Cutaneous Squamous Cell Carcinoma in Patients with Hidradenitis Suppurativa
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
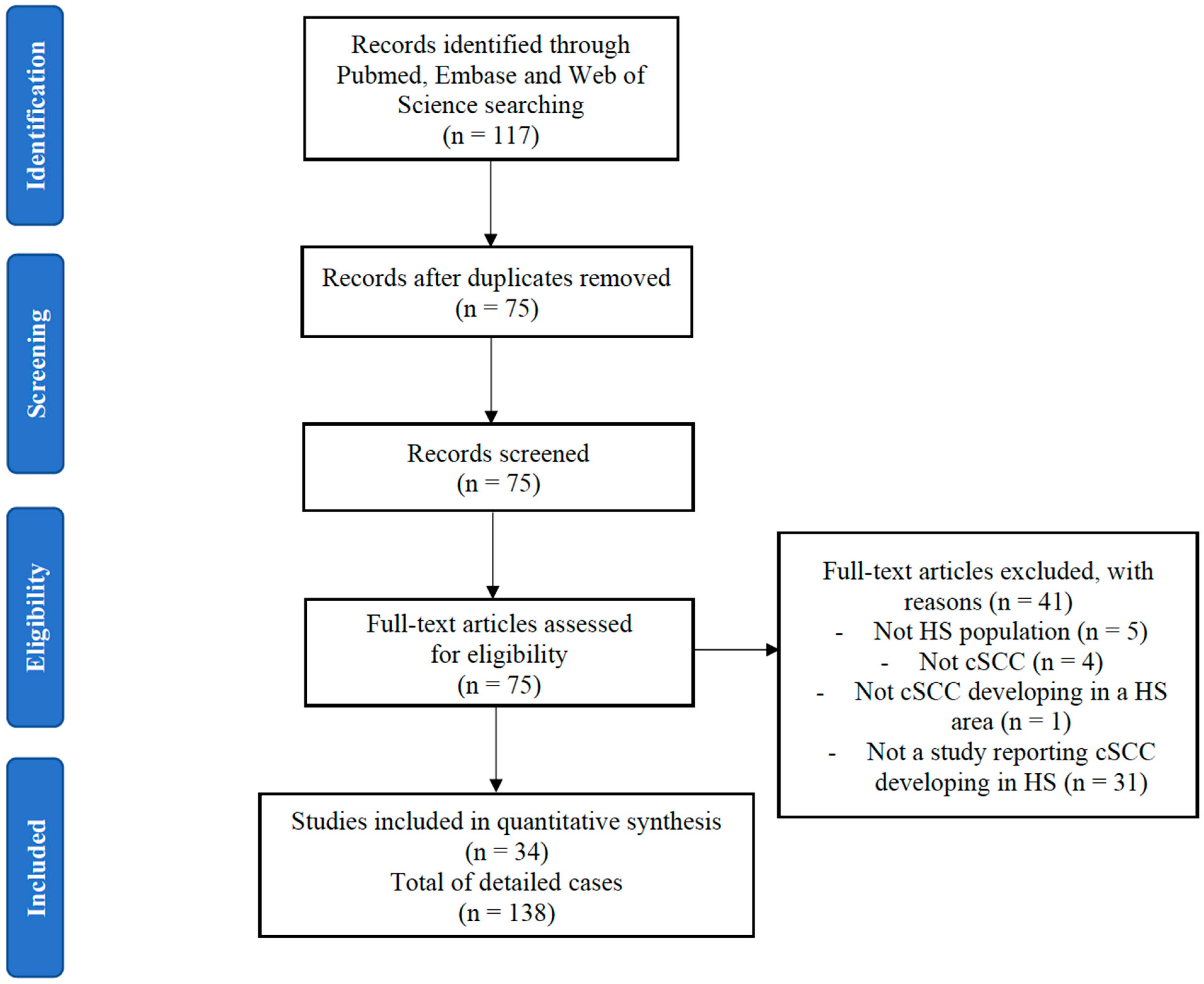
2.1. Study Identification
2.2. Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. cSCC Characteristics
3.3. Associations/Risk Factors with Cutaneous Squamous Cell Carcinoma
3.4. Staging of Squamous Cell Carcinoma and Predictors of Adverse Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saunte, D.M.L.; Jemec, G.B.E. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017, 318, 2019–2032. [Google Scholar] [CrossRef]
- Chapman, S.; Delgadillo, D.; Barber, C.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa: A Review of the Prevalence, Pathogenesis, and Treatment of This Dreaded Complication. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 25–28. [Google Scholar] [CrossRef]
- Lee, E.Y.; Alhusayen, R.; Lansang, P.; Shear, N.; Yeung, J. What Is Hidradenitis Suppurativa? Can. Fam. Phys. 2017, 63, 114–120. [Google Scholar]
- Li, X.; Jiang, L.; Huang, Y.; Ren, Z.; Liang, X.; Wang, P. A Gene Dysfunction Module Reveals the Underlying Pathogenesis of Hidradenitis Suppurativa: An Update. Austr. J. Dermatol. 2020, 61, e10–e14. [Google Scholar] [CrossRef] [PubMed]
- Jfri, A.H.; O’Brien, E.A.; Litvinov, I.V.; Alavi, A.; Netchiporouk, E. Hidradenitis Suppurativa: Comprehensive Review of Predisposing Genetic Mutations and Changes. J. Cutan. Med. Surg. 2019, 23, 519–527. [Google Scholar] [CrossRef]
- Huang, C.; Lai, Z.; He, M.; Zhai, B.; Zhou, L.; Long, X. Successful Surgical Treatment for Squamous Cell Carcinoma Arising from Hidradenitis Suppurativa: A Case Report and Literature Review. Medicine (Baltimore) 2017, 96, e5857. [Google Scholar] [CrossRef]
- Lavogiez, C.; Delaporte, E.; Darras-Vercambre, S.; Martin De Lassalle, E.; Castillo, C.; Mirabel, X.; Laurent, F.; Patenotre, P.; Gheit, T.; Talmant, J.C.; et al. Clinicopathological Study of 13 Cases of Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa. Dermatology (Basel) 2010, 220, 147–153. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Hsiao, J.L.; Lowes, M.A.; Shi, V.Y. A Comparison of International Management Guidelines for Hidradenitis Suppurativa. DRM 2019, 1–16. [Google Scholar] [CrossRef]
- Barresi, V.; Vitarelli, E.; Barresi, G. Acne Inversa Complicated by Squamous Cell Carcinoma in Association with Diffuse Malignant Peritoneal Mesothelioma Arising in the Absence of Predisposing Factors: A Case Report. J. Cutan. Pathol. 2008, 35, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Ben, A.J.; Bouasker, I.; Najah, H.; Zribi, H.; Bedoui, R.; Guesmi, F.; Hani, M.A.; Nouira, R.; Zoghlami, A.; Najah, N. Squamous Cell Carcinoma Arising in Verneuil’s Disease. Tunis Med. 2008, 86, 169–170. [Google Scholar] [PubMed]
- Ben Said, B.; Maitre, S.; Perrot, J.-L.; Labeille, B.; Cambazard, F. Syndrome Paranéoplasique Hypercalcémie–Hyperleucocytose Au Cours Des Carcinomes Épidermoïdes Cutanés. À Propos de Deux Observations. La Revue de Méd. Interne 2010, 31, 309–311. [Google Scholar] [CrossRef]
- Brown, M.D.; Zachary, C.B.; Grekin, R.C.; Swanson, N.A. Genital Tumors: Their Management by Micrographic Surgery. J. Am. Acad. Dermatol. 1988, 18, 115–122. [Google Scholar] [CrossRef]
- Chicarilli, Z.N.M.D. Follicular Occlusion Triad: Hidradenitis Suppurativa, Acne Conglobata, and Dissecting Cellulitis of the Scalp. Ann. Plast. Surg. 1987, 18, 230–237. [Google Scholar] [CrossRef]
- Dessinioti, C.; Plaka, M.; Zisimou, C.; Christofidou, E.; Antoniou, C.; Stratigos, A.J. Advanced Squamous Cell Carcinoma of the Axillae Mimicking Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e421–e423. [Google Scholar] [CrossRef]
- Giesey, R.; Delost, G.R.; Honaker, J.; Korman, N.J. Metastatic Squamous Cell Carcinoma in a Patient Treated with Adalimumab for Hidradenitis Suppurativa. JAAD Case Rep. 2017, 3, 489–491. [Google Scholar] [CrossRef]
- Gur, E.; Neligan, P.C.; Shafir, R.; Reznick, R.; Cohen, M.; Shpitzer, T. Squamous Cell Carcinoma in Perineal Inflammatory Disease. Ann. Plast. Surg. 1997, 38, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Harview, C.L.; Truong, A.K.; Worswick, S.D.; Sarantopoulos, G.P.; Hsiao, J.L. Squamous Cell Carcinoma of the Perineum Masquerading as Necrotizing Hidradenitis Suppurativa. Dermatol. Online J. 2018, 24, 13030/qt3pb602cq. [Google Scholar] [PubMed]
- Jourabchi, N.; Fischer, A.H.; Cimino-Mathews, A.; Waters, K.M.; Okoye, G.A. Squamous Cell Carcinoma Complicating a Chronic Lesion of Hidradenitis Suppurativa: A Case Report and Review of the Literature. Int. Wound J. 2017, 14, 435–438. [Google Scholar] [CrossRef]
- Juviler, P.G.; Patel, A.P.; Qi, Y. Infiltrative Squamous Cell Carcinoma in Hidradenitis Suppurativa: A Case Report for Early Surgical Intervention. Int. J. Surg. Case Rep. 2019, 55, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, J.J.; Shah, K.K.; Hallemeier, C.L.; Baum, C.L.; Davis, M.D.P. Squamous Cell Carcinoma in Perineal, Perianal, and Gluteal Hidradenitis Suppurativa: Experience in 12 Patients. Dermatol. Surg. 2019, 45, 519–526. [Google Scholar] [CrossRef]
- Maalouf, E.; Faye, O.; Poli, F.; Cosnes, A.; Revuz, J. Carcinome Épidermoïde Mortel Sur Hidradénite Suppurée Après Traitement Par Infliximab. Ann. Dermatol. Vénéréol. 2006, 133, 473–474. [Google Scholar] [CrossRef]
- Makris, G.-M.; Poulakaki, N.; Papanota, A.-M.; Kotsifa, E.; Sergentanis, T.N.; Psaltopoulou, T. Vulvar, Perianal and Perineal Cancer After Hidradenitis Suppurativa: A Systematic Review and Pooled Analysis. Dermatol. Surg. 2017, 43, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Manolitsas, T.; Biankin, S.; Jaworski, R.; Wain, G. Vulval Squamous Cell Carcinoma Arising in Chronic Hidradenitis Suppurativa. Gynecol. Oncol. 1999, 75, 285–288. [Google Scholar] [CrossRef]
- McArdle, D.J.T.; McArdle, J.P.; Lee, F.; Mignanelli, E.D. Rare “Inverted” Verrucous Carcinoma (Carcinoma Cuniculatum) of the Sacrogluteal Region: Case Report and Literature Review. Int. J. Surg. Pathol. 2017, 25, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Ishikawa, M.; Mori, T.; Hanami, Y.; Ohtsuka, M.; Yamamoto, T. Huge Squamous Cell Carcinoma Arising on Severe Hidradenitis Suppurativa. Actas Dermosifiliogr 2018, 109, 828. [Google Scholar] [CrossRef] [PubMed]
- Montagliani, L.; Monneuse, O.; Braye, F.; Barth, X.; Claudy, A.; Tissot, E. Maladie de Verneuil et Cancer. J. De Chirurgie J. CHIR 2005, 142, 381–382. [Google Scholar] [CrossRef]
- Obredor, C.; Palermo, M.; Zorraquín, C.; Albertengo, J.C. Perineal suppurative hidradenitis and carcinoma. A case report. Acta Gastroenterol. Latinoam. 2009, 39, 278–281. [Google Scholar] [PubMed]
- Pena, Z.G.; Sivamani, R.K.; Konia, T.H.; Eisen, D.B. Squamous Cell Carcinoma in the Setting of Chronic Hidradenitis Suppurativa; Report of a Patient and Update of the Literature. Dermatol. Online J. 2015, 21, 13030/qt9q9707dp. [Google Scholar]
- Pérez-Diaz, D.; Calvo-Serrano, M.; Mártinez-Hijosa, E.; Fuenmayor-Valera, L.; Muñoz-Jiménez, F.; Turégano-Fuentes, F.; Del Valle, E. Squamous Cell Carcinoma Complicating Perianal Hidradenitis Suppurativa. Int. J. Colorectal Dis. 1995, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Pitch, M.A.; Bryan, D.J.; McMillan, J.; Chavez, L.; Hammes, S.R.; Scott, G.; Mercurio, M.G.; Somers, K.E. A Fatal Case of Parathyroid Hormone-Related Peptide (PTHrP)-Producing Squamous Cell Carcinoma Arising in the Context of Long-Standing Hidradenitis Suppurativa. JAAD Case Rep. 2018, 4, 426–428. [Google Scholar] [CrossRef]
- Powell, H.B.; Googe, P.B.; Sayed, C.J. Squamous Cell Carcinoma Arising in a Chronic Perineal Wound in a Patient with Long-Standing Cutaneous Crohn’s Disease. JAAD Case Rep. 2018, 4, 346–348. [Google Scholar] [CrossRef]
- Rekawek, P.; Mehta, S.; Andikyan, V.; Harmaty, M.; Zakashansky, K. Squamous Cell Carcinoma of the Vulva Arising in the Setting of Chronic Hidradenitis Suppurativa: A Case Report. Gynecol. Oncol. Rep. 2016, 16, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T. Squamous Cell Carcinoma: Complication of Chronic Skin Disorders in Black Patients. J. Natl. Med. Assoc. 1986, 78, 1203–1205. [Google Scholar] [PubMed]
- Roy, C.F.; Roy, S.F.; Ghazawi, F.M.; Patocskai, E.; Bélisle, A.; Dépeault, A. Cutaneous Squamous Cell Carcinoma Arising in Hidradenitis Suppurativa: A Case Report. SAGE Open Med. Case Rep. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Segura Palacios, J.M.; García Montero, P.; Fúnez Liébana, R.; Repiso Jiménez, J.B. Human Papilloma Virus and the Risk of Squamous Cell Carcinoma Arising in Hidradenitis Suppurativa. Actas Dermo-Sifiliográficas (English Edition) 2018, 109, 457–459. [Google Scholar] [CrossRef]
- Sevray, M.; Dupré, P.-F.; Le Flahec, G.; Trimaille, A.; Misery, L.; Brenaut, E. Vulvar Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa in a Young Woman. JAAD Case Rep. 2019, 5, 999–1001. [Google Scholar] [CrossRef]
- Talmant, J.-C.; Bruant-Rodier, C.; Nunziata, A.C.; Rodier, J.F.; Wilk, A. Squamous cell carcinoma arising in Verneuil’s disease: Two cases and literature review. Ann. Chir. Plast. Esthet. 2006, 51, 82–86. [Google Scholar] [CrossRef]
- Yatim, A.; Bohelay, G.; Grootenboer-Mignot, S.; Prost-Squarcioni, C.; Alexandre, M.; Le Roux-Villet, C.; Martin, A.; Maubec, E.; Caux, F. Paraneoplastic Pemphigus Revealed by Anti-Programmed Death-1 Pembrolizumab Therapy for Cutaneous Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa. Front. Med. 2019, 6. [Google Scholar] [CrossRef]
- Yen, C.-F.; Chang, Y.-Y.; Lee, Y.-Y. Image Gallery: Squamous Cell Carcinoma Arising in Long-Standing Hidradenitis Suppurativa. Br. J. Dermatol. 2018, 179, e226. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Tan, C. Squamous Cell Carcinoma Arising in Chronic Hidradenitis Suppurativa: A Lethal Complication to Be Avoided. Acta Oncol. 2017, 56, 497–498. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Short, K.A.; Kalu, G.; Mortimer, P.S.; Higgins, E.M. Vulval Squamous Cell Carcinoma Arising in Chronic Hidradenitis Suppurativa. Clin. Exp. Dermatol. 2005, 30, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Wan, D.; Roostaeian, J.; Rubayi, S. Marjolin Ulcer in Hidradenitis Suppurativa: Case Reports. Ann. Plast. Surg. 2010, 64, 315–317. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Novick, M.; Gard, D.A.; Hardy, S.B.; Spira, M. Burn Scar Carcinoma: A Review and Analysis of 46 Cases. J. Trauma 1977, 17, 809–817. [Google Scholar] [CrossRef]
- Knackstedt, T.J.; Collins, L.K.; Li, Z.; Yan, S.; Samie, F.H. Squamous Cell Carcinoma Arising in Hypertrophic Lichen Planus: A Review and Analysis of 38 Cases. Dermatol. Surg. 2015, 41, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Boozalis, E.; Shah, A.A.; Wigley, F.; Kang, S.; Kwatra, S.G. Morphea and Systemic Sclerosis Are Associated with an Increased Risk for Melanoma and Nonmelanoma Skin Cancer. J. Am. Acad. Dermatol. 2019, 80, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of Nonmelanoma Skin Cancer in Relation to Ambient UV Radiation in White Populations, 1978-2012: Empirical Relationships. JAMA Dermatol. 2014, 150, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wikonkal, N.M.; Brash, D.E. Ultraviolet Radiation Induced Signature Mutations in Photocarcinogenesis. J. Investig. Dermatol. Symp. Proc. 1999, 4, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Ruocco, E.; De Vita, V.; Monfrecola, G. Squamous Cell Carcinoma Arising in Long-Standing Hidradenitis Suppurativa: An Overlooked Facet of the Immunocompromised District. Clin. Dermatol. 2017, 35, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.Y.; Kovarik, C.L.; Lee, R.A. Lymphedematous Verrucous Changes Simulating Squamous Cell Carcinoma in Long-Standing Hidradenitis Suppurativa. Int. J. Dermatol. 2013, 52, 808–812. [Google Scholar] [CrossRef]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The Evolving Relationship of Wound Healing and Tumor Stroma. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Rønnov-Jessen, L.; Petersen, O.W. Induction of Alpha-Smooth Muscle Actin by Transforming Growth Factor-Beta 1 in Quiescent Human Breast Gland Fibroblasts. Implications for Myofibroblast Generation in Breast Neoplasia. Lab. Investig. 1993, 68, 696–707. [Google Scholar] [PubMed]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of Platelet-Derived Growth Factor in Wound Healing. J. Cell. Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses in the Causation of Human Cancers—A Brief Historical Account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Miller, D.L.; Puricelli, M.D.; Stack, M.S. Virology and Molecular Pathogenesis of HPV (Human Papillomavirus)Associated Oropharyngeal Squamous Cell Carcinoma. Biochem. J. 2012, 443, 339–353. [Google Scholar] [CrossRef]
- Flores, R.; Lu, B.; Beibei, L.; Nielson, C.; Abrahamsen, M.; Wolf, K.; Lee, J.-H.; Harris, R.B.; Giuliano, A.R. Correlates of Human Papillomavirus Viral Load with Infection Site in Asymptomatic Men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3573–3576. [Google Scholar] [CrossRef]
- Pham, C.T.; Juhasz, M.; Sung, C.T.; Mesinkovska, N.A. The Human Papillomavirus Vaccine as a Treatment for Human Papillomavirus–Related Dysplastic and Neoplastic Conditions: A Literature Review. J. Am. Acad. Dermatol. 2020, 82, 202–212. [Google Scholar] [CrossRef]
- Mejilla, A.; Li, E.; Sadowski, C.A. Human Papilloma Virus (HPV) Vaccination: Questions and Answers. Can. Pharm. J. 2017, 150, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Panelos, J.; Massi, D. Emerging Role of Notch Signaling in Epidermal Differentiation and Skin Cancer. Cancer Biol. Ther. 2009, 8, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Montaudié, H.; Chiaverini, C.; Sbidian, E.; Charlesworth, A.; Lacour, J.-P. Inherited Epidermolysis Bullosa and Squamous Cell Carcinoma: A Systematic Review of 117 Cases. Orphanet. J. Rare Dis. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Combemale, P.; Bousquet, M.; Kanitakis, J.; Bernard, P. Malignant Transformation of Leg Ulcers: A Retrospective Study of 85 Cases. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Alam, M.; Chen, P.-L.; Daniels, G.A.; DiMaio, D.; Farma, J.M.; Ghosh, K.; Harms, K.; Sekulic, A.; Stebbins, W. NCCN Guidelines Index Table of Contents. 2019, Volume 86. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 4 January 2021).
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Petrow, P.; Scheer-Senyarich, I.; Duvillard, P.; Lacroix, L.; Gelly, J.; Certain, A.; Duval, X.; Crickx, B.; Buffard, V.; et al. Phase II Study of Cetuximab as First-Line Single-Drug Therapy in Patients with Unresectable Squamous Cell Carcinoma of the Skin. J. Clin. Oncol. 2011, 29, 3419–3426. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Number (n) | % |
|---|---|---|
| Total | 138 | 100 |
| Sex (males) (n = 125) | 102 | 81.6 |
| Age, in years (mean, SD) | 53.5 ± 10.2 | |
| HS duration prior to cSCC (mean, SD) | 24.7 ± 11.9 | |
| Race (n = 84) | ||
| White | 70 | 83.3 |
| African-American | 12 | 14.3 |
| Others | 2 | 2.4 |
| Smoking (n = 27) | 22 | 81.5 |
| Hurley stage (n = 79) | ||
| 1 | 1 | 1.3 |
| 2 | 8 | 10.1 |
| 3 | 70 | 88.6 |
| Site of cSCC (n = 131) | ||
| Gluteal and perianal | 115 | 87.8 |
| Inguinal | 4 | 3.1 |
| Vulvar | 7 | 5.3 |
| Scrotal | 2 | 1.5 |
| Scapular | 2 | 1.5 |
| Axillary | 1 | 0.8 |
| Morphology of cSCC (n = 77) | ||
| Ulcer | 53 | 68.8 |
| Nodule/plaque | 12 | 15.6 |
| Verrucous | 12 | 15.6 |
| Histological grade of cSCC (n = 98) | ||
| 0 (in situ) | 2 | 2.0 |
| 1 (well-differentiated) | 45 | 45.9 |
| 1(V) (well-differentiated, verrucous) | 14 | 14.3 |
| 2 (moderately differentiated) | 25 | 25.5 |
| 3 (poorly differentiated) | 12 | 12.2 |
| Immunosuppressive treatment (n = 14) | ||
| Biologic | 4 | 28.6 |
| Nonbiologic * | 6 | 42.9 |
| Biologic and nonbiologic | 3 | 21.4 |
| None | 1 | 7.7 |
| Concomitant autoimmune chronic disorder | ||
| Crohn’s disease | 4 | 2.9 |
| HPV status (n = 38) | ||
| Lesional HPV detected | 12 | 31.6 |
| High-risk HPV | 9 | 75 |
| Metastatic status | ||
| Nodal microscopic metastasis (n = 94) | 43 | 46.2 |
| Site = inguinal (n = 32) | 23 | 71.9 |
| Distant metastasis (n = 99) | 31 | 31.3 |
| Site = lung (n = 28) | 11 | 39.3 |
| cSCC treatment | ||
| Surgical excision (n = 131) | 100 | 76.3 |
| Radiotherapy (n = 50) | 37 | 74.0 |
| Chemotherapy (n = 43) | 26 | 60.5 |
| Recurrence of cSCC post excision (n = 76) | ||
| Same site | 30 | 39.5 |
| Nearby site | 2 | 2.6 |
| cSCC vital status (n = 110) | ||
| Demise due to cSCC or its complications Demise due to cause unrelated to cSCC Time to demise in months (mean, SD) | 56 | 50.9 |
| 4 11.6 ± 15.2 | 3.6 | |
| Duration of follow-up in patients with favorable outcome, in months (mean, SD) | 28 ± 31.3 |
| Variables (Prognostic Factors) | Value | Univariate Cox Proportional Hazards Model | ||
|---|---|---|---|---|
| OR | 95%CI | p | ||
| Sex (n = 89) | 2—females | 1 | ||
| 1—males | 1.9 | 0.673–5.376 | 0.225 | |
| Age (n = 87) | Mean—53.6 | 1 | ||
| >Mean | 1.014 | 0.983–1.046 | 0.376 | |
| Race (n = 56) | 2—African-American | 1 | ||
| 1—White | 0.612 | 0.265–1.414 | 0.251 | |
| Hurley score (n = 51) | 1—1 | - | ||
| 2—2 | 1 | |||
| 3—3 | 2.706 | 0.336–20.036 | 0.330 | |
| Site of cSCC (n = 89) | 1—gluteal/perianal/perineal | 1 | ||
| 2—inguinal | 0.000 | 0.000 | 0.982 | |
| 3—vulvar | 0.713 | 0.097–5.207 | 0.738 | |
| 4—scrotal | 0.000 | 0.000 | 0.987 | |
| 5—scapular | 2.970 | 0.710–12.415 | 0.136 | |
| 6—axillary | - | |||
| Duration of HS prior to cSCC (years) (n = 82) | Mean—24.48 | 1 | ||
| >Mean | 1.024 | 0.998–1.051 | 0.074 | |
| Presence of HPV (n = 29) | 1—no | 1 | ||
| 2—yes | 0.736 | 0.153–3.546 | 0.703 | |
| Past or active smoking | 1—no | 1 | ||
| 2—yes | 2.156 | 0.264–17.624 | 0.474 | |
| Immunosuppressive therapy | 0—NR | 1 | ||
| 1—none | 0.000 | 0.000 | 0.984 | |
| 2—non biologics | 2.262 | 0.802–6,385 | 0.123 | |
| 3—biologics | 3.428 | 1.031–11.404 | 0.045 | |
| 4—combined biologic and nonbiologic | 13.970 | 1.706–114.368 | 0.014 | |
| Morphology (n = 53) | 1—ulcer | 10.378 | 1.386–77.727 | 0.023 |
| 2—nodule/plaque | 2.742 | 0.246–30.567 | 0.412 | |
| 3—verrucous | 1 | |||
| Histologic grade (Broder) (n = 68) | 1—1 (well) | 1 | ||
| 2—2 (mod) | 1.553 | 0.586–4.114 | 0.376 | |
| 3—3 (poorly) | 7.186 | 2.835–18.219 | 0.000 | |
| 4—1(V) (well-verrucous) | 0.346 | 0.071–1.684 | 0.189 | |
| 5—in situ | 0.000 | 0.000 | 0.982 | |
| Palpable lymphadenopathy (n = 20) | 1—no | 1 | ||
| 2—yes | 41.694 | 0.168–10335.3 | 0.185 | |
| Presence of nodal metastasis (n = 71) | 1—no | 1 | ||
| 2—yes | 9.669 | 3.884–24.07 | 0.000 | |
| Site of nodal metastasis (n = 22) | 1—inguinal | 1 | ||
| 2—other | - | |||
| 3—multiple (>1) | 0.911 | 0.261–3.175 | 0.883 | |
| Presence of distant metastasis (n = 72) | 1—no | 1 | ||
| 2—yes | 4.261 | 2.069–8.775 | 0.000 | |
| Site of distant metastasis (n = 20) | 1—lung | 1 | ||
| 2—bone | 0.351 | 0.039–3.190 | 0.352 | |
| 3—other | 2.390 | 0.545–10.47 | 0.248 | |
| 4—multiple (>1) | 0.931 | 0.289–2.99 | 0.905 | |
| Excision (n = 91) | 2—yes | 1 | ||
| 1—no | 8.184 | 4.108–16.30 | 0.000 | |
| Radiotherapy (n = 37) | 1—no | 1 | ||
| 2—yes | 2.469 | 0.703–8.671 | 0.159 | |
| Chemotherapy (n = 32) | 1—no | 1 | ||
| 2—yes | 2.082 | 0.752–5.764 | 0.158 | |
| Recurrent cSCC (n = 32) | 1—no | 1 | ||
| 2—yes, same site | 5.218 | 1.91–14.28 | 0.001 | |
| 3—yes, different site | 7.944 | 1.53–41.34 | 0.014 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racanelli, E.; Jfri, A.; Gefri, A.; O’Brien, E.; Litvinov, I.V.; Zubarev, A.; Savin, E.; Netchiporouk, E. Cutaneous Squamous Cell Carcinoma in Patients with Hidradenitis Suppurativa. Cancers 2021, 13, 1153. https://doi.org/10.3390/cancers13051153
Racanelli E, Jfri A, Gefri A, O’Brien E, Litvinov IV, Zubarev A, Savin E, Netchiporouk E. Cutaneous Squamous Cell Carcinoma in Patients with Hidradenitis Suppurativa. Cancers. 2021; 13(5):1153. https://doi.org/10.3390/cancers13051153
Chicago/Turabian StyleRacanelli, Elysia, Abdulhadi Jfri, Amnah Gefri, Elizabeth O’Brien, Ivan V. Litvinov, Andrey Zubarev, Evgeny Savin, and Elena Netchiporouk. 2021. "Cutaneous Squamous Cell Carcinoma in Patients with Hidradenitis Suppurativa" Cancers 13, no. 5: 1153. https://doi.org/10.3390/cancers13051153
APA StyleRacanelli, E., Jfri, A., Gefri, A., O’Brien, E., Litvinov, I. V., Zubarev, A., Savin, E., & Netchiporouk, E. (2021). Cutaneous Squamous Cell Carcinoma in Patients with Hidradenitis Suppurativa. Cancers, 13(5), 1153. https://doi.org/10.3390/cancers13051153







