History of Extensive Disease Small Cell Lung Cancer Treatment: Time to Raise the Bar? A Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. First Line Chemotherapy in ED-SCLC
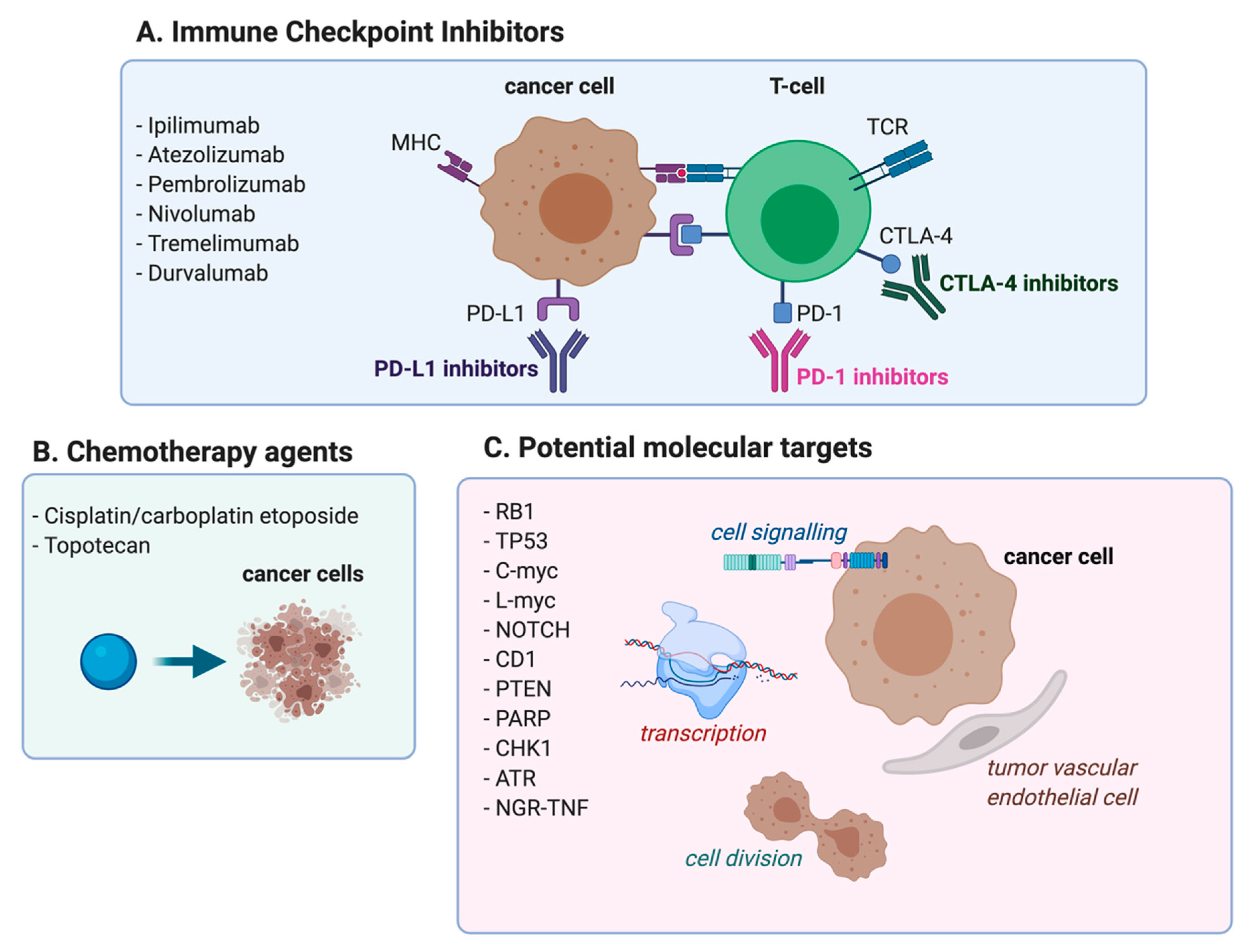
3. Immune Checkpoint Inhibitors in the Treatment of Patients with ED-SCLC
3.1. Immune Checkpoint Inhibitors in First Line Setting-CTLA4 Blockade
3.2. Immune Checkpoint Inhibitors in First-Line Setting-PD-1/PD-L1 Blockade
3.3. Immune Checkpoint Inhibitors as a Maintenance Strategy
3.4. Immune Checkpoint Inhibitors in Recurrent Patients
4. Second Line and Beyond
5. Discussion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepherd, F.A.; Crowley, J.; Van Houtte, P.; Postmus, P.E.; Carney, D.; Chansky, K.; Shaikh, Z.; Goldstraw, P. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals Regarding the Clinical Staging of Small Cell Lung Cancer in the Forthcoming (Seventh) Edition of the Tumor, Node, Metastasis Classification for Lung Cancer. J. Thorac Oncol. 2007, 2, 1067–1077. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.E.; van Meerbeeck, J.; Rami-Porta, R.; Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Gandhi, L.; Ganti, A.K.P.; Govindan, R.; Grecula, J.C.; et al. Small Cell Lung Cancer. J. Natl. Compr. Canc. Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, J.; Sun, T.; Zheng, X.; Li, J.; Sun, H.; Zhou, X.; Zhou, C.; Zhang, H.; Cheng, Z.; et al. Survival Changes in Patients with Small Cell Lung Cancer and Disparities between Different Sexes, Socioeconomic Statuses and Ages. Sci. Rep. 2017, 7, 1339. [Google Scholar] [CrossRef]
- Byers, L.A.; Rudin, C.M. Small Cell Lung Cancer: Where Do We Go from Here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef]
- Roth, B.J.; Johnson, D.H.; Einhorn, L.H.; Schacter, L.P.; Cherng, N.C.; Cohen, H.J.; Crawford, J.; Randolph, J.A.; Goodlow, J.L.; Broun, G.O. Randomized Study of Cyclophosphamide, Doxorubicin, and Vincristine versus Etoposide and Cisplatin versus Alternation of These Two Regimens in Extensive Small-Cell Lung Cancer: A Phase III Trial of the Southeastern Cancer Study Group. J. Clin. Oncol. 1992, 10, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Mascaux, C.; Paesmans, M.; Berghmans, T.; Branle, F.; Lafitte, J.J.; Lemaitre, F.; Meert, A.P.; Vermylen, P.; Sculier, J.P. European Lung Cancer Working Party (ELCWP) A Systematic Review of the Role of Etoposide and Cisplatin in the Chemotherapy of Small Cell Lung Cancer with Methodology Assessment and Meta-Analysis. Lung Cancer 2000, 30, 23–36. [Google Scholar] [CrossRef]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or Cisplatin-Based Chemotherapy in First-Line Treatment of Small-Cell Lung Cancer: The COCIS Meta-Analysis of Individual Patient Data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Morabito, A.; Carillio, G.; Daniele, G.; Piccirillo, M.C.; Montanino, A.; Costanzo, R.; Sandomenico, C.; Giordano, P.; Normanno, N.; Perrone, F.; et al. Treatment of Small Cell Lung Cancer. Crit. Rev. Oncol. Hematol. 2014, 91, 257–270. [Google Scholar] [CrossRef]
- Loehrer, P.J.; Ansari, R.; Gonin, R.; Monaco, F.; Fisher, W.; Sandler, A.; Einhorn, L.H. Cisplatin plus Etoposide with and without Ifosfamide in Extensive Small-Cell Lung Cancer: A Hoosier Oncology Group Study. J. Clin. Oncol. 1995, 13, 2594–2599. [Google Scholar] [CrossRef]
- Pujol, J.L.; Daurès, J.P.; Rivière, A.; Quoix, E.; Westeel, V.; Quantin, X.; Breton, J.L.; Lemarié, E.; Poudenx, M.; Milleron, B.; et al. Etoposide plus Cisplatin with or without the Combination of 4’-Epidoxorubicin plus Cyclophosphamide in Treatment of Extensive Small-Cell Lung Cancer: A French Federation of Cancer Institutes Multicenter Phase III Randomized Study. J. Natl. Cancer Inst. 2001, 93, 300–308. [Google Scholar] [CrossRef]
- Ihde, D.C.; Mulshine, J.L.; Kramer, B.S.; Steinberg, S.M.; Linnoila, R.I.; Gazdar, A.F.; Edison, M.; Phelps, R.M.; Lesar, M.; Phares, J.C. Prospective Randomized Comparison of High-Dose and Standard-Dose Etoposide and Cisplatin Chemotherapy in Patients with Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 1994, 12, 2022–2034. [Google Scholar] [CrossRef]
- Reck, M.; von Pawel, J.; Macha, H.-N.; Kaukel, E.; Deppermann, K.-M.; Bonnet, R.; Ulm, K.; Hessler, S.; Gatzemeier, U. Randomized Phase III Trial of Paclitaxel, Etoposide, and Carboplatin versus Carboplatin, Etoposide, and Vincristine in Patients with Small-Cell Lung Cancer. J. Natl. Cancer Inst. 2003, 95, 1118–1127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masuda, N.; Fukuoka, M.; Kusunoki, Y.; Matsui, K.; Takifuji, N.; Kudoh, S.; Negoro, S.; Nishioka, M.; Nakagawa, K.; Takada, M. CPT-11: A New Derivative of Camptothecin for the Treatment of Refractory or Relapsed Small-Cell Lung Cancer. J. Clin. Oncol. 1992, 10, 1225–1229. [Google Scholar] [CrossRef]
- Kudoh, S.; Fujiwara, Y.; Takada, Y.; Yamamoto, H.; Kinoshita, A.; Ariyoshi, Y.; Furuse, K.; Fukuoka, M. Phase II Study of Irinotecan Combined with Cisplatin in Patients with Previously Untreated Small-Cell Lung Cancer. West Japan Lung Cancer Group. J. Clin. Oncol. 1998, 16, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Nishiwaki, Y.; Kawahara, M.; Negoro, S.; Sugiura, T.; Yokoyama, A.; Fukuoka, M.; Mori, K.; Watanabe, K.; Tamura, T.; et al. Irinotecan plus Cisplatin Compared with Etoposide plus Cisplatin for Extensive Small-Cell Lung Cancer. New Engl. J. Med. 2002, 346, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Bunn, P.A.; Langer, C.; Einhorn, L.; Guthrie, T.; Beck, T.; Ansari, R.; Ellis, P.; Byrne, M.; Morrison, M.; et al. Randomized Phase III Trial Comparing Irinotecan/Cisplatin with Etoposide/Cisplatin in Patients with Previously Untreated Extensive-Stage Disease Small-Cell Lung Cancer. J. Clin. Oncol. 2006, 24, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N.; Natale, R.; Crowley, J.; Lenz, H.J.; Redman, M.W.; Carleton, J.E.; Jett, J.; Langer, C.J.; Kuebler, J.P.; Dakhil, S.R.; et al. Phase III Trial of Irinotecan/Cisplatin Compared with Etoposide/Cisplatin in Extensive-Stage Small-Cell Lung Cancer: Clinical and Pharmacogenomic Results from SWOG S0124. J. Clin. Oncol. 2009, 27, 2530–2535. [Google Scholar] [CrossRef]
- Hermes, A.; Bergman, B.; Bremnes, R.; Ek, L.; Fluge, S.; Sederholm, C.; Sundstrøm, S.; Thaning, L.; Vilsvik, J.; Aasebø, U.; et al. Irinotecan plus Carboplatin versus Oral Etoposide plus Carboplatin in Extensive Small-Cell Lung Cancer: A Randomized Phase III Trial. J. Clin. Oncol. 2008, 26, 4261–4267. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, X.; Zhou, X.; Huang, L.; Huang, R.; Chu, Z.; Zhan, Q. A Meta-Analysis of Randomized Controlled Trials Comparing Irinotecan/Platinum with Etoposide/Platinum in Patients with Previously Untreated Extensive-Stage Small Cell Lung Cancer; Centre for Reviews and Dissemination: UK, 2010. [Google Scholar]
- Fink, T.H.; Huber, R.M.; Heigener, D.F.; Eschbach, C.; Waller, C.; Steinhauer, E.U.; Virchow, J.C.; Eberhardt, F.; Schweisfurth, H.; Schroeder, M.; et al. Topotecan/Cisplatin Compared with Cisplatin/Etoposide as First-Line Treatment for Patients with Extensive Disease Small-Cell Lung Cancer: Final Results of a Randomized Phase III Trial. J. Thorac Oncol. 2012, 7, 1432–1439. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Y.; Hao, X.; Wang, J.; Hu, C.; Han, B.; Liu, X.; Zhang, L.; Wan, H.; Xia, Z.; et al. Randomized Phase III Trial of Amrubicin/Cisplatin versus Etoposide/Cisplatin as First-Line Treatment for Extensive Small-Cell Lung Cancer. BMC Cancer 2016, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Tanaka, K.; Idezuka, J.; Nishizawa, M. Regulatory T Cells in Paraneoplastic Neurological Syndromes. J. Neuroimmunol. 2008, 196, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Passiglia, F.; Ahn, M.-J.; Barlesi, F.; Forde, P.M.; Garon, E.B.; Gettinger, S.; Goldberg, S.B.; Herbst, R.S.; Horn, L.; et al. Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J. Thorac Oncol. 2020, 15, 914–947. [Google Scholar] [CrossRef]
- Hato, S.V.; Khong, A.; de Vries, I.J.M.; Lesterhuis, W.J. Molecular Pathways: The Immunogenic Effects of Platinum-Based Chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-ΚB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef]
- Qiao, M.; Jiang, T.; Ren, S.; Zhou, C. Combination Strategies on the Basis of Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer: Where Do We Stand? Clin. Lung Cancer 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Liu, W.M.; Fowler, D.W.; Smith, P.; Dalgleish, A.G. Pre-Treatment with Chemotherapy Can Enhance the Antigenicity and Immunogenicity of Tumours by Promoting Adaptive Immune Responses. Br. J. Cancer 2010, 102, 115–123. [Google Scholar] [CrossRef]
- Suzuki, E.; Kapoor, V.; Jassar, A.S.; Kaiser, L.R.; Albelda, S.M. Gemcitabine Selectively Eliminates Splenic Gr-1+/CD11b+ Myeloid Suppressor Cells in Tumor-Bearing Animals and Enhances Antitumor Immune Activity. Clin. Cancer Res. 2005, 11, 6713–6721. [Google Scholar] [CrossRef]
- Lazzari, C.; Karachaliou, N.; Bulotta, A.; Viganó, M.; Mirabile, A.; Brioschi, E.; Santarpia, M.; Gianni, L.; Rosell, R.; Gregorc, V. Combination of Immunotherapy with Chemotherapy and Radiotherapy in Lung Cancer: Is This the Beginning of the End for Cancer? Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Reck, M.; Bondarenko, I.; Luft, A.; Serwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Lu, H.; Cuillerot, J.-M.; Lynch, T.J. Ipilimumab in Combination with Paclitaxel and Carboplatin as First-Line Therapy in Extensive-Disease-Small-Cell Lung Cancer: Results from a Randomized, Double-Blind, Multicenter Phase 2 Trial. Ann. Oncol. 2013, 24, 75–83. [Google Scholar] [CrossRef]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.-W.; Akerley, W.; Pietanza, M.C.; Wu, Y.-L.; Zielinski, C.; Thomas, M.; et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without Tremelimumab, plus Platinum-Etoposide versus Platinum-Etoposide Alone in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): Updated Results from a Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.-H.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.; Wang, Y.; Dowlati, A.; Lewis, D.A.; Chen, Y.; Mohindra, A.R.; Razaq, M.; Ahuja, H.G.; Liu, J.; King, D.M.; et al. Randomized Phase II Clinical Trial of Cisplatin/Carboplatin and Etoposide (CE) Alone or in Combination with Nivolumab as Frontline Therapy for Extensive-Stage Small Cell Lung Cancer (ES-SCLC): ECOG-ACRIN EA5161. JCO 2020, 38, 9000. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Pennell, N.A.; Fidler, M.J.; Halmos, B.; Bonomi, P.; Stevenson, J.; Schneider, B.; Sukari, A.; Ventimiglia, J.; Chen, W.; et al. Phase II Study of Maintenance Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer (SCLC). J. Thorac Oncol. 2018, 13, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Kim, H.R.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodriguez-Cid, J.; Schenker, M.; et al. Nivolumab (Nivo) plus Ipilimumab (Ipi), Nivo, or Placebo (Pbo) as Maintenance Therapy in Patients (Pts) with Extensive Disease Small Cell Lung Cancer (ED-SCLC) after First-Line (1L) Platinum-Based Chemotherapy (Chemo): Results from the Double-Blind, Randomized Phase III CheckMate 451 Study. Ann. Oncol. 2019, 30, ii77. [Google Scholar] [CrossRef]
- Ott, P.A.; Elez, E.; Hiret, S.; Kim, D.-W.; Morosky, A.; Saraf, S.; Piperdi, B.; Mehnert, J.M. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 3823–3829. [Google Scholar] [CrossRef]
- Chung, H.C.; Lopez-Martin, J.A.; Kao, S.C.-H.; Miller, W.H.; Ros, W.; Gao, B.; Marabelle, A.; Gottfried, M.; Zer, A.; Delord, J.-P.; et al. Phase 2 Study of Pembrolizumab in Advanced Small-Cell Lung Cancer (SCLC): KEYNOTE-158. JCO 2018, 36, 8506. [Google Scholar] [CrossRef]
- Chung, H.C.; Piha-Paul, S.A.; Lopez-Martin, J.; Schellens, J.H.M.; Kao, S.; Miller, W.H.; Delord, J.-P.; Gao, B.; Planchard, D.; Gottfried, M.; et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J. Thorac Oncol. 2020, 15, 618–627. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab Alone and Nivolumab plus Ipilimumab in Recurrent Small-Cell Lung Cancer (CheckMate 032): A Multicentre, Open-Label, Phase 1/2 Trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Ready, N.; Farago, A.F.; de Braud, F.; Atmaca, A.; Hellmann, M.D.; Schneider, J.G.; Spigel, D.R.; Moreno, V.; Chau, I.; Hann, C.L.; et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J. Thorac Oncol. 2019, 14, 237–244. [Google Scholar] [CrossRef]
- Reck, M.; Vicente, D.; Ciuleanu, T.; Gettinger, S.; Peters, S.; Horn, L.; Audigier-Valette, C.; Pardo, N.; Juan-Vidal, O.; Cheng, Y.; et al. Efficacy and Safety of Nivolumab (Nivo) Monotherapy versus Chemotherapy (Chemo) in Recurrent Small Cell Lung Cancer (SCLC): Results from CheckMate 331. Ann. Oncol. 2018, 29, x43. [Google Scholar] [CrossRef]
- Goldman, J.W.; Dowlati, A.; Antonia, S.J.; Nemunaitis, J.J.; Butler, M.O.; Segal, N.H.; Smith, P.A.; Weiss, J.; Zandberg, D.P.; Xiao, F.; et al. Safety and Antitumor Activity of Durvalumab Monotherapy in Patients with Pretreated Extensive Disease Small-Cell Lung Cancer (ED-SCLC). JCO 2018, 36, 8518. [Google Scholar] [CrossRef]
- Pujol, J.-L.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Uwer, L.; Hureaux, J.; Guisier, F.; Carmier, D.; Madelaine, J.; Otto, J.; et al. A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients With Small Cell Lung Cancer: Results From the IFCT-1603 Trial. J. Thorac Oncol. 2019, 14, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.T.; George, J.; Owonikoko, T.K.; Berns, A.; Brambilla, E.; Byers, L.A.; Carbone, D.; Chen, H.J.; Christensen, C.L.; Dive, C.; et al. New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J. Thorac Oncol. 2020, 15, 520–540. [Google Scholar] [CrossRef]
- Wakuda, K.; Kenmotsu, H.; Naito, T.; Akamatsu, H.; Ono, A.; Shukuya, T.; Nakamura, Y.; Tsuya, A.; Murakami, H.; Takahashi, T.; et al. Efficacy of Rechallenge Chemotherapy in Patients with Sensitive Relapsed Small Cell Lung Cancer. Am. J. Clin. Oncol. 2015, 38, 28–32. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Behera, M.; Chen, Z.; Bhimani, C.; Curran, W.J.; Khuri, F.R.; Ramalingam, S.S. A Systematic Analysis of Efficacy of Second-Line Chemotherapy in Sensitive and Refractory Small-Cell Lung Cancer. J. Thorac Oncol. 2012, 7, 866–872. [Google Scholar] [CrossRef]
- von Pawel, J.; Schiller, J.H.; Shepherd, F.A.; Fields, S.Z.; Kleisbauer, J.P.; Chrysson, N.G.; Stewart, D.J.; Clark, P.I.; Palmer, M.C.; Depierre, A.; et al. Topotecan versus Cyclophosphamide, Doxorubicin, and Vincristine for the Treatment of Recurrent Small-Cell Lung Cancer. J. Clin. Oncol. 1999, 17, 658–667. [Google Scholar] [CrossRef]
- Jotte, R.; Conkling, P.; Reynolds, C.; Galsky, M.D.; Klein, L.; Fitzgibbons, J.F.; McNally, R.; Renschler, M.F.; Oliver, J.W. Randomized Phase II Trial of Single-Agent Amrubicin or Topotecan as Second-Line Treatment in Patients with Small-Cell Lung Cancer Sensitive to First-Line Platinum-Based Chemotherapy. J. Clin. Oncol. 2011, 29, 287–293. [Google Scholar] [CrossRef]
- Gregorc, V.; Cavina, R.; Novello, S.; Grossi, F.; Lazzari, C.; Capelletto, E.; Genova, C.; Salini, G.; Lambiase, A.; Santoro, A. NGR-HTNF and Doxorubicin as Second-Line Treatment of Patients with Small Cell Lung Cancer. Oncologist 2018, 23, 1133-e112. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as Second-Line Treatment for Patients with Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef]
- Calcinotto, A.; Grioni, M.; Jachetti, E.; Curnis, F.; Mondino, A.; Parmiani, G.; Corti, A.; Bellone, M. Targeting TNF-α to Neoangiogenic Vessels Enhances Lymphocyte Infiltration in Tumors and Increases the Therapeutic Potential of Immunotherapy. J. Immunol. 2012, 188, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, Y.; Chlewicki, L.K.; Zhang, Y.; Guo, J.; Liang, W.; Wang, J.; Wang, X.; Fu, Y.-X. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell 2016, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Nau, M.M.; Brooks, B.J.; Battey, J.; Sausville, E.; Gazdar, A.F.; Kirsch, I.R.; McBride, O.W.; Bertness, V.; Hollis, G.F.; Minna, J.D. L-Myc, a New Myc-Related Gene Amplified and Expressed in Human Small Cell Lung Cancer. Nature 1985, 318, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.E.; Makuch, R.W.; Simmons, A.D.; Gazdar, A.F.; Burch, D.; Cashell, A.W. Myc Family DNA Amplification in Small Cell Lung Cancer Patients’ Tumors and Corresponding Cell Lines. Cancer Res. 1988, 48, 5163–5166. [Google Scholar]
- Yokomizo, A.; Tindall, D.J.; Drabkin, H.; Gemmill, R.; Franklin, W.; Yang, P.; Sugio, K.; Smith, D.I.; Liu, W. PTEN/MMAC1 Mutations Identified in Small Cell, but Not in Non-Small Cell Lung Cancers. Oncogene 1998, 17, 475–479. [Google Scholar] [CrossRef]
- Karachaliou, N.; Pilotto, S.; Lazzari, C.; Bria, E.; de Marinis, F.; Rosell, R. Cellular and Molecular Biology of Small Cell Lung Cancer: An Overview. Transl. Lung Cancer Res. 2016, 5, 2–15. [Google Scholar] [CrossRef]
| Author (Ref) | Treatment | Primary End-Point | OS (m) | p | TTP/PFS (m) | p | ORR (%) |
|---|---|---|---|---|---|---|---|
| Ihde [12] | high EP standard EP | RR | 10.7 11.4 | 0.68 | 7.0 6.9 | 0.96 | 86 83 |
| Roth [6] | EP CAV EP/CAV | OS | 4.3 4.0 5.2 | 0.425 | 4.3 4.0 5.2 | 0.052 | 61 51 60 |
| Loehrer [10] | EP VIP | OS | 7.3 9.1 | 0.045 | 6.0 6.8 | 0.039 | 67 73 |
| Pujol [11] | EP PCDE | OS | 9.3 10.5 | 0.0067 | 7.2 6.3 | <0.00001 | 61 76 |
| Reck [13] | TEC CEV | OS | 12.7 11.7 | 0.024 | 8.1 7.5 | 0.033 | 72.1 69.4 |
| Noda [16] | IC EP | OS | 12.8 9.4 | 0.002 | 6.9 4.8 | 0.03 | 84.4 67.5 |
| Hanna [17] | IC EP | OS | 10.2 9.3 | 0.68 | 4.1 4.6 | 0.37 | 48.7 43.6 |
| Lara [18] | IC EP | OS | 9.1 9.9 | 0.071 | 5.8 5.2 | 0.07 | 60 57 |
| Hermes [19] | CBDCA + E (*) CBDCA + I | OS | 8.5 7.1 | 0.02 | - | - | - |
| Fink [21] | TP EP | OS | 44.9 weeks 40.9 weeks | 0.029 | 27.4 weeks 24.3 weeks | 0.01 | 55.5 45.5 |
| Sun [22] | AP EP | OS | 11.8 10.3 | 0.008 | 6.8 5.7 | 0.035 | 69.8 57.3 |
| Author | Treatment | Setting | Primary End-Point | OS (m) | p | PFS (m) | p |
|---|---|---|---|---|---|---|---|
| Reck [32] | Phased I + PC Concurrent I + PC PC | I line | irPFS | 12.5 9.1 10.5 | 0.13 0.41 | 6.4 5.6 5.2 | 0.03 0.11 |
| Reck [33] | Ipilimumab + PE PE | I line | OS | 11 10.9 | 0.37 | 4.6 4.4 | - |
| Horn [34] | Atezolizumab + CE CE | I line | OS PFS | 12.3 10.3 | 0.007 | 5.2 4.3 | 0.02 |
| Goldman [35] | DPE PE DTPE | I line | OS | 12.9 10.5 10.4 | 0.0032 0.0045 | 5.1 5.4 4.9 | - |
| Rudin [36] | Pembrolizumab + PE PE | I line | OS PFS | 10.8 9.7 | 0.0164 | 4.5 4.3 | 0.0023 |
| Leal [37] | Nivolumab + PE PE | I line | PFS | 11.3 9.3 | 0.14 | 5.5 4.6 | 0.012 |
| Gadgeel [38] | Pembrolizumab | maintenance | PFS | 9.6 | - | 1.4 | - |
| Owonikoko [39] | Nivolumab Nivolumab + Ipilimumab | maintenance | OS | 10.4 9.2 | - | 1.9 1.7 | - |
| Ott [40] | Pembrolizumab | II/III line | ORR | 9.7 | - | 1.9 | - |
| Chung [41] | Pembrolizumab | II/III line | ORR | 9.1 | - | 2.0 | - |
| Antonia [43] | Nivolumab Nivolumab 1 mg/kg + Ipilimumab 1 mg/kg Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg | II/III line | ORR | 5.7 4.7 (#) | - | 1.4 1.5 (#) | - |
| Reck [45] | Nivolumab Topotecan/amrubicine | II line | OS | 7.5 8.4 | 0.11 | 1.4 3.8 | - |
| Goldman [46] | Durvalumab | I/II line | safety | 4.8 | - | 1.5 | - |
| Pujol [47] | Atezolizumab Topotecan/PE | II line | ORR at 6 months | 9.5 8.7 | 0.60 | 1.4 4.3 | - |
| Author | Regimen | Primary End-Point | OS (m) | ORR % | PFS (m) |
|---|---|---|---|---|---|
| von Pawel [51] | Topotecan CAV | PFS | 25.0 24.7 | 24.3 18.3 | 3.3 3.0. |
| Jotte [52] | Topotecan Amrubicin | ORR | 7.8 7.5 | 15 44 | 3.3 4.5 |
| Gregorc [53] |
NGR-hTNF +
Doxorubicin | PFS | 13.1 | 25 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, C.; Mirabile, A.; Bulotta, A.; Viganó, M.G.; Ogliari, F.R.; Ippati, S.; DellOca, I.; Santarpia, M.; Lorusso, V.; Reck, M.; et al. History of Extensive Disease Small Cell Lung Cancer Treatment: Time to Raise the Bar? A Review of the Literature. Cancers 2021, 13, 998. https://doi.org/10.3390/cancers13050998
Lazzari C, Mirabile A, Bulotta A, Viganó MG, Ogliari FR, Ippati S, DellOca I, Santarpia M, Lorusso V, Reck M, et al. History of Extensive Disease Small Cell Lung Cancer Treatment: Time to Raise the Bar? A Review of the Literature. Cancers. 2021; 13(5):998. https://doi.org/10.3390/cancers13050998
Chicago/Turabian StyleLazzari, Chiara, Aurora Mirabile, Alessandra Bulotta, Maria Grazia Viganó, Francesca Rita Ogliari, Stefania Ippati, Italo DellOca, Mariacarmela Santarpia, Vincenza Lorusso, Martin Reck, and et al. 2021. "History of Extensive Disease Small Cell Lung Cancer Treatment: Time to Raise the Bar? A Review of the Literature" Cancers 13, no. 5: 998. https://doi.org/10.3390/cancers13050998
APA StyleLazzari, C., Mirabile, A., Bulotta, A., Viganó, M. G., Ogliari, F. R., Ippati, S., DellOca, I., Santarpia, M., Lorusso, V., Reck, M., & Gregorc, V. (2021). History of Extensive Disease Small Cell Lung Cancer Treatment: Time to Raise the Bar? A Review of the Literature. Cancers, 13(5), 998. https://doi.org/10.3390/cancers13050998






