Value of the Lung Immune Prognostic Index in Patients with Non-Small Cell Lung Cancer Initiating First-Line Atezolizumab Combination Therapy: Subgroup Analysis of the IMPOWER150 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Predictor and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Population
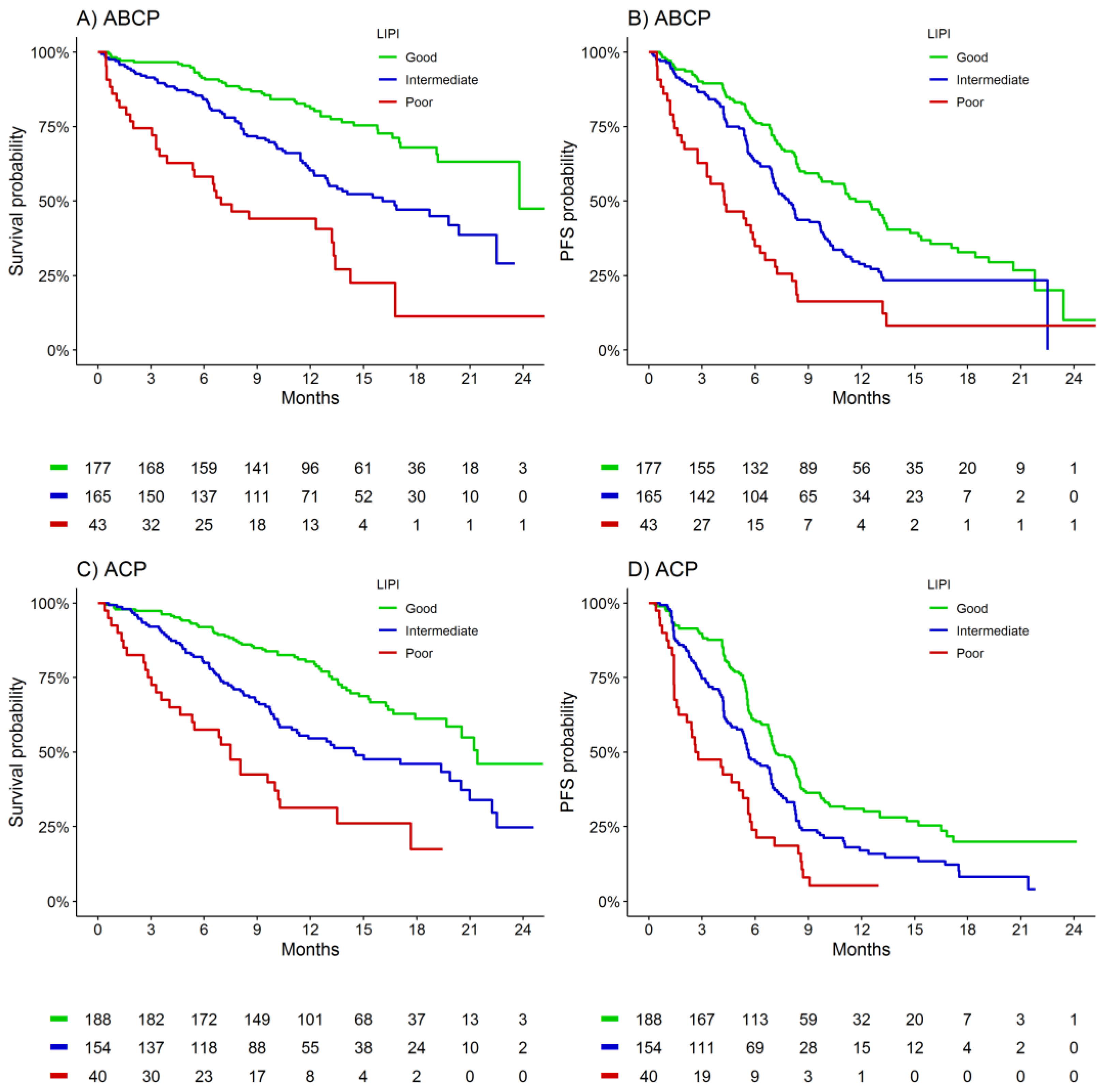
3.2. Prognostic Association
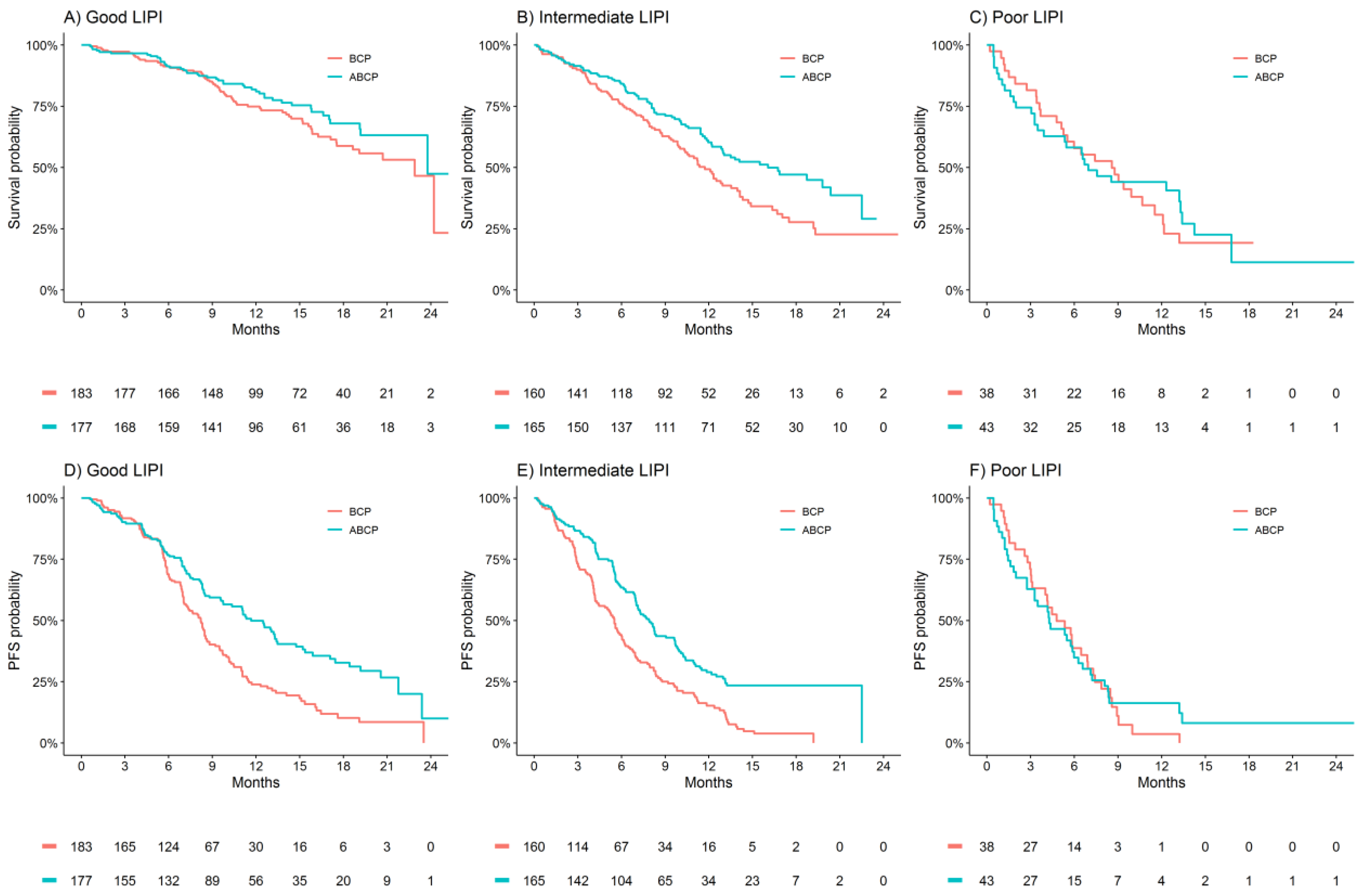
3.3. Treatment Benefit Differences by LIPI Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non–Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Chalabi, M.; Cardona, A.; Nagarkar, D.; Scala, A.D.; Gandara, D.; Rittmeyer, A.; Albert, M.; Powles, T.; Kok, M.; Herrera, F. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Kichenadasse, G.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Concomitant Antibiotic Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Eur. Urol. 2020, 78, 540–543. [Google Scholar] [CrossRef]
- Elkrief, A.; DeRosa, L.; Kroemer, G.; Zitvogel, L.; Routy, B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: A new independent prognostic factor? Ann. Oncol. 2019, 30, 1572–1579. [Google Scholar] [CrossRef]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Kichenadasse, G.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Baseline tumor size and survival outcomes in lung cancer patients treated with immune checkpoint inhibitors. Semin. Oncol. 2019, 46, 380–384. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Wagner, J.; Kichenadasse, G.; Modi, N.; Rowland, A.; Sorich, M.J. Patient-reported outcomes as a prognostic marker of survival in patients with advanced nonsmall cell lung cancer treated with immunotherapy. Int. J. Cancer 2020, 147, 3085–3089. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Wiese, M.D.; Gurney, H.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br. J. Cancer 2017, 117, 913–920. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Yu, Z.; Zheng, W.; Cai, Z.; Shi, L.; Yu, X.; Lou, G.; Hong, W.; Zhang, Y.; et al. Prognostic Value of the Lung Immune Prognostic Index May Differ in Patients Treated with Immune Checkpoint Inhibitor Monotherapy or Combined With Chemotherapy for Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 572853. [Google Scholar] [CrossRef]
- Meyers, D.E.; Stukalin, I.; Vallerand, I.A.; Lewinson, R.T.; Suo, A.; Dean, M.; North, S.; Pabani, A.; Cheng, T.; Heng, D.Y.; et al. The Lung Immune Prognostic Index Discriminates Survival Outcomes in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors. Cancers 2019, 11, 1713. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Kazandjian, D.; Gong, Y.; Keegan, P.; Pazdur, R.; Blumenthal, G.M. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1481–1485. [Google Scholar] [CrossRef]
- Kazandjian, D.; Gong, Y.; Blumenthal, G.M. Application of the Lung Immune Prognostic Index from Research to Clinical Practice—Reply. JAMA Oncol. 2020, 6, 300. [Google Scholar] [CrossRef]
- Ruiz-Bañobre, J.; Areses-Manrique, M.C.; Mosquera-Martínez, J.; Cortegoso, A.; Afonso-Afonso, F.J.; De Dios-Álvarez, N.; Fernández-Núñez, N.; Azpitarte-Raposeiras, C.; Amenedo, M.; Santomé, L.; et al. Evaluation of the lung immune prognostic index in advanced non-small cell lung cancer patients under nivolumab monotherapy. Transl. Lung Cancer Res. 2019, 8, 1078–1085. [Google Scholar] [CrossRef]
- Sonehara, K.; Tateishi, K.; Komatsu, M.; Yamamoto, H.; Hanaoka, M. Lung immune prognostic index as a prognostic factor in patients with small cell lung cancer. Thorac. Cancer 2020, 11, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, Z.; Jia, W.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Wang, L.; Cui, P.; et al. Association of the Pretreatment Lung Immune Prognostic Index with Survival Outcomes in Advanced Hepatocellular Carcinoma Patients Treated with PD-1 Inhibitors. J. Hepatocell. Carcinoma 2020, 7, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Pauline, P.; Auclin, E.; Mezquita, L.; Chanza, N.M.; Dumont, C.; Rodriguez-Vida, A.; Lozano, R.; Vera, B.; Ratta, R.; Baciarello, G.; et al. Association of the Lung Immune Prognostic Index with outcome in patients with metastatic urothelial cancer treated with immune checkpoint inhibitor. J. Clin. Oncol. 2020, 38, 545. [Google Scholar] [CrossRef]
- Vozy, A.; Simonaggio, A.; Auclin, E.; Mezquita, L.; Baldini, C.; Martin-Romano, P.; Pistilli, B.; Gazzah, A.; Bahleda, R.; Ribrag, V.; et al. Applicability of the lung immune prognostic index (LIPI) to metastatic triple negative breast cancer (mTNBC) patients treated with immune checkpoint targeted monoclonal antibodies (ICT mAbs). Ann. Oncol. 2018, 29, viii94. [Google Scholar] [CrossRef]
- Minami, S.; Ihara, S.; Komuta, K. Pretreatment Lung Immune Prognostic Index Is a Prognostic Marker of Chemotherapy and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. World J. Oncol. 2019, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sorich, M.J.; Rowland, A.; Karapetis, C.S.; Hopkins, A.M. Response to the Letter to the Editor: Lung Immune Prognostic Index for Outcome Prediction to Immunotherapy in Patients With NSCLC. J. Thorac. Oncol. 2019, 14, e208. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.K.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Simon, R.M.; Paik, S.; Hayes, D.F. Use of Archived Specimens in Evaluation of Prognostic and Predictive Biomarkers. J. Natl. Cancer Inst. 2009, 101, 1446–1452. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Révész, D.; Engelhardt, E.G.; Tamminga, J.J.; Schramel, F.M.N.H.; Onwuteaka-Philipsen, B.; Van De Garde, E.M.W.; Steyerberg, E.W.; Jansma, E.P.; De Vet, H.C.W.; Coupé, V.M.H. Decision support systems for incurable non-small cell lung cancer: A systematic review. BMC Med Inform. Decis. Mak. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Modi, N.D.; Sorich, M.J.; Rowland, A.; Logan, J.M.; McKinnon, R.A.; Kichenadasse, G.; Wiese, M.D.; Hopkins, A.M. A literature review of treatment-specific clinical prediction models in patients with breast cancer. Crit. Rev. Oncol. 2020, 148, 102908. [Google Scholar] [CrossRef]
| Treatment Arm/Risk Group | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Median (95%CI) (Months) | HR (95%CI) | p | c | Median (95%CI) (Months) | HR (95%CI) | p | c | |
| ABCP | <0.001 | 0.63 | <0.001 | 0.59 | ||||
| Good LIPI (n = 177) | 24 (24-NA) | 1.00 | 12 (10–13) | 1.00 | ||||
| Intermediate LIPI (n = 165) | 16 (13-NA) | 2.16 (1.47–3.18) | 8 (7–10) | 1.47 (1.11–1.95) | ||||
| Poor LIPI (n = 43) | 7 (4–13) | 5.28 (3.20–8.69) | 4 (3–7) | 3.02 (2.03–4.50) | ||||
| ACP | <0.001 | 0.63 | <0.001 | 0.58 | ||||
| Good LIPI (n = 188) | 21 (20-NA) | 1.00 | 7 (7–8) | 1.00 | ||||
| Intermediate LIPI (n = 154) | 15 (11–21) | 1.94 (1.37–2.76) | 6 (5–7) | 1.50 (1.17–1.93) | ||||
| Poor LIPI (n = 40) | 7 (5–14) | 5.05 (3.13–8.13) | 3 (2–6) | 2.86 (1.96–4.18) | ||||
| Outcome | Good LIPI HR (95% CI) | Intermediate LIPI HR (95% CI) | Poor LIPI HR (95% CI) | P(interaction) |
|---|---|---|---|---|
| ABCP versus BCP | ||||
| n = 360 | n = 325 | n = 81 | ||
| OS | 0.78 (0.53–1.15) | 0.67 (0.49–0.91) | 0.87 (0.51–1.47) | 0.66 |
| PFS | 0.59 (0.45–0.77) | 0.52 (0.40–0.66) | 0.89 (0.56–1.42) | 0.13 |
| ACP versus BCP | ||||
| n = 371 | n = 314 | n = 78 | ||
| OS | 0.90 (0.63–1.29) | 0.76 (0.56–1.04) | 1.08 (0.63–1.84) | 0.51 |
| PFS | 0.96 (0.76–1.22) | 0.89 (0.70–1.14) | 1.45 (0.91–2.33) | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopkins, A.M.; Kichenadasse, G.; Abuhelwa, A.Y.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Value of the Lung Immune Prognostic Index in Patients with Non-Small Cell Lung Cancer Initiating First-Line Atezolizumab Combination Therapy: Subgroup Analysis of the IMPOWER150 Trial. Cancers 2021, 13, 1176. https://doi.org/10.3390/cancers13051176
Hopkins AM, Kichenadasse G, Abuhelwa AY, McKinnon RA, Rowland A, Sorich MJ. Value of the Lung Immune Prognostic Index in Patients with Non-Small Cell Lung Cancer Initiating First-Line Atezolizumab Combination Therapy: Subgroup Analysis of the IMPOWER150 Trial. Cancers. 2021; 13(5):1176. https://doi.org/10.3390/cancers13051176
Chicago/Turabian StyleHopkins, Ashley M., Ganessan Kichenadasse, Ahmad Y. Abuhelwa, Ross A. McKinnon, Andrew Rowland, and Michael J. Sorich. 2021. "Value of the Lung Immune Prognostic Index in Patients with Non-Small Cell Lung Cancer Initiating First-Line Atezolizumab Combination Therapy: Subgroup Analysis of the IMPOWER150 Trial" Cancers 13, no. 5: 1176. https://doi.org/10.3390/cancers13051176
APA StyleHopkins, A. M., Kichenadasse, G., Abuhelwa, A. Y., McKinnon, R. A., Rowland, A., & Sorich, M. J. (2021). Value of the Lung Immune Prognostic Index in Patients with Non-Small Cell Lung Cancer Initiating First-Line Atezolizumab Combination Therapy: Subgroup Analysis of the IMPOWER150 Trial. Cancers, 13(5), 1176. https://doi.org/10.3390/cancers13051176






