The Exciting New Field of HER2-Low Breast Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Rationale for Targeting HER2-Low BC with Anti-HER2 Agents
2.1. Trastuzumab
2.2. Pertuzumab
2.3. Nelipepimut-S
2.4. Trastuzumab-Emtansine
3. Mechanisms of Action and Clinical Efficacy of the Novel Anti-HER2 Drugs in HER2-Low BC
3.1. Trastuzumab-Deruxtecan
3.2. Trastuzumab-Duocarmazine
3.3. XMT-1522
3.4. Zenocutuzumab
4. Exploiting Combination Treatments in HER2-Low Breast Cancer
4.1. Immunotherapy
4.2. Endocrine Therapies
4.3. CDK4/6i
4.4. Other Combinations
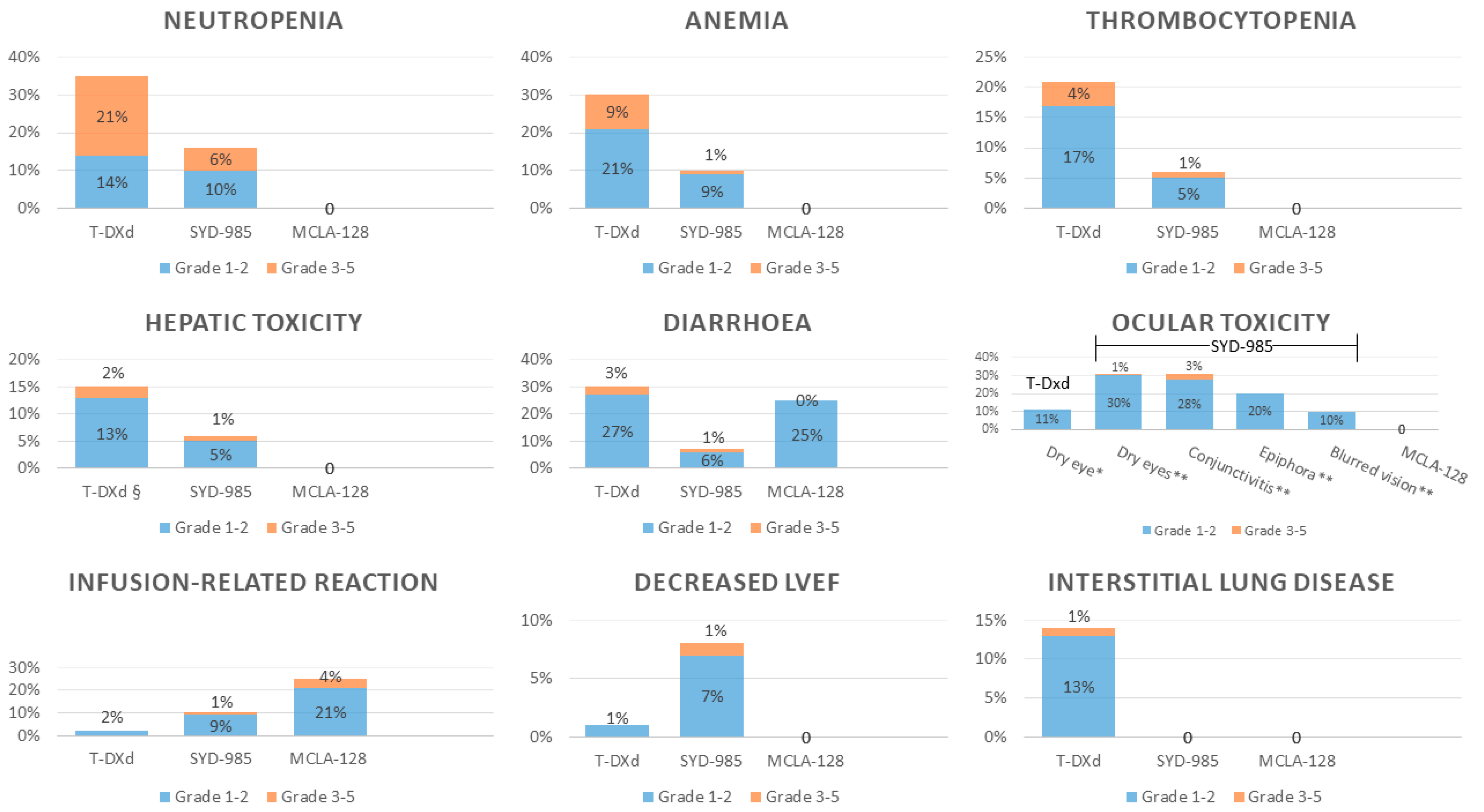
5. Safety
5.1. Hematological Toxicity
5.2. Hepatic Toxicity
5.3. Gastrointestinal Toxicity
5.4. Pulmonary Toxicity
5.5. Ocular Toxicity
5.6. Cardiotoxicity
5.7. Neuropathy
5.8. Infusion-Related Reactions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mendes, D.; Alves, C.; Afonso, N.; Cardoso, F.; Passos-Coelho, J.L.; Costa, L.; Andrade, S.; Batel-Marques, F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res. 2015, 17, 140. [Google Scholar] [CrossRef]
- Viani, G.A.; Afonso, S.L.; Stefano, E.J.; De Fendi, L.I.; Soares, F.V. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer 2007, 7, 153. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A. The HER-2/neuOncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy. Stem Cells 1998, 16, 413–428. [Google Scholar] [CrossRef]
- Mass, R.; Press, M.; Anderson, S.; Murphy, M.; Slamon, D. Improved survival benefit from Herceptin (trastuzumab) in patients selected by fluorescence in situ hybridization (FISH). Proc. Am. Soc. Clin. Oncol. 2001, 20, 22a-abstract. [Google Scholar]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and Safety of Trastuzumab as a Single Agent in First-Line Treatment of HER2-Overexpressing Metastatic Breast Cancer. J. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Mass, R.D.; Press, M.F.; Anderson, S.; Cobleigh, M.A.; Vogel, C.L.; Dybdal, N.; Leiberman, G.; Slamon, D.J. Evaluation of Clinical Outcomes According to HER2 Detection by Fluorescence In Situ Hybridization in Women with Metastatic Breast Cancer Treated with Trastuzumab. Clin. Breast Cancer 2005, 6, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef]
- Denduluri, N.; Chavez-MacGregor, M.; Telli, M.L.; Eisen, A.; Graff, S.L.; Hassett, M.J.; Holloway, J.N.; Hurria, A.; King, T.A.; Lyman, G.H.; et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2433–2443. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Lopez, B.A.; Barrios, C.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Kumar, S.; Hui, P.; Rimm, D.L.; Gershkovich, P. A Retrospective Population-Based Comparison of HER2 Immunohistochemistry and Fluorescence In Situ Hybridization in Breast Carcinomas: Impact of 2007 American Society of Clinical Oncology/ College of American Pathologists Criteria. Arch. Pathol. Lab. Med. 2014, 138, 213–219. [Google Scholar] [CrossRef]
- Lal, P.; Salazar, P.A.; Hudis, C.A.; Ladanyi, M.; Chen, B. HER-2 Testing in Breast Cancer Using Immunohistochemical Analysis and Fluorescence In Situ Hybridization: A Single-Institution Experience of 2,279 Cases and Comparison of Dual-Color and Single-Color Scoring. Am. J. Clin. Pathol. 2004, 121, 631–636. [Google Scholar] [CrossRef]
- Giuliani, S.; Ciniselli, C.M.; Leonardi, E.; Polla, E.; DeCarli, N.; Luchini, C.; Cantaloni, C.; Gasperetti, F.; Cazzolli, D.; Berlanda, G.; et al. In a cohort of breast cancer screened patients the proportion of HER2 positive cases is lower than that earlier reported and pathological characteristics differ between HER2 3+ and HER2 2+/Her2 amplified cases. Virchows Archiv 2016, 469, 45–50. [Google Scholar] [CrossRef]
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based Estimate of the Prevalence of HER-2 Positive Breast Cancer Tumors for Early Stage Patients in the US. Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2+. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Sarotto, I.; Maggiorotto, F.; Berchialla, P.; Kubatzki, F.; Tomasi, N.; Redana, S.; Martinello, R.; Valabrega, G.; Aglietta, M.; et al. Moderate Immunohistochemical Expression of HER-2 (2+) Without HER-2 Gene Amplification Is a Negative Prognostic Factor in Early Breast Cancer. Oncologist 2012, 17, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, H.; Ignatov, T.; Bürger, E.; Kantelhardt, E.J.; Fettke, F.; Thomssen, C.; Costa, S.D.; Ignatov, A. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr.-Relat. Cancer 2015, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease, M.Z.; Woodward, W.A.; Nicolas, M.M.; Corley, L.J.; Fuller, G.N.; Esteva, F.J.; Tucker, S.L.; Buchholz, T.A. Even Low-level HER2 Expression May be Associated with Worse Outcome in Node-positive Breast Cancer. Am. J. Surg. Pathol. 2009, 33, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Caswell-Jin, J.L.; Plevritis, S.K.; Tian, L.; Cadham, C.J.; Xu, C.; Stout, N.K.; Sledge, G.W.; Mandelblatt, J.S.; Kurian, A.W. Change in Survival in Metastatic Breast Cancer with Treatment Advances: Meta-Analysis and Systematic Review. JNCI Cancer Spectr. 2018, 2, pky062. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Banerji, U.; Van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; MacPherson, I.R.; Boni, V.; Rolfo, C.; E De Vries, E.G.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Pistilli, B.; Wildiers, H.; Hamilton, E.P.; Ferreira, A.A.; Dalenc, F.; Vidal, M.; Gavilá, J.; Goncalves, A.; Murias, C.; Mouret-Reynier, M.-A.; et al. Clinical activity of MCLA-128 (zenocutuzumab) in combination with endocrine therapy (ET) in ER+/HER2-low, non-amplified metastatic breast cancer (MBC) patients (pts) with ET-resistant disease who had progressed on a CDK4/6 inhibitor (CDK4/6i). J. Clin. Oncol. 2020, 38, 1037. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A.; Linette, G.P.; Stec, J.; Clark, E.; Ayers, M.; Symmans, W.F.; Pusztai, L.; Bloom, K.J. The HER-2/ neu Gene and Protein in Breast Cancer 2003: Biomarker and Target of Therapy. Oncologist 2003, 8, 307–325. [Google Scholar] [CrossRef]
- Gianni, L.; Lladó, A.; Bianchi, G.; Cortes, J.; Kellokumpu-Lehtinen, P.-L.; Cameron, D.A.; Miles, D.; Salvagni, S.; Wardley, A.; Goeminne, J.-C.; et al. Open-Label, Phase II, Multicenter, Randomized Study of the Efficacy and Safety of Two Dose Levels of Pertuzumab, a Human Epidermal Growth Factor Receptor 2 Dimerization Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer. J. Clin. Oncol. 2010, 28, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Hickerson, A.; Clifton, G.T.; Hale, D.F.; Peace, K.M.; Holmes, J.P.; Vreeland, T.J.; Litton, J.K.; Murthy, R.K.; Lukas, J.J.; Mittendorf, E.A.; et al. Final analysis of nelipepimut-S plus GM-CSF with trastuzumab versus trastuzumab alone to prevent recurrences in high-risk, HER2 low-expressing breast cancer: A prospective, randomized, blinded, multicenter phase IIb trial. J. Clin. Oncol. 2019, 37, 1. [Google Scholar] [CrossRef]
- Filho, O.M.; Viale, G.; Trippa, L.; Li, T.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.; Waks, A.G.; et al. HER2 heterogeneity as a predictor of response to neoadjuvant T-DM1 plus pertuzumab: Results from a prospective clinical trial. J. Clin. Oncol. 2019, 37, 502. [Google Scholar] [CrossRef]
- Ithimakin, S.; Day, K.C.; Malik, F.; Zen, Q.; Dawsey, S.J.; Bersano-Begey, T.F.; Quraishi, A.A.; Ignatoski, K.W.; Daignault, S.; Davis, A.; et al. HER2 Drives Luminal Breast Cancer Stem Cells in the Absence of HER2 Amplification: Implications for Efficacy of Adjuvant Trastuzumab. Cancer Res. 2013, 73, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Sledge, G.; Geyer, C.E.G., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, C.; Wolmark, N. HER2Status and Benefit from Adjuvant Trastuzumab in Breast Cancer. N. Engl. J. Med. 2008, 358, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Reinholz, M.M.; Hillman, D.W.; Tenner, K.S.; Schroeder, M.J.; Davidson, N.E.; Martino, S.; Sledge, G.W.; Harris, L.N.; Gralow, J.R.; et al. HER2and Chromosome 17 Effect on Patient Outcome in the N9831 Adjuvant Trastuzumab Trial. J. Clin. Oncol. 2010, 28, 4307–4315. [Google Scholar] [CrossRef]
- Burris, H.A.; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II Study of the Antibody Drug Conjugate Trastuzumab-DM1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2) –Positive Breast Cancer After Prior HER2-Directed Therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Lorusso, P.; Miller, K.D.; Modi, S.; Yardley, D.; Rodriguez, G.; Guardino, E.; Lu, M.; Zheng, M.; Girish, S.; et al. A Phase II Study of Trastuzumab Emtansine in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer Who Were Previously Treated With Trastuzumab, Lapatinib, an Anthracycline, a Taxane, and Capecitabine. J. Clin. Oncol. 2012, 30, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Colleoni, M.; Bisagni, G.; Mansutti, M.; Zamagni, C.; Del Mastro, L.; Zambelli, S.; Frassoldati, A.; Barlera, S.; Valagussa, P.; et al. Ki67 during and after neoadjuvant trastuzumab, pertuzumab and palbociclib plus or minus fulvestrant in HER2 and ER-positive breast cancer: The NA-PHER2 Michelangelo study. J. Clin. Oncol. 2019, 37, 527. [Google Scholar] [CrossRef]
- Hamilton, E.; Shapiro, C.L.; Petrylak, D.; Boni, V.; Martin, M.; Del Conte, G.; Cortes, J.; Agarwal, L.; Arkenau, H.-T.; Tan, A.R.; et al. Trastuzumab Deruxtecan (T-DXd; DS-8201) with Nivolumab in Patients with HER2-Expressing, Advanced Breast Cancer: A 2-Part, Phase 1b, Multicenter, Open-Label Study; SABCS: San Antonio, TX, USA, 2020. [Google Scholar]
- Eiger, D.; Pondé, N.F.; De Azambuja, E. Pertuzumab in HER2-positive early breast cancer: Current use and perspectives. Futur. Oncol. 2019, 15, 1823–1843. [Google Scholar] [CrossRef]
- Friess, T.S.; Bauer, A.M.B. In vivo activity of recombinant humanized monoclonal antibody 2C4 in xenografts is independent of tumor type and degree of HER2 overexpression. Eur. J. Cancer 2002, 38, S149. [Google Scholar] [CrossRef]
- Fisk, B.; Blevins, T.L.; Wharton, J.T.; Ioannides, C.G. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J. Exp. Med. 1995, 181, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Patel, R.; Holmes, J.P.; Hueman, M.T.; Mittendorf, E.A.; Craig, D.; Stojadinovic, A.; Ponniah, S.; et al. The Impact of HER2/neu Expression Level on Response to the E75 Vaccine: From U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin. Cancer Res. 2009, 15, 2895–2904. [Google Scholar] [CrossRef] [PubMed]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Mao, Z.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Junttila, T.T.; Li, G.; Parsons, K.; Phillips, G.L.; Sliwkowski, M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 2011, 128, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Shafi, H.; Astvatsaturyan, K.; Chung, F.; Mirocha, J.; Schmidt, M.; Bose, S. Clinicopathological significance of HER2/neu genetic heterogeneity in HER2/neu non-amplified invasive breast carcinomas and its concurrent axillary metastasis. J. Clin. Pathol. 2013, 66, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Van Der Lee, M.M.; Groothuis, P.G.; Ubink, R.; Van Der Vleuten, M.A.; Van Achterberg, T.A.; Loosveld, E.M.; Damming, D.; Jacobs, D.C.; Rouwette, M.; Egging, D.F.; et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol. Cancer Ther. 2015, 14, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Le Joncour, V.; Martins, A.; Puhka, M.; Isola, J.; Salmikangas, M.; Laakkonen, P.; Joensuu, H.; Barok, M. A Novel Anti-HER2 Antibody–Drug Conjugate XMT-1522 for HER2-Positive Breast and Gastric Cancers Resistant to Trastuzumab Emtansine. Mol. Cancer Ther. 2019, 18, 1721–1730. [Google Scholar] [CrossRef]
- Geuijen, C.A.; De Nardis, C.; Maussang, D.; Rovers, E.; Gallenne, T.; Hendriks, L.J.; Visser, T.; Nijhuis, R.; Logtenberg, T.; De Kruif, J.; et al. Unbiased Combinatorial Screening Identifies a Bispecific IgG1 that Potently Inhibits HER3 Signaling via HER2-Guided Ligand Blockade. Cancer Cell 2018, 33, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS -8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Modi, S.; Ohtani, S.; Lee, C.; Wang, Y.; Saxena, K.; Cameron, D.A. Abstract OT1-07-02: A phase 3, multicenter, randomized, open-label trial of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice in HER2-low breast cancer (DESTINY-Breast04). Ongoing Clin. Trials 2020, 80, OT1-07. [Google Scholar] [CrossRef]
- Bardia, A.; Barrios, C.; Dent, R. Trastuzumab Deruxtecan (T-DXd; DS-8201) vs Investigator’s Choice of Chemotherapy in Patients with Hormone Receptor-Positive (HR+), HER2 Low Metastatic Breast Cancer Whose Disease has Progressed on Endocrine Therapy in the Metastatic Setting: A Randomized; SABCS: San Antonio, TX, USA, 2020. [Google Scholar]
- Dokter, W.; Ubink, R.; Van Der Lee, M.; Van Der Vleuten, M.; Van Achterberg, T.; Jacobs, D.; Loosveld, E.; Dobbelsteen, D.V.D.; Egging, D.; Mattaar, E.; et al. Preclinical Profile of the HER2-Targeting ADC SYD983/SYD985: Introduction of a New Duocarmycin-Based Linker-Drug Platform. Mol. Cancer Ther. 2014, 13, 2618–2629. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Barve, M.A.; Bardia, A.; Beeram, M.; Bendell, J.C.; Mosher, R.; Hailman, E.; Bergstrom, D.A.; Burris, H.A.; Soliman, H.H. Phase 1 dose escalation of XMT-1522, a novel HER2-targeting antibody-drug conjugate (ADC), in patients (pts) with HER2-expressing breast, lung and gastric tumors. J. Clin. Oncol. 2018, 36, 2546. [Google Scholar] [CrossRef]
- Alsina, M.; Boni, V.; Schellens, J.H.; Moreno, V.; Bol, K.; Westendorp, M.; Sirulnik, L.A.; Tabernero, J.; Calvo, E. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: Final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC). J. Clin. Oncol. 2017, 35, 2522. [Google Scholar] [CrossRef]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med Oncol. 2019, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Giobbe-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Abstract GS2-06: Phase Ib/II study evaluating safety and efficacy of pembrolizumab and trastuzumab in patients with trastuzumab-resistant HER2-positive metastatic breast cancer: Results from the PANACEA (IBCSG 45-13/BIG 4-13/KEYNOTE-014) study. Gen. Sess. Abstr. 2018, 78, GS2-06. [Google Scholar] [CrossRef]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody–Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.; Jacob, W.; Cejalvo, J.M.; Ceppi, M.; James, I.; Hasmann, M.; Crown, J.; Cervantes, A.; Weisser, M.; Bossenmaier, B. Direct estrogen receptor (ER) / HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PLoS ONE 2017, 12, e0177331. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Hamilton, E.; Loi, S.; Schmid, P.; Darilay, A.; Gao, C.; Patel, G.; Wrona, M.; Andre, F. Trastuzumab Deruxtecan (T-DXd; DS-8201) in Combination with Other Anticancer Agents in Patients with HER2-Low Metastatic Breast Cancer: A Phase 1b, Open-label, Multicenter, Dose-Finding and Dose-Expansion Study (DESTINY-Breast08); SABCS: San Antonio, TX, USA, 2020. [Google Scholar]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Cox, D.; Knudsen, E.S. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 2014, 5, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Wang, Q.; Watt, A.C.; Tolaney, S.M.; Dillon, D.A.; Li, W.; Ramm, S.; Palmer, A.C.; Yuzugullu, H.; Varadan, V.; et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016, 29, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Bisagni, G.; Colleoni, M.; Del Mastro, L.; Zamagni, C.; Mansutti, M.; Zambetti, M.; Frassoldati, A.; De Fato, R.; Valagussa, P.; et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): An exploratory, open-label, phase 2 study. Lancet Oncol. 2018, 19, 249–256. [Google Scholar] [CrossRef]
- Rinnerthaler, G.; Gampenrieder, S.P.; Greil, R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef]
- Diéras, V.; Harbeck, N.; Budd, G.T.; Greenson, J.K.; Guardino, A.E.; Samant, M.; Chernyukhin, N.; Smitt, M.C.; Krop, I.E. Trastuzumab Emtansine in Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer: An Integrated Safety Analysis. J. Clin. Oncol. 2014, 32, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, F.; Ellis, P.; Anton, A.; Wuerstlein, R.; Delaloge, S.; Bonneterre, J.; Quenel-Tueux, N.; Linn, S.C.; Irahara, N.; Donica, M.; et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: Primary results from the KAMILLA study cohort 1. Eur. J. Cancer 2019, 109, 92–102. [Google Scholar] [CrossRef]
- Saliba, F.; Hagipantelli, R.; Misset, J.L.; Bastian, G.; Vassal, G.; Bonnay, M.; Herait, P.; Cote, C.; Mahjoubi, M.; Mignard, D.; et al. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: A prospective assessment. J. Clin. Oncol. 1998, 16, 2745–2751. [Google Scholar] [CrossRef]
- Powell, C.; Camidge; Gemma, A.; Kusumoto, M.; Baba, T.; Kuwano, K.; Bankier, A.; Kiura, K.; Tamura, K.; Modi, S.; et al. Abstract P6-17-06: Characterization, monitoring and management of interstitial lung disease in patients with metastatic breast cancer: Analysis of data available from multiple studies of DS-8201a, a HER2-targeted antibody drug conjugate with a topoisomera. Poster Sess. Abstr. 2019, 79, P6-17. [Google Scholar] [CrossRef]
- Pondé, N.F.; Lambertini, M.; De Azambuja, E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open 2016, 1, e000073. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H. Management and Preparedness for Infusion and Hypersensitivity Reactions. Oncologist 2007, 12, 601–609. [Google Scholar] [CrossRef] [PubMed]

| Author | Study Design | Study Population | N | Treatment | Main Efficacy Results |
|---|---|---|---|---|---|
| Single anti-HER2 agents | |||||
| Fehrenbacher et al. NSABP B-47 [18] | Phase 3, randomized (1:1) trial | High-risk early BC-negative for HER2 by FISH and with IHC 1+ or 2+ | 3270 | Adjuvant ChT with or without trastuzumab | 5-year iDFS: 89.8% vs. 89.2%, HR 0.98; 95% CI, 0.76–1.25; p = 0.85/ OS: 94.8% vs. 96.3%, HR 1.33; 95% CI, 0.90–1.95; p = 0.15 |
| Gianni L et al. [28] | Phase 2, randomized (1:1) trial | HER2-low metastatic BC | 78 | Pertuzumab (420 mg q3w vs. 1050 mg q3w) | CBR (CR + PR + SD at 24 weeks): 9.8% in 420 mg q3w arm vs. 5.4% in 1050 mg q3w arm Median time to progression: 6.1 weeks (both arms) |
| Burris et al. [35] | Phase 2, single-arm trial | HER2-positive metastatic BC (including HER2-low BC after central assessment) | 112 pts (21 HER2-low) | Trastuzumab emtansine (T-DM1) | ORR: 4.8% (95% CI, 1.0–21.8%) vs. 33.8% (95% CI, 23.2–44.9%) Median PFS: 2.6 mo (95% CI, 1.4–3.9 mo) vs. 8.2 mo (95% CI, 4.4 mo to NE) |
| Krop et al. [36] | Phase 2, single-arm study | HER2-positive metastatic BC (including HER2-low BC after retrospective re-evaluation) | 110 pts (15 HER2-low) | Trastuzumab emtansine (T-DM1) | ORR: 20% (95% CI, 5.7–44.9) vs. 41.3% (95% CI 30.4–52.8) Median PFS: 2.8 mo (95% CI 1.3-NE) vs. 7.3 (95% CI, 4.6–12.3) |
| Modi et al. [24] | Phase 1, dose-expansion study | HER2-low BC refractory to standard therapies | 54 | Trastuzumab–deruxtecan (T-DXd) (DS8201a) | ORR: 37% (95% CI, 24.3–51.3%) Median DoR: 10.4 mo (95% CI, 8.8 mo-NE) |
| Banerji et al. [25] | Phase 1 dose-expansion study | Advanced BC, gastric, urothelial, or endometrial cancer with at least HER2 IHC 1+ | 146 (47 HER2-low BC) | Trastuzumab duocarmazine (SYD985) | ORR: 28% (95% CI, 13.8–46.8%) in HR+ HER2-low BC, 40% (95% CI, 16.3–67.6%) in HR- HER2-low BC |
| Combination therapies | |||||
| Hickerson et al. [29] | Phase II, randomized (1:1), blinded, placebo-controlled | Node-positive (or negative if HR-negative) HER2-low BC patients after standard adjuvant therapy | 275 | Nelipepimut-S + trastuzumab vs. placebo + GM-CSF + trastuzumab | 24-month DFS rate: 89.9% in the vaccine arm vs. 83.8% in the control arm (HR = 0.62; 95% CI = 0.31–1.25; p = 0.18); 24-month DFS rate in the subgroup of TNBC: 92.6% vs. 70.2%, respectively (HR = 0.26; 95% CI = 0.08–0.81; p = 0.013) |
| Gianni et al. [37] | Phase II, multicenter, multicohort trial | Cohort C: HR-positive/HER2-low early BC | 23 | Trastuzumab + pertuzumab + fulvestrant + palbociclib | Baseline mean Ki67: 32.4% Mean Ki67 at week 2: 2.6% (mean a change of −29.5; p < 0.001) Mean Ki67 at surgery: 7.5% (mean change of −19.3; p < 0.001) |
| Pistilli et al. [26] | Phase 2 study | ER+/HER2-low metastatic BC refractory to ET/CDK4/6i | 50 | Zenocutuzumab (MCLA-128) + ET | CBR (CR + PR + SD at 24 weeks): 16.7% (90% CI 8.6–28.1) |
| Hamilton et al. [38] | 2-part, phase 1b study | Cohort 2: HER2-low BC after standard therapy | 16 | Trastuzumab–deruxtecan + nivolumab | Confirmed ORR by independent central review: 38% (95% CI, 15–65); DoR not evaluable |
| Antibody-Drug Conjugate | T-DM1 | SYD-986 | T-Dxd |
|---|---|---|---|
| HER2 targeting vehicle | Trastuzumab | Trastuzumab | Trastuzumab |
| Linker | Non-cleavable | Cleavable | Cleavable |
| Drug–antibody ratio | 3.5:1 | 2.8:1 | 8:1 |
| Cytotoxic moiety | Maytansine derivative | Seco-DUBA | Exatecan derivative |
| Cytotoxic moiety MoA | Antimicrotubule (mitotic poison) | Alkylating agent | Topoisomerase I inhibitor |
| Diffusible cytotoxic moiety? |  |  |  |
| Bystander killing effect? |  |  |  |
| Targets HER2-positive or homogenous tumors? |  |  |  |
| Targets HER2-low or heterogeneous tumors? |  |  |  |
| Drugs Tested | Study Design | Patient Population | Primary Endpoint | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| Trastuzumab–deruxtecan + pembrolizumab | Phase Ib, open-label, two-part, multicenter, nonrandomized, multiple-dose | Advanced BC (HER2-positive and HER2-low) and HER2-positive NSCLC | Phase I: MTD Phase II: ORR | Recruiting | NCT04042701 |
| Trastuzumab–deruxtecan + nivolumab | Phase Ib, multicenter, two-part, open-label | Advanced BC (HER2-positive and HER2-low) and urothelial cancer | Phase I: MTD Phase II: ORR | Ongoing | NCT03523572 |
| Trastuzumab–deruxtecan + durvalumab | Phase Ib/II, two-stage, open-label, multicenter | Arm 6: Advanced TNBC with low HER2 expression | Safety | Recruiting | NCT03742102 |
| Trastuzumab–deruxtecan + durvalumab + paclitaxel | Phase Ib, open-label, modular, dose-finding and dose-expansion | Module 2: advanced HER2-low BC | Safety and tolerability | Not yet recruiting | NCT04556773 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiger, D.; Agostinetto, E.; Saúde-Conde, R.; de Azambuja, E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers 2021, 13, 1015. https://doi.org/10.3390/cancers13051015
Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers. 2021; 13(5):1015. https://doi.org/10.3390/cancers13051015
Chicago/Turabian StyleEiger, Daniel, Elisa Agostinetto, Rita Saúde-Conde, and Evandro de Azambuja. 2021. "The Exciting New Field of HER2-Low Breast Cancer Treatment" Cancers 13, no. 5: 1015. https://doi.org/10.3390/cancers13051015
APA StyleEiger, D., Agostinetto, E., Saúde-Conde, R., & de Azambuja, E. (2021). The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers, 13(5), 1015. https://doi.org/10.3390/cancers13051015






