveland, C.; Riediger, L.; Manne, S.; Fairlie, D.P.; Gorczyca, W.; Almanza, O.; et al. Complement C5a receptor facilitates cancer metastasis by altering t-cell responses in the metastatic niche. Cancer Res. 2014, 74, 3454–3465. [Google Scholar] [CrossRef]
- Ytting, H.; Jensenius, J.C.; Christensen, I.J.; Thiel, S.; Nielsen, H.J. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand. J. Gastroenterol. 2004, 39, 674–679. [Google Scholar] [CrossRef]
- Corrales, L.; Ajona, D.; Rafail, S.; Lasarte, J.J.; Riezu-Boj, J.I.; Lambris, J.D.; Rouzaut, A.; Pajares, M.J.; Montuenga, L.M.; Pio, R. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J. Immunol. 2012, 189, 4674–4683. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Pajares, M.J.; Chiara, M.; Rodrigo, J.P.; Jantus-Lewintre, E.; Camps, C.; Suarez, C.; Bagán, J.; Montuenga, L.; Pio, R. Complement activation product C4d in oral and oropharyngeal squamous cell carcinoma. Oral Dis. 2015, 21, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Zheng, Z.; Wang, C.; Yu, Y. Significance of C4d deposition in the follicular lymphoma and MALT lymphoma and their relationship with follicular dendritic cells. Pathol. Res. Pract. 2007, 203, 163–167. [Google Scholar] [CrossRef]
- Bouwens, T.A.M.; Trouw, L.A.; Veerhuis, R.; Dirven, C.M.F.; Lamfers, M.L.M.; Al-khawaja, H. Complement activation in Glioblastoma Multiforme pathophysiology: Evidence from serum levels and presence of complement activation products in tumor tissue. J. Neuroimmunol. 2015, 278, 271–276. [Google Scholar] [CrossRef]
- Niculescu, F.; Rus, H.G.; Retegan, M.; Vlaicu, R. Persistent Complement Activation on Tumor Cells in Breast Cancer. Am. J. Pathol. 1992, 140, 1039–1043. [Google Scholar]
- Chen, J.; Yang, W.; Sun, H.; Yang, X.; Wu, Y. C5b-9 Staining Correlates With Clinical and Tumor Stage in Gastric Adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 470–475. [Google Scholar] [CrossRef]
- Yamakawa, M.; Yamada, K.; Tsuge, T.; Ohrui, H.; Ogata, T.; Dobashi, M.; Imai, Y. Protection of Thyroid Cancer Cells by Complement-Regulatory Factors. Cancer 1994, 73, 2808–2817. [Google Scholar] [CrossRef]
- Bjørge, L.; Hakulinen, J.; Vintermyr, O.K.; Jarva, H.; Jensen, T.S.; Iversen, O.E.; Meri, S. Ascitic complement system in ovarian cancer. Br. J. Cancer 2005, 92, 895–905. [Google Scholar] [CrossRef]
- Fishelson, Z.; Kirschfink, M. Complement C5b-9 and cancer: Mechanisms of cell damage, cancer counteractions, and approaches for intervention. Front. Immunol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maestri, C.A.; Nisihara, R.; Mendes, H.W.; Jensenius, J.; Thiel, S.; Messias-Reason, I.; De Carvalho, N.S. MASP-1 and MASP-2 serum levels are associated with worse prognostic in cervical cancer progression. Front. Immunol. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Ytting, H.; Christensen, I.J.; Thiel, S.; Jensenius, J.C.; Nielsen, H.J. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: Relation to recurrence and mortality. Clin. Cancer Res. 2005, 11, 1441–1446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swierzko, A.S.; Szala, A.; Sawicki, S.; Szemraj, J.; Sniadecki, M.; Sokolowska, A.; Kaluzynski, A.; Wydra, D.; Cedzynski, M. Mannose-Binding Lectin (MBL) and MBL-associated serine protease-2 (MASP-2) in women with malignant and benign ovarian tumours. Cancer Immunol. Immunother. 2014, 63, 1129–1140. [Google Scholar] [CrossRef]
- Fishelson, Z.; Donin, N.; Zell, S.; Schultz, S.; Kirschfink, M. Obstacles to cancer immunotherapy: Expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 2003, 40, 109–123. [Google Scholar] [CrossRef]
- Olcina, M.M.; Balanis, N.G.; Kim, R.K.; Aksoy, B.A.; Kodysh, J.; Thompson, M.J.; Hammerbacher, J.; Graeber, T.G.; Giaccia, A.J. Mutations in an Innate Immunity Pathway Are Associated with Poor Overall Survival Outcomes and Hypoxic Signaling in Cancer. Cell Rep. 2018, 25, 3721–3732.e6. [Google Scholar] [CrossRef]
- Ong, H.T.; Timm, M.M.; Greipp, P.R.; Witzig, T.E.; Dispenzieri, A.; Russell, S.J.; Peng, K.W. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp. Hematol. 2006, 34, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.; Descamps, G.; Tessoulin, B.; Chiron, D.; Eveillard, M.; Godon, C.; Le Bris, Y.; Vabret, A.; Bellanger, C.; Maillet, L.; et al. P53 regulates CD46 expression and measles virus infection in myeloma cells. Blood Adv. 2018, 2, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.S.; Durrant, L.G.; Madjd, Z.; Ellis, I.O.; Scholefield, J.H.; Spendlove, I. Expression of the membrane complement regulatory protein CD59 (protectin) is associated with reduced survival in colorectal cancer patients. Cancer Immunol. Immunother. 2006, 55, 973–980. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy. Front. Immunol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Ajona, D.; Castaño, Z.; Garayoa, M.; Zudaire, E.; Pajares, M.J.; Martinez, A.; Cuttitta, F.; Montuenga, L.M.; Pio, R. Expression of complement factor H by lung cancer cells: Effects on the activation of the alternative pathway of complement. Cancer Res. 2004, 64, 6310–6318. [Google Scholar] [CrossRef]
- Ajona, D.; Hsu, Y.-F.; Corrales, L.; Montuenga, L.M.; Pio, R. Down-Regulation of Human Complement Factor H Sensitizes Non-Small Cell Lung Cancer Cells to Complement Attack and Reduces In Vivo Tumor Growth. J. Immunol. 2007, 178, 5991–5998. [Google Scholar] [CrossRef]
- Okroj, M.; Hsu, Y.F.; Ajona, D.; Pio, R.; Blom, A.M. Non-small cell lung cancer cells produce a functional set of complement factor I and its soluble cofactors. Mol. Immunol. 2008, 45, 169–179. [Google Scholar] [CrossRef]
- Laskowski, J.; Renner, B.; Pickering, M.C.; Serkova, N.J.; Smith-Jones, P.M.; Clambey, E.T.; Nemenoff, R.A.; Thurman, J.M. Complement factor H–deficient mice develop spontaneous hepatic tumors. J. Clin. Investig. 2020, 130, 4039–4054. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Ortiz-Espinosa, S.; Pio, R.; Lecanda, F. Complement in Metastasis: A Comp in the Camp. Front. Immunol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Cardone, J.; Friec, G.L.; Vantourout, P.; Roberts, A.; Fuchs, A.; Jackson, I.; Suddason, T.; Lord, G.; Atkinson, J.P.; Cope, A.; et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 2010, 11, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Kolev, M.; Le Friec, G.; Leung, M.; Bertram, P.G.; Fara, A.F.; Subias, M.; Pickering, M.C.; Drouet, C.; Meri, S.; et al. Intracellular Complement Activation Sustains T Cell Homeostasis and Mediates Effector Differentiation. Immunity 2013, 39, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.; Dimeloe, S.; Le Friec, G.; Navarini, A.; Arbore, G.; Povoleri, G.A.; Fischer, M.; Belle, R.; Loeliger, J.; Develioglu, L.; et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 2015, 42, 1033–1047. [Google Scholar] [CrossRef]
- Cho, M.S.; Vasquez, H.G.; Rupaimoole, R.; Pradeep, S.; Wu, S.; Zand, B.; Han, H.D.; Rodriguez-Aguayo, C.; Bottsford-Miller, J.; Huang, J.; et al. Autocrine Effects of Tumor-Derived Complement. Cell Rep. 2014, 6, 1085–1095. [Google Scholar] [CrossRef]
- Bulla, R.; Tripodo, C.; Rami, D.; Ling, G.S.; Agostinis, C.; Guarnotta, C.; Zorzet, S.; Durigutto, P.; Botto, M.; Tedesco, F. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Riihilä, P.; Nissinen, L.; Farshchian, M.; Kivisaari, A.; Ala-aho, R.; Kallajoki, M.; Grénman, R.; Meri, S.; Peltonen, S.; Peltonen, J.; et al. Complement factor I promotes progression of cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2015, 135, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Nissinen, L.; Farshchian, M.; Kallajoki, M.; Kivisaari, A.; Meri, S.; Grénman, R.; Peltonen, S.; Peltonen, J.; Pihlajaniemi, T.; et al. Complement Component C3 and Complement Factor B Promote Growth of Cutaneous Squamous Cell Carcinoma. Am. J. Pathol. 2017, 187, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, J.; Wang, D.; Geng, S. Complement C3a promotes proliferation, migration and stemness in cutaneous squamous cell carcinoma. J. Cell. Mol. Med. 2019, 23, 3097–3107. [Google Scholar] [CrossRef]
- Nunez-Cruz, S.; Gimotty, P.A.; Guerra, M.W.; Connolly, D.C.; Wu, Y.Q.; DeAngelis, R.A.; Lambris, J.D.; Coukos, G.; Scholler, N. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia 2012, 14, 994–1004. [Google Scholar] [CrossRef]
- Yoneda, M.; Imamura, R.; Nitta, H.; Taniguchi, K.; Saito, F.; Kikuchi, K.; Ogi, H.; Tanaka, T.; Katabuchi, H.; Nakayama, H.; et al. Enhancement of cancer invasion and growth via the C5a-C5a receptor system: Implications for cancer promotion by autoimmune diseases and association with cervical cancer invasion. Oncol. Lett. 2019, 17, 913–920. [Google Scholar] [CrossRef]
- Maeda, Y.; Kawano, Y.; Wada, Y.; Yatsuda, J.; Motoshima, T.; Murakami, Y.; Kikuchi, K.; Imamura, T.; Eto, M. C5aR is frequently expressed in metastatic renal cell carcinoma and plays a crucial role in cell invasion via the ERK and PI3 kinase pathways. Oncol. Rep. 2015, 33, 1844–1850. [Google Scholar] [CrossRef]
- Hu, W.H.; Hu, Z.; Shen, X.; Dong, L.-Y.; Zhou, W.-Z.; Yu, X.-X. C5a receptor enhances hepatocellular carcinoma cell invasiveness via activating ERK1/2-mediated epithelial-mesenchymal transition. Exp. Mol. Pathol. 2016, 100, 101–108. [Google Scholar] [CrossRef]
- Ajona, D.; Zandueta, C.; Corrales, L.; Moreno, H.; Pajares, M.J.; Ortiz-Espinosa, S.; Martínez-Terroba, E.; Perurena, N.; De Miguel, F.J.; Jantus-Lewintre, E.; et al. Blockade of the complement C5a/C5aR1 axis impairs lung cancer bone metastasis by CXCL16-mediated effects. Am. J. Respir. Crit. Care Med. 2018, 197, 1164–1176. [Google Scholar] [CrossRef]
- Abdelbaset-Ismail, A.; Borkowska-Rzeszotek, S.; Kubis, E.; Bujko, K.; Brzeźniakiewicz-Janus, K.; Bolkun, L.; Kloczko, J.; Moniuszko, M.; Basak, G.W.; Wiktor-Jedrzejczak, W.; et al. Activation of the complement cascade enhances motility of leukemic cells by downregulating expression of HO-1. Leukemia 2017, 31, 446–458. [Google Scholar] [CrossRef]
- Nitta, H.; Wada, Y.; Kawano, Y.; Murakami, Y.; Irie, A.; Taniguchi, K.; Kikuchi, K.; Yamada, G.; Suzuki, K.; Honda, J.; et al. Enhancement of Human Cancer Cell Motility and Invasiveness by Anaphylatoxin C5a via Aberrantly Expressed C5a Receptor ( CD88 ). Clin. Cancer Res. 2004, 19, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Rupaimoole, R.; Choi, H.-J.; Noh, K.; Chen, J.; Hu, Q.; Sood, A.K.; Afshar-Kharghan, V. Complement Component 3 Is Regulated by TWIST1 and Mediates Epithelial—Mesenchymal Transition. J. Immunol. 2016, 196, 1412–1418. [Google Scholar] [CrossRef]
- Kochanek, D.M.; Ghouse, S.M.; Karbowniczek, M.M.; Markiewski, M.M. Complementing cancer metastasis. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Block, I.; Müller, C.; Sdogati, D.; Pedersen, H.; List, M.; Jaskot, A.M.; Syse, S.D.; Lund Hansen, P.; Schmidt, S.; Christiansen, H.; et al. CFP suppresses breast cancer cell growth by TES-mediated upregulation of the transcription factor DDIT3. Oncogene 2019, 38, 4560–4573. [Google Scholar] [CrossRef]
- Bandini, S.; Curcio, C.; Macagno, M.; Quaglino, E.; Arigoni, M.; Lanzardo, S.; Hysi, A.; Barutello, G.; Consolino, L.; Longo, D.L.; et al. Early onset and enhanced growth of autochthonous mammary carcinomas in C3-deficient Her2/neu transgenic mice. Oncoimmunology 2013, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bandini, S.; Macagno, M.; Hysi, A.; Lanzardo, S.; Conti, L.; Bello, A.; Riccardo, F.; Ruiu, R.; Merighi, I.F.; Forni, G.; et al. The non-inflammatory role of C1q during Her2/neu-driven mammary carcinogenesis. Oncoimmunology 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Martin, C.B.; Soon, N.L.; Martin, B.K. Expression of complement protein C5a in a murine mammary cancer model: Tumor regression by interference with the cell cycle. Cancer Immunol. Immunother. 2005, 54, 1026–1037. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.-N.; Liu, Q.; Yu, Y.; Guo, J.; Wang, K.; Xing, B.-C.; Zheng, Q.-F.; Campa, M.J.; Patz, E.F.; et al. Autocrine complement inhibits IL10-dependent T-cell-mediated antitumor immunity to promote tumor progression. Cancer Discov. 2016, 6, 1022–1035. [Google Scholar] [CrossRef]
- Ajona, D.; Ortiz-Espinosa, S.; Moreno, H.; Lozano, T.; Pajares, M.J.; Agorreta, J.; Bértolo, C.; Lasarte, J.J.; Vicent, S.; Hoehlig, K.; et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 2017, 7, 694–703. [Google Scholar] [CrossRef]
- Downs-Canner, S.; Magge, D.; Ravindranathan, R.; O’Malley, M.E.; Francis, L.; Liu, Z.; Sheng Guo, Z.; Obermajer, N.; Bartlett, D.L. Complement Inhibition: A Novel Form of Immunotherapy for Colon Cancer. Ann. Surg. Oncol. 2016, 23, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Li, L.; Li, L.; Lv, X.; Zhou, D.; Wang, Q.; Chen, J.; Yang, C.; Xu, E.; Dai, W.; et al. C5aR1 is a master regulator in colorectal tumorigenesis via immune modulation. Theranostics 2020, 10, 8619–8632. [Google Scholar] [CrossRef]
- Gunn, L.; Ding, C.; Liu, M.; Ma, Y.; Qi, C.; Cai, Y.; Hu, X.; Aggarwal, D.; Zhang, H.; Yan, J. Opposing Roles for Complement Component C5a in Tumor Progression and the Tumor Microenvironment. J. Immunol. 2012, 189, 2985–2994. [Google Scholar] [CrossRef]
- Kwak, J.W.; Laskowski, J.; Li, H.Y.; McSharry, M.V.; Sippel, T.R.; Bullock, B.L.; Johnson, A.M.; Poczobutt, J.M.; Neuwelt, A.J.; Malkoski, S.P.; et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 2018, 78, 143–156. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Xu, W.; Zheng, X.; Yi, X.; Huang, L.; Wang, Y.; Wu, K. Activated hepatic stellate cells (HSCs) exert immunosuppressive effects in hepatocellular carcinoma by producing complement C3. Onco. Targets. Ther. 2020, 13, 1497–1505. [Google Scholar] [CrossRef]
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, Y.-Y.; Wang, Y.; Han, G.C.; Wang, R.X.; Xiao, H.; Li, X.Y.; Hou, C.M.; Ma, Y.F.; Sheng, D.S.; et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1β/IL-17A axis. Mucosal Immunol. 2015, 8, 1275–1284. [Google Scholar] [CrossRef]
- Nabizadeh, J.A.; Manthey, H.D.; Steyn, F.J.; Chen, W.; Widiapradja, A.; Md Akhir, F.N.; Boyle, G.M.; Taylor, S.M.; Woodruff, T.M.; Rolfe, B.E. The Complement C3a Receptor Contributes to Melanoma Tumorigenesis by Inhibiting Neutrophil and CD4 + T Cell Responses. J. Immunol. 2016, 196, 4783–4792. [Google Scholar] [CrossRef]
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Rep. 2020, 31, 107628. [Google Scholar] [CrossRef]
- Bonavita, E.; Gentile, S.; Rubino, M.; Maina, V.; Papait, R.; Kunderfranco, P.; Greco, C.; Feruglio, F.; Molgora, M.; Laface, I.; et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell 2015, 160, 700–714. [Google Scholar] [CrossRef]
- Piao, C.; Cai, L.; Qiu, S.; Jia, L.; Song, W.; Du, J. Complement 5a enhances hepatic metastases of colon cancer via monocyte chemoattractant protein-1-mediated inflammatory cell infiltration. J. Biol. Chem. 2015, 290, 10667–10676. [Google Scholar] [CrossRef] [PubMed]
- Contractor, T.; Kobayashi, S.; da Silva, E.; Clausen, R.; Chan, C.; Vosburgh, E.; Tang, L.H.; Levine, A.J.; Harris, C.R. Sexual dimorphism of liver metastasis by murine pancreatic neuroendocrine tumors is affected by expression of complement C5. Oncotarget 2016, 7, 30585–30596. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.; Zhang, W.M.; Li, T.T.; Zhang, C.C.; Qiu, S.; Liu, Y.; Liu, S.; Jin, M.; Jia, L.X.; Song, W.C.; et al. Complement 5a stimulates macrophage polarization and contributes to tumor metastases of colon cancer. Exp. Cell Res. 2018, 366, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Medler, T.R.; Murugan, D.; Horton, W.; Kumar, S.; Cotechini, T.; Forsyth, A.M.; Leyshock, P.; Leitenberger, J.J.; Kulesz-Martin, M.; Margolin, A.A.; et al. Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy. Cancer Cell 2018, 34, 561–578. [Google Scholar] [CrossRef]
- Janelle, V.; Langlois, M.P.; Tarrab, E.; Lapierre, P.; Poliquin, L.; Lamarre, A. Transient complement inhibition promotes a tumor-specific immune response through the implication of natural killer cells. Cancer Immunol. Res. 2014, 2, 200–206. [Google Scholar] [CrossRef]
- Liu, C.-F.; Min, X.-Y.; Wang, N.; Wang, J.-X.; Ma, N.; Dong, X.; Zhang, B.; Wu, W.; Li, Z.F.; Zhou, W.; et al. Complement receptor 3 has negative impact on tumor surveillance through suppression of natural killer cell function. Front. Immunol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ding, J.Y.; Lu, C.L.; Lin, Z.W.; Chu, Y.W.; Zhao, G.Y.; Guo, J.; Ge, D. Overexpression of CD88 predicts poor prognosis in non-small-cell lung cancer. Lung Cancer 2013, 81, 259–265. [Google Scholar] [CrossRef]
- Yuan, K.; Ye, J.; Liu, Z.; Ren, Y.; He, W.; Xu, J.; He, Y.; Yuan, Y. Complement C3 overexpression activates JAK2/STAT3 pathway and correlates with gastric cancer progression. J. Exp. Clin. Cancer Res. 2020, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.Q.; Zhang, L.; Tang, M.; Cao, X.; Xu, G.L.; Wu, Y.Z. Complement C5a/C5aR pathway potentiates the pathogenesis of gastric cancer by down-regulating p21 expression. Cancer Lett. 2018, 412, 30–36. [Google Scholar] [CrossRef]
- Lin, K.; He, S.; He, L.; Chen, J.; Cheng, X.; Zhang, G.; Zhu, B. Complement component 3 is a prognostic factor of non-small cell lung cancer. Mol. Med. Rep. 2014, 10, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Borras, J.M.; Lievens, Y.; Barton, M.; Corral, J.; Ferlay, J.; Bray, F.; Grau, C. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother. Oncol. 2016, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Surace, L.; Lysenko, V.; Fontana, A.O.; Cecconi, V.; Janssen, H.; Bicvic, A.; Okoniewski, M.; Pruschy, M.; Dummer, R.; Neefjes, J.; et al. Complement Is a Central Mediator of Radiotherapy-Induced Tumor-Specific Immunity and Clinical Response. Immunity 2015, 42, 767–777. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. Role of T lymphocytes in tumor response to radiotherapy. Front. Oncol. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Eriksson, D.; Stigbrand, T. Radiation-induced cell death mechanisms. Tumor Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.S.; Schneider, R.J.; Formenti, S.C.; Dustin, M.L.; et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008, 181, 3099–3107. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; De Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Song, H.; He, C.; Knaak, C.; Guthridge, J.M.; Holers, V.M.; Tomlinson, S. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J. Clin. Investig. 2003, 111, 1875–1885. [Google Scholar] [CrossRef]
- Engelman, R.M.; Rousou, J.A.; Flack, J.E.; Deaton, D.W.; Kalfin, R.; Das, D.K. Influence of steroids on complement and cytokine generation after cardiopulmonary bypass. Ann. Thorac. Surg. 1995, 60, 801–804. [Google Scholar] [CrossRef]
- Coulpier, M.; Andreev, S.; Lemercier, C.; Dauchel, H.; Lees, O.; Fontaine, M.; Ripoche, J. Activation of the endothelium by IL-la and glucocorticoids results in major increase of complement C3 and factor B production and generation of C3a. Clin. Exp. Immunol. 1995, 101, 142–149. [Google Scholar] [CrossRef]
- Lappin, D.F.; Whaley, K. Modulation of complement gene expression by glucocorticoids. Biochem. J. 1991, 280, 117–123. [Google Scholar] [CrossRef]
- Schleimer, R.P. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc. Am. Thorac. Soc. 2004, 1, 222–230. [Google Scholar] [CrossRef]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Illidge, T.M. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology 2015, 4, 1–4. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Melis, M.H.M.; Wilkinson, R.W.; Adlard, A.L.; Stratford, I.J.; Honeychurch, J.; Illidge, T.M. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood 2013, 121, 251–259. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Q.; Liao, J.Y.; Song, E.; Xia, Q.; Pan, J.; Li, Y.; Li, J.; Zhou, B.; Ye, Y.; et al. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell 2020, 180, 1081–1097.e24. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Roumenina, L.T. B cells and complement at the forefront of chemotherapy. Nat. Rev. Clin. Oncol. 2020, 17, 393–394. [Google Scholar] [CrossRef]
- Murray, K.P.; Mathure, S.; Kaul, R.; Khan, S.; Carson, L.F.; Twiggs, L.B.; Martens, M.G.; Kaul, A. Expression of complement regulatory proteins - CD 35, CD 46, CD 55, and CD 59 - In benign and malignant endometrial tissue. Gynecol. Oncol. 2000, 76, 176–182. [Google Scholar] [CrossRef]
- Saygin, C.; Wiechert, A.; Rao, V.S.; Alluri, R.; Connor, E.; Thiagarajan, P.S.; Hale, J.S.; Li, Y.; Chumakova, A.; Jarrar, A.; et al. CD55 regulates self-renewal and cisplatin resistance in endometrioid tumors. J. Exp. Med. 2017, 214, 2715–2732. [Google Scholar] [CrossRef]
- Hammond, E.M.; Asselin, M.C.; Forster, D.; O’Connor, J.P.B.; Senra, J.M.; Williams, K.J. The Meaning, Measurement and Modification of Hypoxia in the Laboratory and the Clinic. Clin. Oncol. 2014, 26, 277–288. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomedicine 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Brizel, D.M.; Dodge, R.K.; Clough, R.W.; Dewhirst, M.W. Oxygenation of head and neck cancer: Changes during radiotherapy and impact on treatment outcome. Radiother. Oncol. 1999, 53, 113–117. [Google Scholar] [CrossRef]
- Höckel, M.; Schlenger, K.; Aral, B.; Mitze, M.; Schäffer, U.; Vaupel, P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996, 56, 4509–4515. [Google Scholar]
- Koch, S.; Mayer, F.; Honecker, F.; Schittenhelm, M.; Bokemeyer, C. Efficacy of cytotoxic agents used in the treatment of testicular germ cell tumours under normoxic and hypoxic conditions in vitro. Br. J. Cancer 2003, 89, 2133–2139. [Google Scholar] [CrossRef]
- Strese, S.; Fryknäs, M.; Larsson, R.; Gullbo, J. Effects of hypoxia on human cancer cell line chemosensitivity. BMC Cancer 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Okroj, M.; Corrales, L.; Stokowska, A.; Pio, R.; Blom, A.M. Hypoxia increases susceptibility of non-small cell lung cancer cells to complement attack. Cancer Immunol. Immunother. 2009, 58, 1771–1780. [Google Scholar] [CrossRef]
- Wenger, R.H.; Rolfs, A.; Marti, H.H.; Bauer, C.; Gassmann, M. Hypoxia, a novel inducer of acute phase gene expression in a human hepatoma cell line. J. Biol. Chem. 1995, 270, 27865–27870. [Google Scholar] [CrossRef] [PubMed]
- Louis, N.A.; Hamilton, K.E.; Kong, T.; Colgan, S.P. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 2005, 19, 950–959. [Google Scholar] [CrossRef]
- Olcina, M.M.; Kim, R.K.; Melemenidis, S.; Graves, E.E.; Giaccia, A.J. The tumour microenvironment links complement system dysregulation and hypoxic signalling. Br. J. Radiol. 2019, 91, 20180069. [Google Scholar]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Lynam-Lennon, N.; Reynolds, J.V.; Pidgeon, G.P.; Lysaght, J.; Marignol, L.; Maher, S.G. Alterations in DNA Repair Efficiency are Involved in the Radioresistance of Esophageal Adenocarcinoma. Radiat. Res. 2010, 174, 703–711. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018, 73, 1–10. [Google Scholar] [CrossRef]
- Lynam-Lennon, N.; Bibby, B.A.S.; Mongan, A.M.; Marignol, L.; Paxton, C.N.; Geiersbach, K.; Bronner, M.P.; Sullivan, J.O.; Reynolds, J.V.; Maher, S.G. Low MiR-187 Expression Promotes Resistance to Chemoradiation Therapy In Vitro and Correlates with Treatment Failure in Patients with Esophageal Adenocarcinoma. Mol. Med. 2016, 22, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Kurumizaka, H.; Ikawa, S.; Nakada, M.; Eda, K.; Kagawa, W.; Takata, M.; Takeda, S.; Yokoyama, S.; Shibata, T. Homologous-pairing activity of the human DNA-repair proteins Xrcc3.Rad51C. Proc. Natl. Acad. Sci. USA 2001, 98, 5538–5543. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.S.; Chang, W.S.; Hsu, P.C.; Chen, C.C.; Cheng, S.P.; Wang, Y.C.; Tsai, C.W.; Shen, T.C.; Bau, D.T. The contribution of XRCC3 genotypes to childhood acute lymphoblastic leukemia. Cancer Manag. Res. 2018, 10, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Kuricova, M.; Naccarati, A.; Kumar, R.; Koskinen, M.; Sanyal, S.; Dusinska, M.; Tulinska, J.; Vodickova, L.; Liskova, A.; Jahnova, E.; et al. DNA repair and cyclin D1 polymorphisms and styrene-induced genotoxicity and immunotoxicity. Toxicol. Appl. Pharmacol. 2005, 207, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Huang, X.; Floberg, J.M.; Elhammali, A.E.; McCormick, M.L.; Patti, G.J.; Spitz, D.R.; Schwarz, J.K. Radio-resistant cervical cancers are sensitive to inhibition of glycolysis and redox metabolism. Cancer Res. 2018, 78, 1392–1403. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Mustafi, S.B.; Xiong, X.; Dwivedi, S.K.D.; Nesin, V.; Saha, S.; Zhang, M.; Dhanasekaran, D.; Jayaraman, M.; Mannel, R.; et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Cascone, T.; McKenzie, J.A.; Mbofung, R.M.; Punt, S.; Wang, Z.; Xu, C.; Williams, L.J.; Wang, Z.; Bristow, C.A.; Carugo, A.; et al. Increased Tumor Glycolysis Characterizes Immune Resistance to Adoptive T Cell Therapy. Cell Metab. 2018, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, B.; Zaal, E.A.; Zecha, J.; Wu, W.; Berkers, C.R.; Kuster, B.; Lemeer, S. Lapatinib resistance in breast cancer cells is accompanied by phosphorylation-mediated reprogramming of glycolysis. Cancer Res. 2017, 77, 1842–1853. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Low, S.H.H.; Soh, C.; Kamal Mustapa, N.; Beloueche-Babari, M.; Koh, K.X.; Loh, J.; Soong, R. Increased drug resistance is associated with reduced glucose levels and an enhanced glycolysis phenotype. Br. J. Pharmacol. 2014, 171, 3255–3267. [Google Scholar] [CrossRef]
- Hess, C.; Kemper, C. Complement-Mediated Regulation of Metabolism and Basic Cellular Processes. Immunity 2016, 45, 240–254. [Google Scholar] [CrossRef]
- Kolev, M.; Kemper, C. Keeping it all going-complement meets metabolism. Front. Immunol. 2017, 8, 1. [Google Scholar] [CrossRef]
- West, E.E.; Kemper, C. Complement and T Cell Metabolism: Food for Thought. Immunometabolism 2019, 1–19. [Google Scholar]
- Arbore, G.; West, E.E.; Spolski, R.; Robertson, A.A.B.; Klos, A.; Rheinheimer, C.; Dutow, P.; Woodruff, T.M.; Yu, Z.X.; O’Neill, L.A.; et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome actvity in CD4+ T cells. Science 2016, 352, 1247–1262. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Fresno Vara, J.Á.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. P13K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.G.; Niculescu, F.I.; Shin, M.L. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol. Rev. 2001, 180, 49–55. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.G.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR Activation of Human T Cells Induces the Production of Exosomes Bearing the TCR/CD3/ζ Complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Sugimoto, H.; Connell, J.T.O.; Kato, N.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; Lucci, A.; et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.A.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012, 10, 1–7. [Google Scholar] [CrossRef]
- Van den Boorn, J.G.; Daßler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Barros, F.M.; Carneiro, F.; Machado, J.C.; Melo, S.A. Exosomes and Immune Response in cancer: Friends or Foes? Front. Immunol. 2018, 9, 730. [Google Scholar] [CrossRef]
- Corrado, C.; Raimondo, S.; Chiesi, A.; Ciccia, F.; De Leo, G.; Alessandro, R. Exosomes as intercellular signaling organelles involved in health and disease: Basic science and clinical applications. Int. J. Mol. Sci. 2013, 14, 5338–5366. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Andreola, G.; Rivoltini, L.; Castelli, C.; Huber, V.; Perego, P.; Deho, P.; Squarcina, P.; Accornero, P.; Lozupone, F.; Lugini, L.; et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 2002, 195, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-Derived Microvesicles Promote Regulatory T Cell Expansion and Induce Apoptosis in Tumor-Reactive Activated CD8+ T Lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Karasu, E.; Eisenhardt, S.U.; Harant, J.; Huber-Lang, M. Extracellular vesicles: Packages sent with complement. Front. Immunol. 2018, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Végh, P.; Prechl, J.; Kerekes, K.; Kovács, J.; Csikós, G.; Bajtay, Z.; Erdei, A. B lymphocytes and macrophages release cell membrane deposited C3-fragments on exosomes with T cell response-enhancing capacity. Mol. Immunol. 2008, 45, 2343–2351. [Google Scholar] [CrossRef]
- Clayton, A.; Harris, C.L.; Court, J.; Mason, M.D.; Morgan, B.P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003, 33, 522–531. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Xu, L.; Zhan, S.; Xiao, Y.; Gao, Y.; Wu, B.; Ge, W. Protein content and functional characteristics of serum-purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. Int. J. Cancer 2017, 140, 900–913. [Google Scholar] [CrossRef]
- Balbinotti, H.; Cadore, N.A.; Dutra, C.S.; Silva, E.D.D.A.; Ferreira, H.B.; Zaha, A.; Monteiro, K.M. Protein profiling of extracellular vesicles associated with cisplatin resistance in lung cancer. Anticancer Res. 2020, 40, 5509–5516. [Google Scholar] [CrossRef] [PubMed]
- Pilzer, D.; Gasser, O.; Moskovich, O.; Schifferli, J.A.; Fishelson, Z. Emission of membrane vesicles: Roles in complement resistance, immunity and cancer. Springer Semin. Immunopathol. 2005, 27, 375–387. [Google Scholar] [CrossRef]
- Pilzer, D.; Fishelson, Z. Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int. Immunol. 2005, 17, 1239–1248. [Google Scholar] [CrossRef]
- Aung, T.; Chapuy, B.; Vogel, D.; Wenzel, D.; Oppermann, M.; Lahmann, M.; Weinhage, T.; Menck, K.; Hupfeld, T.; Koch, R.; et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl. Acad. Sci. USA 2011, 108, 15336–15341. [Google Scholar] [CrossRef] [PubMed]
- Safaei, R.; Larson, B.J.; Cheng, T.C.; Gibson, M.A.; Otani, S.; Naerdemann, W.; Howell, S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005, 4, 1595–1604. [Google Scholar] [CrossRef]
- Kelleher, R.; Balu-Iyer, S.; Loyall, J.; Sacca, A.J.; Shenoy, G.N.; Peng, P.; Iyer, V.; Fathallah, A.M.; Berenson, C.S.; Wallace, P.K.; et al. Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade. Cancer Immunol. Res. 2015, 3, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Unnewehr, H.; Rittirsch, D.; Sarma, J.V.; Zetoune, F.; Flierl, M.A.; Perl, M.; Denk, S.; Weiss, M.; Schneider, M.E.; Monk, P.N.; et al. Changes and Regulation of the C5a Receptor on Neutrophils during Septic Shock in Humans. J. Immunol. 2013, 190, 4215–4225. [Google Scholar] [CrossRef]
- Reis, E.S.; Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019, 15. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Zhao, Y.; Xiong, H.; Sneh, T.; Fan, Y.; Wang, J.; Zhou, X.; Gong, C. Complement and coagulation cascades pathway correlates with chemosensitivity and overall survival in patients with soft tissue sarcoma. Eur. J. Pharmacol. 2020, 879, 173121. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, A.; Bachleitner-Hofmann, T.; Baumann, S.; Marchetti-Deschmann, M.; Rech-Weichselbraun, I.; Burghuber, C.; Pluschnig, U.; Bartsch, R.; Graf, A.; Greil, R.; et al. Modulation of plasma complement by the initial dose of epirubicin/docetaxel therapy in breast cancer and its predictive value. Br. J. Cancer 2010, 103, 1201–1208. [Google Scholar] [CrossRef]
- Maher, S.G.; McDowell, D.T.; Collins, B.C.; Muldoon, C.; Gallagher, W.M.; Reynolds, J.V. Serum Proteomic Profiling Reveals That Pretreatment Complement Protein Levels are Predictive of Esophageal Cancer Patient Response to Neoadjuvant Chemoradiation. Ann. Surg. 2011, 254, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 1–18. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Dranoff, G. Immune Therapy for Cancer. Annu. Rev. Immunol. 2009, 27, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Maloney, D.G.; Grillo-López, A.J.; White, C.A.; Bodkin, D.; Schilder, R.J.; Neidhart, J.A.; Janakiraman, N.; Foon, K.A.; Liles, T.-M.; Dallaire, B.K.; et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 1997, 90, 2188–2195. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- De Weers, M.; Tai, Y.-T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.H.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a Novel Therapeutic Human CD38 Monoclonal Antibody, Induces Killing of Multiple Myeloma and Other Hematological Tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef]
- Taylor, R.P.; Lindorfer, M.A. Cytotoxic mechanisms of immunotherapy: Harnessing complement in the action of anti-tumor monoclonal antibodies. Semin. Immunol. 2016, 28, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.B.; Henner, D.; Wong, W.L.T.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Taylor, R.P.; Lindorfer, M.A. The role of complement in mAb-based therapies of cancer. Methods 2014, 65, 18–27. [Google Scholar] [CrossRef]
- Derer, S.; Beurskens, F.J.; Rösner, T.; Peipp, M.; Valerius, T. Complement in antibody-based tumor therapy. Crit. Rev. Immunol. 2014, 34, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; Zaffaroni, L.; Vaccari, T.; Lazzari, M.; Borleri, G.-M.; Bernasconi, S.; Tedesco, F.; Rambaldi, A.; Introna, M. Biologic response of Blymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000, 95, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Veeramani, S.; Weiner, G.J. Complement in Monoclonal Antibody Therapy of Cancer. Immunol Res. 2014, 59, 203–210. [Google Scholar] [CrossRef]
- Wang, S.Y.; Weiner, G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin. Biol. Ther. 2008, 8, 759–768. [Google Scholar] [CrossRef]
- Van Spriel, A.B.; Leusen, J.H.W.; Van Egmond, M.; Dijkman, H.B.P.M.; Assmann, K.J.M.; Mayadas, T.N.; Van de Winkel, J.G.J. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 2001, 97, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Leusen, J.H.W.; Boross, P. Regulation of complement and modulation of its activity in monoclonal antibody therapy of cancer. MAbs 2014, 6, 1133–1144. [Google Scholar] [CrossRef]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B Cells In Vivo by a Chimeric Mouse Human Monoclonal Antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, A.; Junnikkala, S.; Meri, S. Rituximab (anti-CD20) therapy of B-cell lymphomas: Direct complement killing is superior to cellular effector mechanisms. Scand. J. Immunol. 2000, 51, 634–641. [Google Scholar] [CrossRef]
- Gennari, R.; Menard, S.; Fagnoni, F.; Ponchio, L.; Scelsi, M.; Tagliabue, E.; Castiglioni, F.; Villani, L.; Magalotti, C.; Gibelli, N.; et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 2004, 10, 5650–5655. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubata, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E.; Eagar, T.N.; Obu, T.; Wu, J.; Tang, Q.; Azuma, M.; Krummel, M.F.; Bluestone, J.A. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 2009, 10, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, T.S.; Hu-Lieskovan, S.; Ribas, A. Mechanisms of Resistance to PD-1 and PD-L1 blockade. Cancer J. 2018, 24, 47–53. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Berman, D.M.; Aznar, M.A.; Korman, A.J.; Gracia, J.L.P.; Haanen, J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 2015, 15, 457–472. [Google Scholar] [CrossRef]
- Weber, J.S.; Sznol, M.; Sullivan, R.J.; Blackmon, S.; Boland, G.; Kluger, H.M.; Halaban, R.; Bacchiocchi, A.; Ascierto, P.A.; Capone, M.; et al. A Serum Protein Signature Associated with Outcome after Anti–PD-1 Therapy in Metastatic Melanoma. Cancer Immunol. Res. 2018, 6, 79–86. [Google Scholar] [CrossRef] [PubMed]
- An, L.L.; Gorman, J.V.; Stephens, G.; Swerdlow, B.; Warrener, P.; Bonnell, J.; Mustelin, T.; Fung, M.; Kolbeck, R. Complement C5a induces PD-L1 expression and acts in synergy with LPS through Erk1/2 and JNK signaling pathways. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pio, R.; Ajona, D.; Lambris, J.D. Complement inhibition: A promising concept for cancer treatment. Semin. Immunol. 2013, 25, 54–64. [Google Scholar] [CrossRef]
- Kolev, M.; Markiewski, M.M. Targeting complement-mediated immunoregulation for cancer immunotherapy. Semin. Immunol. 2018, 37, 85–97. [Google Scholar] [CrossRef]
- Naing, A.; Papadopoulos, K.P.; Autio, K.A.; Ott, P.A.; Patel, M.R.; Wong, D.J.; Falchook, G.S.; Pant, S.; Whiteside, M.; Rasco, D.R.; et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol. 2016, 34, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Infante, J.R.; Papadopoulos, K.P.; Chan, I.H.; Shen, C.; Ratti, N.P.; Rojo, B.; Autio, K.A.; Wong, D.J.; Patel, M.R.; et al. PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8+ T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell 2018, 34, 775–791. [Google Scholar] [CrossRef]
- Zha, H.; Han, X.; Zhu, Y.; Yang, F.; Li, Y.; Li, Q.; Guo, B.; Zhu, B. Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. Oncoimmunology 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Zha, H.; Wang, X.; Zhu, Y.; Chen, D.; Han, X.; Yang, F.; Gao, J.; Hu, C.; Shu, C.; Feng, Y.; et al. Intracellular activation of complement C3 leads to PD-L1 antibody treatment resistance by modulating tumor-associated macrophages. Cancer Immunol. Res. 2019, 7, 193–207. [Google Scholar] [CrossRef]
- Massard, C.; Cassier, P.; Bendell, J.C.; Marie, D.B.; Blery, M.; Morehouse, C.; Ascierto, M.; Zerbib, R.; Mitry, E.; Tolcher, A.W. Preliminary results of STELLAR-001, a dose escalation phase I study of the anti-C5aR, IPH5401, in combination with durvalumab in advanced solid tumours. Ann. Oncol. 2019, 30, v492. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; He, Y.W. The Complement Receptors C3aR and C5aR Are a New Class of Immune Checkpoint Receptor in Cancer Immunotherapy. Front. Immunol. 2019, 10, 1574. [Google Scholar] [CrossRef]
- Peng, W.; McKenzie, J.A.; Hwu, P. Complementing T-cell function: An inhibitory role of the complement system in T-cell-mediated antitumor immunity. Cancer Discov. 2016, 6, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.H.; van der Touw, W.; Paz-Artal, E.; Li, M.O.; Heeger, P.S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 2013, 210, 257–268. [Google Scholar] [CrossRef]
- Mamidi, S.; Höne, S.; Teufel, C.; Sellner, L.; Zenz, T.; Kirschfink, M. Neutralization of membrane complement regulators improves complement-dependent effector functions of therapeutic anticancer antibodies targeting leukemic cells. Oncoimmunology 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Hu, W.; Ge, X.; You, T.; Xu, T.; Zhang, J.; Wu, G.; Peng, Z.; Chorev, M.; Aktas, B.H.; Halperin, J.A.; et al. Human CD59 inhibitor sensitizes rituximab-resistant lymphoma cells to complement-mediated cytolysis. Cancer Res. 2011, 71, 2298–2307. [Google Scholar] [CrossRef]
- Zhao, W.-P.; Zhu, B.; Duan, Y.-Z.; Chen, Z.-T. Neutralization of complement regulatory proteins CD55 and CD59 augments therapeutic effect of herceptin against lung carcinoma cells. Oncol. Rep. 2009, 21, 1405–1411. [Google Scholar]
- Wang, Y.; Yang, Y.J.; Wang, Z.; Liao, J.; Liu, M.; Zhong, X.-R.; Zheng, H.; Wang, Y.-P. CD55 and CD59 expression protects HER2-overexpressing breast cancer cells from trastuzumab-induced complement-dependent cytotoxicity. Oncol. Lett. 2017, 14, 2961–2969. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.; Towner, L.; Donev, R. Complement in cancer and cancer immunotherapy. Arch. Immunol. Ther. Exp. (Warsz). 2011, 59, 407–419. [Google Scholar] [CrossRef]
- Bowen, W.S.; Svrivastava, A.K.; Batra, L.; Barsoumian, H.; Shirwan, H. Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines 2018, 17, 207–215. [Google Scholar] [CrossRef]
- Morgan, E.L.; Morgan, B.N.; Stein, E.A.; Vitrs, E.L.; Thoman, M.L.; Sanderson, S.D.; Phillips, J.A. Enhancement of in vivo and in vitro immune functions by a conformationally biased, response-selective agonist of human C5a: Implications for a novel adjuvant in vaccine design. Vaccine 2010, 28, 463–469. [Google Scholar] [CrossRef]
- Hegde, G.V.; Meyers-Clark, E.; Joshi, S.S.; Sanderson, S.D. A conformationally-biased, response-selective agonist of C5a acts as a molecular adjuvant by modulating antigen processing and presentation activities of human dendritic cells. Int. Immunopharmacol. 2008, 8, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Hurtgen, B.J.; Bellecourt, M.; Sanderson, S.D.; Morgan, E.L.; Cole, G.T. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine 2012, 30, 4681–4690. [Google Scholar] [CrossRef]
- Floreani, A.A.; Gunselman, S.J.; Heires, A.J.; Hauke, R.J.; Tarantolo, S.; Jackson, J.D. Novel C5a agonist-based dendritic cell vaccine in a murine model of melanoma. Cell Cycle 2007, 6, 2835–2839. [Google Scholar] [CrossRef]
- Kollessery, G.; Nordgren, T.M.; Mittal, A.K.; Joshi, S.S.; Sanderson, S.D. Tumor-specific peptide-based vaccines containing the conformationally biased, response-selective C5a agonists EP54 and EP67 protect against aggressive large B cell lymphoma in a syngeneic murine model. Vaccine 2011, 29, 5904–5910. [Google Scholar] [CrossRef]
- Platt, J.L.; Silva, I.; Balin, S.J.; Lefferts, A.R.; Farkash, E.; Ross, T.M.; Carroll, M.C.; Cascalho, M. C3d regulates immune checkpoint blockade and enhances antitumor immunity. JCI insight 2017, 2, e90201. [Google Scholar] [CrossRef]
- Xu, G.L.; Zhang, K.Q.; Guo, B.; Zhao, T.-T.; Yang, F.; Jiang, M.; Wang, Q.H.; Shang, Y.H.; Wu, Y.Z. Induction of protective and therapeutic antitumor immunity by a DNA vaccine with C3d as a molecular adjuvant. Vaccine 2010, 28, 7221–7227. [Google Scholar] [CrossRef]
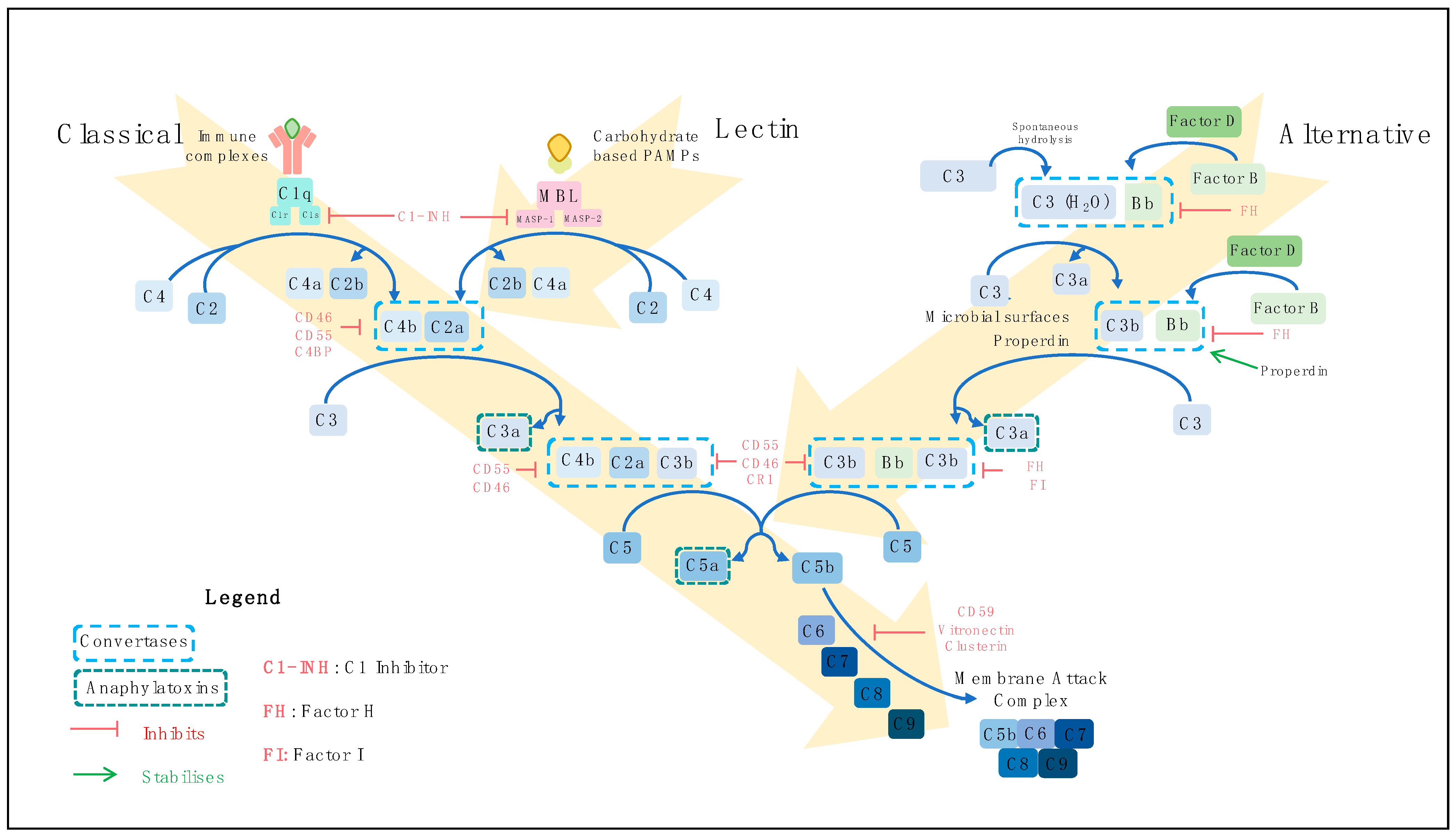
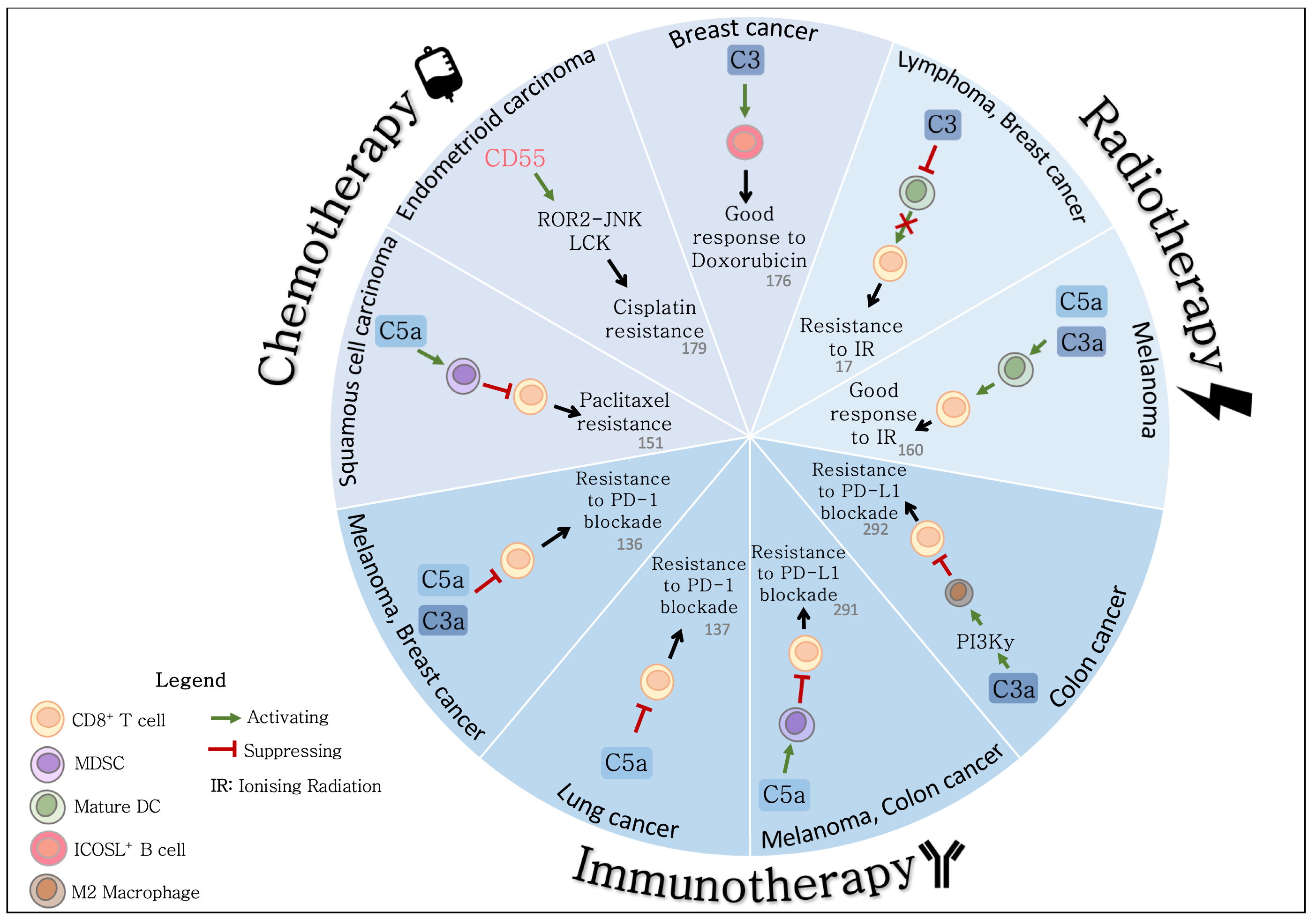
| Regulator | Alternative Name (s) | Distribution | Function | Reference |
|---|---|---|---|---|
| CD35 | Complement receptor 1 (CR1) | Primarily lymphocytes, erythrocytes, phagocytes, dendritic cells | Cofactor for C3b and C4b degradation by Factor H Accelerates C3 and C5 convertases | [51,52,53,54,55] |
| CD46 | Membrane cofactor protein (MCP) | All nucleated cells | Cofactor for C3b and C4b degradation by Factor H | [56,57,58] |
| CD55 | Decay accelerating factor (DAF) | Ubiquitously expressed | Accelerates decay of C3 and C5 convertases | [59,60,61] |
| CD59 | Membrane-inhibitor of reactive lysis (MIRL), MAC inhibitory protein (MAC-IP), Protectin | Ubiquitously expressed | Binds C5b-C9 to prevent polymerization of C9 | [62,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, R.M.; Cannon, A.; Reynolds, J.V.; Lysaght, J.; Lynam-Lennon, N. Complement in Tumourigenesis and the Response to Cancer Therapy. Cancers 2021, 13, 1209. https://doi.org/10.3390/cancers13061209
O’Brien RM, Cannon A, Reynolds JV, Lysaght J, Lynam-Lennon N. Complement in Tumourigenesis and the Response to Cancer Therapy. Cancers. 2021; 13(6):1209. https://doi.org/10.3390/cancers13061209
Chicago/Turabian StyleO’Brien, Rebecca M., Aoife Cannon, John V. Reynolds, Joanne Lysaght, and Niamh Lynam-Lennon. 2021. "Complement in Tumourigenesis and the Response to Cancer Therapy" Cancers 13, no. 6: 1209. https://doi.org/10.3390/cancers13061209
APA StyleO’Brien, R. M., Cannon, A., Reynolds, J. V., Lysaght, J., & Lynam-Lennon, N. (2021). Complement in Tumourigenesis and the Response to Cancer Therapy. Cancers, 13(6), 1209. https://doi.org/10.3390/cancers13061209





