Fertility-Sparing Surgery in Gynecologic Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Cervical Cancer
1.2. Ovarian Cancer
1.3. Endometrial Cancer
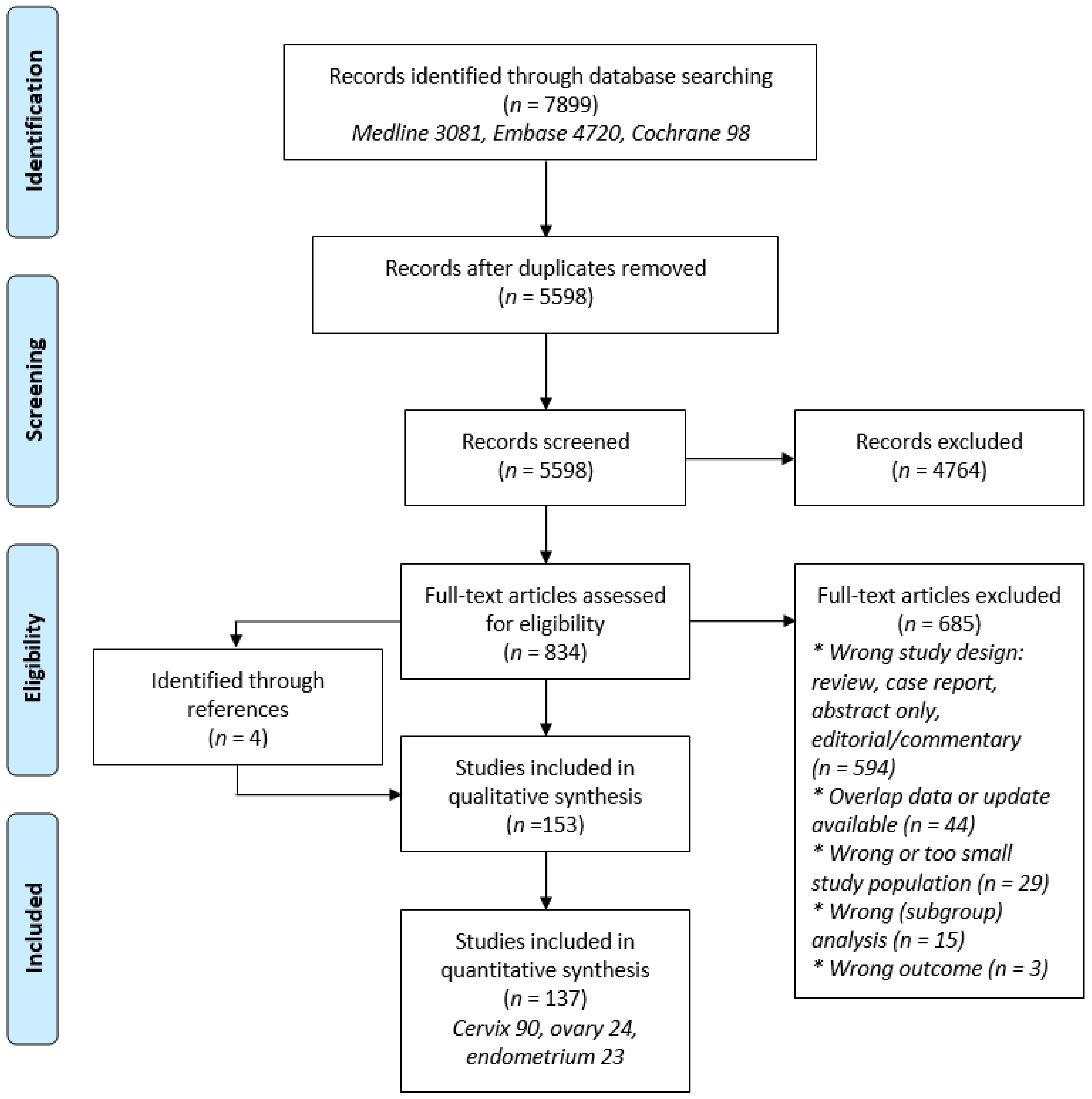
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis and Analysis
3. Results
3.1. Study Characteristics
3.2. Patient Characteristics and Treatment Modalities
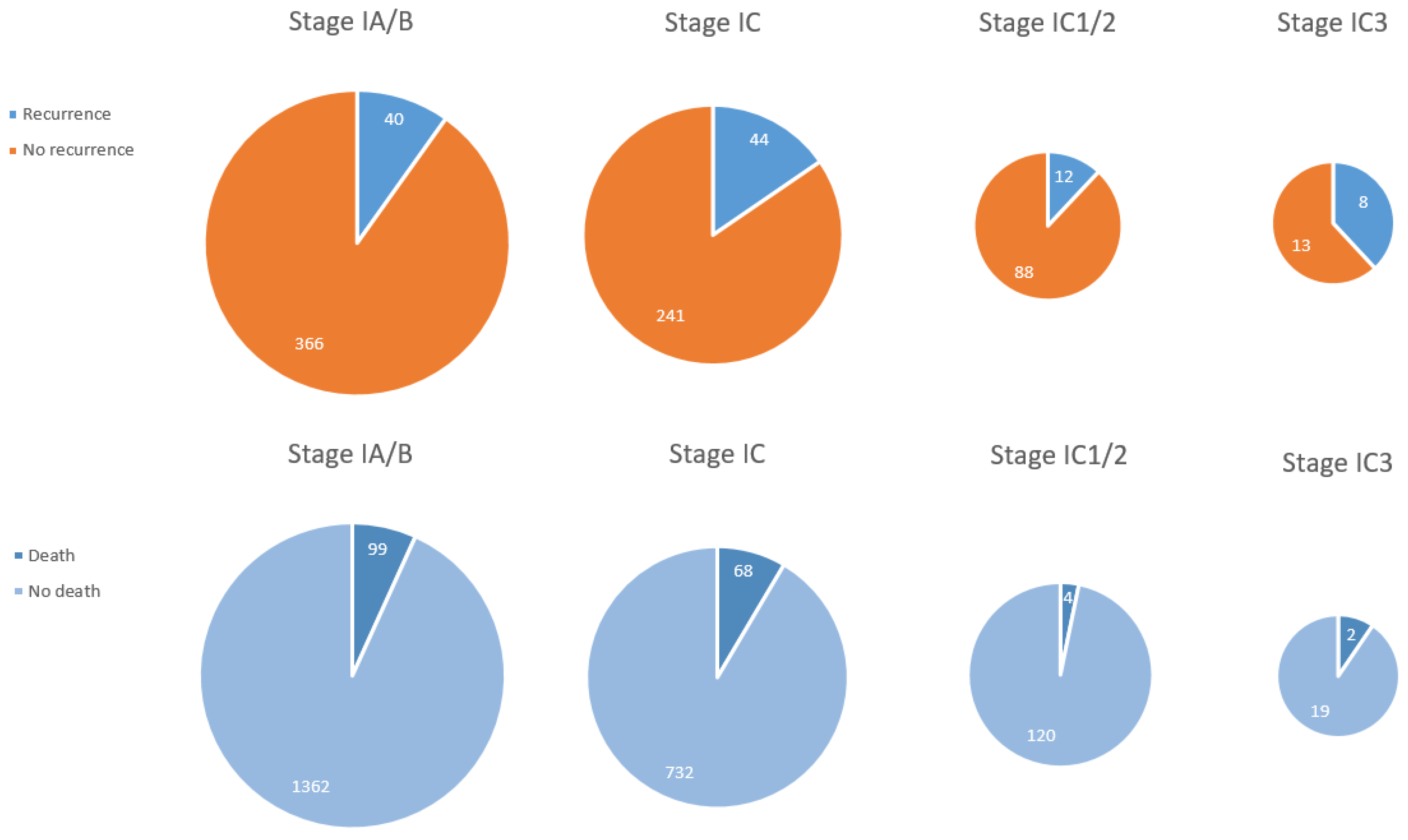
3.3. Oncological Outcome
3.4. Reproductive Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, International Agency for Research on Cancer (IARC), Global Cancer Observatory (GCO). Available online: https://gco.iarc.fr (accessed on 17 November 2020).
- The Netherlands Cancer Registry, the Netherlands Comprehensive Cancer Organisation (IKNL). Available online: https://www.iknl.nl/netherlands-cancer-registry (accessed on 17 November 2020).
- Moser, E.C.; Meunier, F. Cancer survivorship: A positive side-effect of more successful cancer treatment. EJC Suppl. 2014, 12, 1–4. [Google Scholar] [CrossRef]
- Duffy, C.; Allen, S. Medical and psychosocial aspects of fertility after cancer. Cancer J. 2009, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Iavazzo, C.; Fotiou, A.; Psomiadou, V.; Douligeris, A.; Vorgias, G.; Kalinoglou, N. Short- and long term outcomes after abdominal radical trachelectomy versus radical hysterectomy for early stage cervical cancer: A systematic review of the literature and meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Maulard, A.; Pautier, P.; Chargari, C.; Gouy, S.; Morice, P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: A systematic review of the literature. Fertil. Steril. 2016, 106, 1195–1211.e5. [Google Scholar] [CrossRef] [PubMed]
- Plante, M. Bulky Early-Stage Cervical Cancer (2-4 cm Lesions): Upfront Radical Trachelectomy or Neoadjuvant Chemotherapy Followed by Fertility-Preserving Surgery: Which Is the Best Option? Int. J. Gynecol. Cancer 2015, 25, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.Y.; Yang, J.X.; Wu, X.H.; Chen, Y.L.; Li, L.; Liu, K.J.; Cui, M.H.; Xie, X.; Wu, Y.M.; Kong, B.H.; et al. Comparisons of vaginal and abdominal radical trachelectomy for early-stage cervical cancer: Preliminary results of a multi-center research in China. Br. J. Cancer 2013, 109, 2778–2782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laios, A.; Kasius, J.; Tranoulis, A.; Gryparis, A.; Ind, T. Obstetric Outcomes in Women With Early Bulky Cervical Cancer Downstaged by Neoadjuvant Chemotherapy to Allow for Fertility-Sparing Surgery: A Meta-analysis and Metaregression. Int. J. Gynecol. Cancer 2018, 28, 794–801. [Google Scholar] [CrossRef]
- Plante, M.; van Trommel, N.; Lheureux, S.; Oza, A.M.; Wang, L.; Sikorska, K.; Ferguson, S.E.; Han, K.; Amant, F. FIGO 2018 stage IB2 (2-4 cm) Cervical cancer treated with Neo-adjuvant chemotherapy followed by fertility Sparing Surgery (CONTESSA); Neo-Adjuvant Chemotherapy and Conservative Surgery in Cervical Cancer to Preserve Fertility (NEOCON-F). A PMHC, DGOG, GCIG/CCRN and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Epithelial Ovarian Cancer. Dutch National Guideline, Version 2.3. Available online: www.oncoline.nl (accessed on 17 November 2020).
- Bentivegna, E.; Gouy, S.; Maulard, A.; Pautier, P.; Leary, A.; Colombo, N.; Morice, P. Fertility-sparing surgery in epithelial ovarian cancer: A systematic review of oncological issues. Ann. Oncol. 2016, 27, 1994–2004. [Google Scholar] [CrossRef]
- Liu, D.C.J.; Gao, A.; Wang, Z.; Cai, L. Fertility sparing surgery vs radical surgery for epithelial ovarian cancer: A meta-analysis of overall survival and disease-free survival. BMC Cancer 2020, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, K.R.; Kim, Y.T.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Kim, S.M.; et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur. J. Cancer 2013, 49, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.C.; Fader, A.N.; Carson, K.A.; Bristow, R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol. Oncol. 2012, 125, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Gershenson, D.M. Fertility-sparing therapy for young women with endometrial cancer. Expert Rev. Anticancer Ther. 2006, 6, 27–32. [Google Scholar] [CrossRef]
- Wells, G.A.; O’Connell, B.S.D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 November 2020).
- Abu-Rustum, N.R.; Sonoda, Y. Fertility-sparing surgery in early-stage cervical cancer: Indications and applications. J. Natl. Compr. Cancer Netw 2010, 8, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Tohma, Y.A.; Sahin, H.; Kocaman, E.; Tunc, M.; Haberal, A.N. Oncological and obstetric outcomes after fertility-sparing radical abdominal trachelectomy for early stage cervical cancer: A tertiary centre’s 10 years’ experience. J. Obstet. Gynaecol. 2019, 39, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Lecuru, F.; Magaud, L.; Ngo, C.; Huchon, C.; Bats, A.S.; Mathevet, P. Perioperative morbidity of radical trachelectomy with lymphadenectomy in early-stage cervical cancer: A French prospective multicentric cohort. J. Gynecol. Oncol. 2019, 30, e34. [Google Scholar] [CrossRef]
- Beiner, M.E.; Hauspy, J.; Rosen, B.; Murphy, J.; Laframboise, S.; Nofech-Mozes, S.; Ismiil, N.; Rasty, G.; Khalifa, M.A.; Covens, A. Radical vaginal trachelectomy vs. radical hysterectomy for small early stage cervical cancer: A matched case-control study. Gynecol. Oncol. 2008, 110, 168–171. [Google Scholar] [CrossRef]
- Bekkers, R.L.; Keyser, K.G.; Bulten, J.; Hanselaar, A.G.; Schijf, C.P.; Boonstra, H.; Massuger, L.F. The value of loop electrosurgical conization in the treatment of stage IA1 microinvasive carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 2002, 12, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, M.; Barrett, J.; Seaward, G.; Covens, A. Pregnancy outcomes in patients after radical trachelectomy. Am. J. Obstet. Gynecol. 2003, 189, 1378–1382. [Google Scholar] [CrossRef]
- Biliatis, I.K.A.; Patel, A.; Ratnavelu, N.; Cross, P.; Chattopadhyay, S.; Galaal, K.; Naik, R. Small volume stage 1B1 cervical cancer: Is radical surgery still necessary? Gynecol. Oncol. 2012, 126, 73–77. [Google Scholar] [CrossRef]
- Bisseling, K.C.; Bekkers, R.L.; Rome, R.M.; Quinn, M.A. Treatment of microinvasive adenocarcinoma of the uterine cervix: A retrospective study and review of the literature. Gynecol. Oncol. 2007, 107, 424–430. [Google Scholar] [CrossRef]
- Bogani, G.; Chiappa, V.; Vinti, D.; Somigliana, E.; Filippi, F.; Murru, G.; Murgia, F.; Martinelli, F.; Ditto, A.; Raspagliesi, F. Long-term results of fertility-sparing treatment for early-stage cervical cancer. Gynecol. Oncol. 2019, 154, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bratila, E.; Bratila, C.P.; Coroleuca, C.B. Radical Vaginal Trachelectomy with Laparoscopic Pelvic Lymphadenectomy for Fertility Preservation in Young Women with Early-Stage Cervical Cancer. Indian J. Surg. 2016, 78, 265–270. [Google Scholar] [CrossRef]
- Capilna, M.E.; Ioanid, N.; Scripcariu, V.; Gavrilescu, M.M.; Szabo, B. Abdominal radical trachelectomy: A Romanian series. Int. J. Gynecol. Cancer 2014, 24, 615–619. [Google Scholar] [CrossRef]
- Chernyshova, A.; Kolomiets, L.; Chekalkin, T.; Chernov, V.; Sinilkin, I.; Gunther, V.; Marchenko, E.; Baigonakova, G.; Kang, J.H. Fertility-Sparing Surgery Using Knitted TiNi Mesh Implants and Sentinel Lymph Nodes: A 10-Year Experience. J. Investig. Surg. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jung, S.G.; Park, H.; Lee, S.Y.; Lee, C.; Hwang, Y.Y.; Kim, S.J. Fertility preservation by photodynamic therapy combined with conization in young patients with early stage cervical cancer: A pilot study. Photodiagnosis Photodyn. Ther. 2014, 11, 420–425. [Google Scholar] [CrossRef]
- Cui, R.R.; Chen, L.; Tergas, A.I.; Hou, J.Y.; St Clair, C.M.; Neugut, A.I.; Ananth, C.V.; Hershman, D.L.; Wright, J.D. Trends in Use and Survival Associated With Fertility-Sparing Trachelectomy for Young Women With Early-Stage Cervical Cancer. Obstet. Gynecol. 2018, 131, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Dargent, D.; Martin, X.; Sacchetoni, A.; Mathevet, P. Laparoscopic vaginal radical trachelectomy: A treatment to preserve the fertility of cervical carcinoma patients. Cancer 2000, 88, 1877–1882. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, Y.; Li, D.; Zhang, X.; Guo, H.; Wang, F.; Sheng, X. Abdominal radical trachelectomy guided by sentinel lymph node biopsy for stage IB1 cervical cancer with tumors >2 cm. Oncotarget 2017, 8, 3422–3429. [Google Scholar] [CrossRef]
- Diaz, J.P.; Sonoda, Y.; Leitao, M.M.; Zivanovic, O.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Oncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol. Oncol. 2008, 111, 255–260. [Google Scholar] [CrossRef]
- Du, X.L.; Sheng, X.G.; Jiang, T.; Li, Q.S.; Yu, H.; Pan, C.X.; Lu, C.H.; Wang, C.; Song, Q.Q. Sentinel lymph node biopsy as guidance for radical trachelectomy in young patients with early stage cervical cancer. BMC Cancer 2011, 11, 157. [Google Scholar] [CrossRef]
- Ebisawa, K.; Takano, M.; Fukuda, M.; Fujiwara, K.; Hada, T.; Ota, Y.; Kurotsuchi, S.; Kanao, H.; Andou, M. Obstetric outcomes of patients undergoing total laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol. Oncol. 2013, 131, 83–86. [Google Scholar] [CrossRef]
- Fanfani, F.; Landoni, F.; Gagliardi, M.L.; Fagotti, A.; Preti, E.; Moruzzi, M.C.; Monterossi, G.; Scambia, G. Sexual and Reproductive Outcomes in Early Stage Cervical Cancer Patients after Excisional Cone as a Fertility-sparing Surgery: An Italian Experience. J. Reprod. Infertil. 2014, 15, 29–34. [Google Scholar]
- Van Gent, M.D.; van den Haak, L.W.; Gaarenstroom, K.N.; Peters, A.A.; van Poelgeest, M.I.; Trimbos, J.B.; de Kroon, C.D. Nerve-sparing radical abdominal trachelectomy versus nerve-sparing radical hysterectomy in early-stage (FIGO IA2-IB) cervical cancer: A comparative study on feasibility and outcome. Int. J. Gynecol. Cancer 2014, 24, 735–743. [Google Scholar] [CrossRef]
- Gil-Ibanez, B.; Glickman, A.; Del Pino, M.; Boada, D.; Fuste, P.; Diaz-Feijoo, B.; Pahisa, J.; Torne, A. Vaginal fertility-sparing surgery and laparoscopic sentinel lymph node detection in early cervical cancer. Retrospective study with 15 years of follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 23–27. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Chen, X.; Sun, L.; Chen, K.; Sheng, X. Surgical and Oncologic Outcomes of Radical Abdominal Trachelectomy Versus Hysterectomy for Stage IA2-IB1 Cervical Cancer. J. Minim Invasive Gynecol. 2019, 26, 484–491. [Google Scholar] [CrossRef]
- Hauerberg, L.; Hogdall, C.; Loft, A.; Ottosen, C.; Bjoern, S.F.; Mosgaard, B.J.; Nedergaard, L.; Lajer, H. Vaginal Radical Trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol. Oncol. 2015, 138, 304–310. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.M.; Zhao, Q.; Wang, T.; Wang, Y.; Kong, W.M.; Song, F.; Duan, W.; Zhu, L.; Zhang, W.Y. Clinical value of cold knife conization as conservative management in patients with microinvasive cervical squamous cell cancer (stage IA1). Int. J. Gynecol. Cancer 2014, 24, 1306–1311. [Google Scholar] [CrossRef]
- Helpman, L.; Grisaru, D.; Covens, A. Early adenocarcinoma of the cervix: Is radical vaginal trachelectomy safe? Gynecol. Oncol. 2011, 123, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Hertel, H.; Kohler, C.; Grund, D.; Hillemanns, P.; Possover, M.; Michels, W.; Schneider, A.; German Association of Gynecologic, O. Radical vaginal trachelectomy (RVT) combined with laparoscopic pelvic lymphadenectomy: Prospective multicenter study of 100 patients with early cervical cancer. Gynecol. Oncol. 2006, 103, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Johansen, G.; Lonnerfors, C.; Falconer, H.; Persson, J. Reproductive and oncologic outcome following robot-assisted laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol. Oncol. 2016, 141, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, Y.; Nishio, H.; Miyakoshi, K.; Sato, S.; Sugiyama, J.; Matsumoto, T.; Tanaka, K.; Ochiai, D.; Minegishi, K.; Hamatani, T.; et al. Pregnancy Outcomes After Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer: A 13-Year Experience in a Single Tertiary-Care Center. Int. J. Gynecol. Cancer 2016, 26, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.Y.; Kim, D.Y.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Fertility-sparing laparoscopic radical trachelectomy for young women with early stage cervical cancer. BJOG 2010, 117, 340–347. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, C.H.; Lim, M.C.; Chang, S.J.; Kim, Y.B.; Kim, M.A.; Kim, T.J.; Park, S.Y.; Kim, B.G.; Song, Y.S.; et al. Safe criteria for less radical trachelectomy in patients with early-stage cervical cancer: A multicenter clinicopathologic study. Ann. Surg. Oncol. 2012, 19, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ishioka, S.; Endo, T.; Baba, T.; Mizuuchi, M.; Takada, S.; Saito, T. Possibility of less radical treatment for patients with early invasive uterine cervical cancer. J. Obstet. Gynaecol. Res 2016, 42, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Abu-Rustum, N.R.; Chi, D.S.; Gardner, G.J.; Leitao, M.M., Jr.; Carter, J.; Barakat, R.R.; Sonoda, Y. Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol. Oncol. 2012, 125, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Lanowska, M.; Mangler, M.; Speiser, D.; Bockholdt, C.; Schneider, A.; Kohler, C.; Vasiljeva, J.; Al-Hakeem, M.; Vercellino, G.F. Radical vaginal trachelectomy after laparoscopic staging and neoadjuvant chemotherapy in women with early-stage cervical cancer over 2 cm: Oncologic, fertility, and neonatal outcome in a series of 20 patients. Int. J. Gynecol. Cancer 2014, 24, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, W.Y.; Lee, J.W.; Kim, H.S.; Choi, Y.L.; Ahn, G.H.; Lee, J.H.; Kim, B.G.; Bae, D.S. Conization using electrosurgical conization and cold coagulation for international federation of gynecology and obstetrics stage IA1 squamous cell carcinomas of the uterine cervix. Int. J. Gynecol. Cancer 2009, 19, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.M.; Son, W.S.; You, H.J.; Kim, D.Y.; Kim, J.H.; Kim, Y.T.; Nam, J.H. The efficacy of conservative management after conization in patients with stage IA1 microinvasive cervical carcinoma. Acta Obstet. Gynecol. Scand 2009, 88, 209–215. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, Y.L.; Chiang, Y.C.; Cheng, C.Y.; Lai, Y.L.; Tai, Y.J.; Hsu, H.C.; Hwa, H.L.; Cheng, W.F. Outcome and Subsequent Pregnancy after Fertility-Sparing Surgery of Early-Stage Cervical Cancers. Int. J. Environ. Res. Public Health 2020, 17, 7103. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, H.; Zang, R.; Zhou, Y.; Ju, X.; Ke, G.; Wu, X. Radical abdominal trachelectomy for cervical malignancies: Surgical, oncological and fertility outcomes in 62 patients. Gynecol. Oncol. 2011, 121, 565–570. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Jiang, Z.; Xia, L.; Ju, X.; Chen, X.; Wu, X. Oncological results and recurrent risk factors following abdominal radical trachelectomy: An updated series of 333 patients. BJOG 2019, 126, 1169–1174. [Google Scholar] [CrossRef]
- Li, X.; Xia, L.; Chen, X.; Fu, Y.; Wu, X. Simple conization and pelvic lymphadenectomy in early-stage cervical cancer: A retrospective analysis and review of the literature. Gynecol. Oncol. 2020, 158, 231–235. [Google Scholar] [CrossRef]
- Lindsay, R.B.K.; Shanbhag, S.; Tolhurst, J.; Millan, D.; Siddiqui, N. Fertility conserving management of early cervical cancer: Our experience of LLETZ and pelvic lymph node dissection. Int. J. Gynecol. Cancer 2014, 24, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lintner, B.; Saso, S.; Tarnai, L.; Novak, Z.; Palfalvi, L.; Del Priore, G.; Smith, J.R.; Ungar, L. Use of abdominal radical trachelectomy to treat cervical cancer greater than 2 cm in diameter. Int. J. Gynecol. Cancer 2013, 23, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Z.; Xiao, M.; Liu, C.; Zhang, Z. The Surgical Morbidity and Oncological Outcome of Total Laparoscopic Radical Trachelectomy Versus Total Laparoscopic Radical Hysterectomy For Early Stage Cervical Cancer: A Retrospective Study With 11-Year Follow-Up. Onco. Targets Ther. 2019, 12, 7941–7947. [Google Scholar] [CrossRef]
- Ma, L.K.; Cao, D.Y.; Yang, J.X.; Liu, J.T.; Shen, K.; Lang, J.H. Pregnancy outcome and obstetric management after vaginal radical trachelectomy. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3019–3024. [Google Scholar] [PubMed]
- Machida, H.; Iwata, T.; Okugawa, K.; Matsuo, K.; Saito, T.; Tanaka, K.; Morishige, K.; Kobayashi, H.; Yoshino, K.; Tokunaga, H.; et al. Fertility-sparing trachelectomy for early-stage cervical cancer: A proposal of an ideal candidate. Gynecol. Oncol. 2020, 156, 341–348. [Google Scholar] [CrossRef]
- Machida, H.; Mandelbaum, R.S.; Mikami, M.; Enomoto, T.; Sonoda, Y.; Grubbs, B.H.; Paulson, R.J.; Roman, L.D.; Wright, J.D.; Matsuo, K. Characteristics and outcomes of reproductive-aged women with early-stage cervical cancer: Trachelectomy vs hysterectomy. Am. J. Obstet. Gynecol. 2018, 219, 461.e1–461.e18. [Google Scholar] [CrossRef]
- Malmsten, C.; Hellberg, P.; Bergmark, K.; Dahm-Kahler, P. Long-term fertility, oncological, and quality-of-life outcomes after trachelectomy in early stage cervical cancer. Arch. Gynecol. Obstet. 2019, 299, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Maneo, A.; Sideri, M.; Scambia, G.; Boveri, S.; Dell’anna, T.; Villa, M.; Parma, G.; Fagotti, A.; Fanfani, F.; Landoni, F. Simple conization and lymphadenectomy for the conservative treatment of stage IB1 cervical cancer. An Italian experience. Gynecol. Oncol. 2011, 123, 557–560. [Google Scholar] [CrossRef]
- Maneo, A.; Chiari, S.; Bonazzi, C.; Mangioni, C. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecol. Oncol. 2008, 111, 438–443. [Google Scholar] [CrossRef]
- Mangler, M.; Lanowska, M.; Kohler, C.; Vercellino, F.; Schneider, A.; Speiser, D. Pattern of cancer recurrence in 320 patients after radical vaginal trachelectomy. Int. J. Gynecol. Cancer 2014, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Marchiole, P.; Benchaib, M.; Buenerd, A.; Lazlo, E.; Dargent, D.; Mathevet, P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): A comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH). Gynecol. Oncol. 2007, 106, 132–141. [Google Scholar] [CrossRef]
- Matsuo, K.; Chen, L.; Mandelbaum, R.S.; Melamed, A.; Roman, L.D.; Wright, J.D. Trachelectomy for reproductive-aged women with early-stage cervical cancer: Minimally invasive surgery versus laparotomy. Am. J. Obstet. Gynecol. 2019, 220, 469.e1–469.e13. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Mikami, M.; Enomoto, T.; Roman, L.D.; Wright, J.D. Trachelectomy for stage IB1 cervical cancer with tumor size >2 cm: Trends and characteristics in the United States. J Gynecol. Oncol. 2018, 29, e85. [Google Scholar] [CrossRef]
- Muraji, M.; Sudo, T.; Nakagawa, E.; Ueno, S.; Wakahashi, S.; Kanayama, S.; Yamada, T.; Yamaguchi, S.; Fujiwara, K.; Nishimura, R. Type II versus type III fertility-sparing abdominal radical trachelectomy for early-stage cervical cancer: A comparison of feasibility of surgical outcomes. Int. J. Gynecol. Cancer 2012, 22, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Kasuga, A.; Hara-Yamashita, A.; Ikeda, Y.; Asai-Sato, M.; Nakao, T.; Hayashi, C.; Takeya, C.; Adachi, K.; Tsuruga, T.; et al. Reconstructed uterine length is critical for the prevention of cervical stenosis following abdominal trachelectomy in cervical cancer patients. J. Obstet. Gynaecol. Res. 2020, 46, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Nick, A.M.; Frumovitz, M.M.; Soliman, P.T.; Schmeler, K.M.; Ramirez, P.T. Fertility sparing surgery for treatment of early-stage cervical cancer: Open vs. robotic radical trachelectomy. Gynecol. Oncol. 2012, 124, 276–280. [Google Scholar] [CrossRef]
- Nishio, H.; Fujii, T.; Sugiyama, J.; Kuji, N.; Tanaka, M.; Hamatani, T.; Miyakoshi, K.; Minegishi, K.; Tsuda, H.; Iwata, T.; et al. Reproductive and obstetric outcomes after radical abdominal trachelectomy for early-stage cervical cancer in a series of 31 pregnancies. Hum. Reprod. 2013, 28, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, K.; Yahata, H.; Sonoda, K.; Kodama, K.; Yagi, H.; Ohgami, T.; Yasunaga, M.; Onoyama, I.; Kaneki, E.; Asanoma, K.; et al. Evaluation of adjuvant chemotherapy after abdominal trachelectomy for cervical cancer: A single-institution experience. Int. J. Clin. Oncol. 2020, 26, 216–224. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Reproductive outcomes after laparoscopic radical trachelectomy for early-stage cervical cancer. J Gynecol. Oncol. 2014, 25, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Joo, W.D.; Chang, S.J.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Long-term outcomes after fertility-sparing laparoscopic radical trachelectomy in young women with early-stage cervical cancer: An Asan Gynecologic Cancer Group (AGCG) study. J. Surg. Oncol. 2014, 110, 252–257. [Google Scholar] [CrossRef]
- Plante, M.; Gregoire, J.; Renaud, M.C.; Roy, M. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol. Oncol. 2011, 121, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Renaud, M.C.; Sebastianelli, A.; Gregoire, J. Simple vaginal trachelectomy in women with early-stage low-risk cervical cancer who wish to preserve fertility: The new standard of care? Int. J. Gynecol. Cancer 2020, 30, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.K.; Papadopoulos, A.J.; Montalto, S.A.; Coutts, M.; Culora, G.; Kodampur, M.; Mehra, G.; Devaja, O. Fertility-sparing surgery for early cervical cancer-approach to less radical surgery. Int. J. Gynecol. Cancer 2012, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Renaud, M.C.; Plante, M.; Roy, M. Combined laparoscopic and vaginal radical surgery in cervical cancer. Gynecol. Oncol. 2000, 79, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Rendon, G.J.; Lopez Blanco, A.; Aragona, A.; Saadi, J.M.; Di Guilmi, J.; Arab Eblen, C.; Heredia Munoz, F.; Pareja, R. Oncological and obstetrical outcomes after neo-adjuvant chemotherapy followed by fertility-sparing surgery in patients with cervical cancer >/=2 cm. Int. J. Gynecol. Cancer 2020, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Pluta, M.; Strnad, P.; Hrehorcak, M.; Chmel, R.; Skapa, P.; Robova, H. A less radical treatment option to the fertility-sparing radical trachelectomy in patients with stage I cervical cancer. Gynecol. Oncol. 2008, 111 (Suppl. 2), S116–S120. [Google Scholar] [CrossRef] [PubMed]
- Robova, H.; Halaska, M.J.; Pluta, M.; Skapa, P.; Matecha, J.; Lisy, J.; Rob, L. Oncological and pregnancy outcomes after high-dose density neoadjuvant chemotherapy and fertility-sparing surgery in cervical cancer. Gynecol. Oncol. 2014, 135, 213–216. [Google Scholar] [CrossRef]
- Saadi, J.; Minig, L.; Noll, F.; Saraniti, G.; Cardenas-Rebollo, J.M.; Perrotta, M. Four Surgical Approaches to Cervical Excision during Laparoscopic Radical Trachelectomy for Early Cervical Cancer. J. Minim Invasive Gynecol. 2017, 24, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Saso, S.; Ghaem-Maghami, S.; Chatterjee, J.; Naji, O.; Farthing, A.; Mason, P.; McIndoe, A.; Hird, V.; Ungar, L.; Del Priore, G.; et al. Abdominal radical trachelectomy in West London. BJOG 2012, 119, 187–193. [Google Scholar] [CrossRef]
- Shah, J.S.; Jooya, N.D.; Woodard, T.L.; Ramirez, P.T.; Fleming, N.D.; Frumovitz, M. Reproductive counseling and pregnancy outcomes after radical trachelectomy for early stage cervical cancer. J Gynecol. Oncol. 2019, 30, e45. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H. Challenging dogma: Radical conservation surgery for early stage cervical cancer in order to retain fertility. Ann. R Coll. Surg. Engl. 2009, 91, 181–187. [Google Scholar] [CrossRef]
- Shinkai, S.; Ishioka, S.; Mariya, T.; Fujibe, Y.; Kim, M.; Someya, M.; Saito, T. Pregnancies after vaginal radical trachelectomy (RT) in patients with early invasive uterine cervical cancer: Results from a single institute. BMC Pregnancy Childbirth 2020, 20, 248. [Google Scholar] [CrossRef]
- Slama, J.; Cerny, A.; Dusek, L.; Fischerova, D.; Zikan, M.; Kocian, R.; Germanova, A.; Cibula, D. Results of less radical fertility-sparing procedures with omitted parametrectomy for cervical cancer: 5years of experience. Gynecol. Oncol. 2016, 142, 401–404. [Google Scholar] [CrossRef]
- Sonoda, Y.; Chi, D.S.; Carter, J.; Barakat, R.R.; Abu-Rustum, N.R. Initial experience with Dargent’s operation: The radical vaginal trachelectomy. Gynecol. Oncol. 2008, 108, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Sopracordevole, F.; Chiossi, G.; Barbero, M.; Cristoforoni, P.; Ghiringhello, B.; Frega, A.; Tortolani, F.; Boselli, F.; Clemente, N.; Ciavattini, A.; et al. Surgical approach and long-term clinical outcome in women with microinvasive cervical cancer. Anticancer Res. 2014, 34, 4345–4349. [Google Scholar]
- Speiser, D.; Mangler, M.; Kohler, C.; Hasenbein, K.; Hertel, H.; Chiantera, V.; Gottschalk, E.; Lanowska, M. Fertility outcome after radical vaginal trachelectomy: A prospective study of 212 patients. Int. J. Gynecol. Cancer 2011, 21, 1635–1639. [Google Scholar] [CrossRef]
- Spoozak, L.; Lewin, S.N.; Burke, W.M.; Deutsch, I.; Sun, X.; Herzog, T.J.; Wright, J.D. Microinvasive adenocarcinoma of the cervix. Am. J. Obstet. Gynecol. 2012, 206, 80.e1–80.e6. [Google Scholar] [CrossRef]
- Tamauchi, S.; Kajiyama, H.; Sakata, J.; Sekiya, R.; Suzuki, S.; Mizuno, M.; Utsumi, F.; Niimi, K.; Kotani, T.; Shibata, K.; et al. Oncologic and obstetric outcomes of early stage cervical cancer with abdominal radical trachelectomy: Single-institution experience. J. Obstet. Gynaecol. Res. 2016, 42, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Ramirez, P.T.; Ferreyra, H.; Saadi, J.; Franco, G.; Goldsman, M.; Perrotta, M. Abdominal radical trachelectomy: A safe and feasible option for fertility preservation in developing countries. J. Low Genit. Tract. Dis. 2013, 17, 378–384. [Google Scholar] [CrossRef]
- Tokunaga, H.; Watanabe, Y.; Niikura, H.; Nagase, S.; Toyoshima, M.; Shiro, R.; Yokoyama, Y.; Mizunuma, H.; Ohta, T.; Nishiyama, H.; et al. Outcomes of abdominal radical trachelectomy: Results of a multicenter prospective cohort study in a Tohoku Gynecologic Cancer Unit. Int. J. Clin. Oncol. 2015, 20, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Tomao, F.; Maruccio, M.; Preti, E.P.; Boveri, S.; Ricciardi, E.; Zanagnolo, V.; Landoni, F. Conization in Early Stage Cervical Cancer: Pattern of Recurrence in a 10-Year Single-Institution Experience. Int. J. Gynecol. Cancer 2017, 27, 1001–1008. [Google Scholar] [CrossRef]
- Tseng, J.H.; Aloisi, A.; Sonoda, Y.; Gardner, G.J.; Zivanovic, O.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Long-Term Oncologic Outcomes of Uterine-Preserving Surgery in Young Women With Stage Ib1 Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Uzan, C.; Gouy, S.; Desroque, D.; Pomel, C.; Duvillard, P.; Balleyguier, C.; Haie-Meder, C.; Morice, P. Analysis of a continuous series of 34 young patients with early-stage cervical cancer selected for a vaginal radical trachelectomy: Should “staging” conization be systematically performed before this procedure? Int. J. Gynecol. Cancer 2013, 23, 331–336. [Google Scholar] [CrossRef]
- Vieira, M.A.; Rendon, G.J.; Munsell, M.; Echeverri, L.; Frumovitz, M.; Schmeler, K.M.; Pareja, R.; Escobar, P.F.; Reis, R.D.; Ramirez, P.T. Radical trachelectomy in early-stage cervical cancer: A comparison of laparotomy and minimally invasive surgery. Gynecol. Oncol. 2015, 138, 585–589. [Google Scholar] [CrossRef]
- Wang, A.; Cui, G.; Jin, C.; Wang, Y.; Tian, X. Multicenter research on tumor and pregnancy outcomes in patients with early-stage cervical cancer after fertility-sparing surgery. J. Int. Med. Res. 2019, 47, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bi, Y.; Wu, H.; Wu, M.; Li, L. Oncologic and obstetric outcomes after conization for adenocarcinoma in situ or stage IA1 cervical cancer. Sci. Rep. 2020, 10, 19920. [Google Scholar] [CrossRef]
- Wethington, S.L.; Cibula, D.; Duska, L.R.; Garrett, L.; Kim, C.H.; Chi, D.S.; Sonoda, Y.; Abu-Rustum, N.R. An international series on abdominal radical trachelectomy: 101 patients and 28 pregnancies. Int. J. Gynecol. Cancer 2012, 22, 1251–1257. [Google Scholar] [CrossRef]
- Wright, J.D.; Nathavithrana, R.; Lewin, S.N.; Sun, X.; Deutsch, I.; Burke, W.M.; Herzog, T.J. Fertility-conserving surgery for young women with stage IA1 cervical cancer: Safety and access. Obstet. Gynecol. 2010, 115, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Liu, Z.; Fu, X.; Li, Y.; Che, H.; Mo, R.; Song, L. Long-term outcomes of radical vaginal trachelectomy and laparoscopic pelvic lymphadenectomy after neoadjuvant chemotherapy for the IB1 cervical cancer: A series of 60 cases. Int. J. Surg. 2016, 29, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.; Choi, C.H.; Lee, Y.Y.; Kim, T.J.; Lee, J.W.; Kim, B.G.; Bae, D.S. Perioperative Outcomes of Radical Trachelectomy in Early-Stage Cervical Cancer: Vaginal Versus Laparoscopic Approaches. Int. J. Gynecol. Cancer 2015, 25, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.I.; Kobayashi, E.; Kodama, M.; Hashimoto, K.; Ueda, Y.; Sawada, K.; Tomimatsu, T.; Kimura, T. Oncological and Reproductive Outcomes of Abdominal Radical Trachelectomy. Anticancer Res. 2020, 40, 5939–5947. [Google Scholar] [CrossRef]
- Zhang, D.; Ge, H.; Li, J.; Wu, X. A new method of surgical margin assuring for abdominal radical trachelectomy in frozen section. Eur. J. Cancer 2015, 51, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Ge, H.; Ju, X.; Chen, X.; Tang, J.; Wu, X. Surgical and pathological outcomes of abdominal radical trachelectomy versus hysterectomy for early-stage cervical cancer. Int. J. Gynecol. Cancer 2014, 24, 1312–1318. [Google Scholar] [CrossRef]
- Zusterzeel, P.L.; Pol, F.J.; van Ham, M.; Zweemer, R.P.; Bekkers, R.L.; Massuger, L.F.; Verheijen, R.H. Vaginal Radical Trachelectomy for Early-Stage Cervical Cancer: Increased Recurrence Risk for Adenocarcinoma. Int. J. Gynecol. Cancer 2016, 26, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.H.; Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T. Feasibility and safety of fertility-sparing surgery in epithelial ovarian cancer with dense adhesion: A long-term result from a single institution. J. Gynecol. Oncol. 2020, 31, e85. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.F.; Zhang, Y.; Yang, B.; Ai, J.H.; Wang, X.Y.; Cheng, X.D.; Li, K.Z. Oncological and Reproductive Outcomes of Fertility-sparing Surgery in Women with Early-stage Epithelial Ovarian Carcinoma: A Multicenter Retrospective Study. Curr Med. Sci. 2020, 40, 745–752. [Google Scholar] [CrossRef]
- Colombo, N.; Parma, G.; Lapresa, M.T.; Maggi, F.; Piantanida, P.; Maggioni, A. Role of conservative surgery in ovarian cancer: The European experience. Int. J. Gynecol. Cancer 2005, 15 (Suppl. 3), 206–211. [Google Scholar] [CrossRef]
- Crafton, S.M.; Cohn, D.E.; Llamocca, E.N.; Louden, E.; Rhoades, J.; Felix, A.S. Fertility-sparing surgery and survival among reproductive-age women with epithelial ovarian cancer in 2 cancer registries. Cancer 2020, 126, 1217–1224. [Google Scholar] [CrossRef]
- Ditto, A.; Martinelli, F.; Bogani, G.; Lorusso, D.; Carcangiu, M.; Chiappa, V.; Reato, C.; Donfrancesco, C.; De Carrillo, K.J.; Raspagliesi, F. Long-term safety of fertility sparing surgery in early stage ovarian cancer: Comparison to standard radical surgical procedures. Gynecol. Oncol. 2015, 138, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, I.; Abd-Allah, M.; Ramzy, S.; Mohamed, A.M.; Saber, A. Outcome of fertility preserving surgery in early stage ovarian cancer. J. Egypt Natl. Cancer Inst. 2013, 25, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Fruscio, R.; Ceppi, L.; Corso, S.; Galli, F.; Dell’Anna, T.; Dell’Orto, F.; Giuliani, D.; Garbi, A.; Chiari, S.; Mangioni, C.; et al. Long-term results of fertility-sparing treatment compared with standard radical surgery for early-stage epithelial ovarian cancer. Br. J. Cancer 2016, 115, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Cromi, A.; Fanfani, F.; Malzoni, M.; Ditto, A.; De Iaco, P.; Uccella, S.; Gallotta, V.; Raspagliesi, F.; Scambia, G. Laparoscopic fertility-sparing surgery for early ovarian epithelial cancer: A multi-institutional experience. Gynecol. Oncol. 2016, 141, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Gouy, S.; Saidani, M.; Maulard, A.; Bach-Hamba, S.; Bentivegna, E.; Leary, A.; Pautier, P.; Devouassoux-Shisheboran, M.; Genestie, C.; Morice, P. Results of Fertility-Sparing Surgery for Expansile and Infiltrative Mucinous Ovarian Cancers. Oncologist 2018, 23, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, L.R.; Liang, Z.Q.; Meng, Y.G.; Guo, H.Y.; Qu, P.P.; Ma, C.L.; Xu, C.J.; Yuan, B.B. Clinical outcomes of fertility-sparing treatments in young patients with epithelial ovarian carcinoma. J. Zhejiang Univ. Sci. B 2011, 12, 787–795. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, J.; Yu, M.; Xie, W.; Cao, D.; Wu, M.; Pan, L.; Huang, H.; You, Y.; Shen, K. Oncofertility in patients with stage I epithelial ovarian cancer: Fertility-sparing surgery in young women of reproductive age. World J. Surg. Oncol. 2017, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Johansen, G.; Dahm-Kahler, P.; Staf, C.; Floter Radestad, A.; Rodriguez-Wallberg, K.A. A Swedish Nationwide prospective study of oncological and reproductive outcome following fertility-sparing surgery for treatment of early stage epithelial ovarian cancer in young women. BMC Cancer 2020, 20, 1009. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshikawa, N.; Kawai, M.; Mizuno, K.; Yamamuro, O.; Nagasaka, T.; Shibata, K.; Kikkawa, F. Fertility-sparing surgery and oncologic outcome among patients with early-stage ovarian cancer ~propensity score- matched analysis~. BMC Cancer 2019, 19, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Hahn, H.S.; Kim, T.J.; Lee, I.H.; Lim, K.T.; Lee, K.H.; Shim, J.U.; Mok, J.E. Fertility preservation in patients with early epithelial ovarian cancer. J Gynecol. Oncol. 2009, 20, 44–47. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, Y.R.; Kim, T.H.; Kim, H.S.; Kim, M.A.; Kim, J.W.; Park, N.H.; Song, Y.S. Safety of fertility-sparing surgery in primary mucinous carcinoma of the ovary. Cancer Res. Treat 2015, 47, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leblanc, E.; Rey, A.; Baron, M.; Querleu, D.; Blanchot, J.; Duvillard, P.; Lhomme, C.; Castaigne, D.; Classe, J.M.; et al. Gcclcc; Sfog, Conservative treatment in epithelial ovarian cancer: Results of a multicentre study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer) and SFOG (Societe Francaise d’Oncologie Gynecologique). Hum. Reprod. 2005, 20, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.J.; Lajer, H.; Mosgaard, B.J.; Bach Hamba, S.; Morice, P.; Gouy, S.; Hussein, Y.; Soslow, R.A.; Schlappe, B.A.; Zhou, Q.C.; et al. International Study of Primary Mucinous Ovarian Carcinomas Managed at Tertiary Medical Centers. Int. J. Gynecol. Cancer 2018, 28, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Mastroyannis, S.A.; Haggerty, A.F.; Giuntoli, R.L., 2nd; Morgan, M.A.; Ko, E.M.; Latif, N.A. Fertility preserving surgery for high-grade epithelial ovarian carcinoma confined to the ovary. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 63–70. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: Oncologic safety and reproductive outcomes. Gynecol. Oncol. 2008, 110, 345–353. [Google Scholar] [CrossRef]
- Park, J.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Outcomes of fertility-sparing surgery among young women with FIGO stage I clear cell carcinoma of the ovary. Int. J. Gynaecol. Obstet. 2016, 134, 49–52. [Google Scholar] [CrossRef]
- Ratanasrithong, P.; Benjapibal, M. Pregnancy Outcomes after Conservative Surgery for Early-Stage Ovarian Neoplasms. Asian Pac. J. Cancer Prev. 2017, 18, 2083–2087. [Google Scholar] [CrossRef]
- Satoh, T.; Hatae, M.; Watanabe, Y.; Yaegashi, N.; Ishiko, O.; Kodama, S.; Yamaguchi, S.; Ochiai, K.; Takano, M.; Yokota, H.; et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: A proposal for patient selection. J. Clin. Oncol. 2010, 28, 1727–1732. [Google Scholar] [CrossRef]
- Schilder, J.M.; Thompson, A.M.; DePriest, P.D.; Ueland, F.R.; Cibull, M.L.; Kryscio, R.J.; Modesitt, S.C.; Lu, K.H.; Geisler, J.P.; Higgins, R.V.; et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol. Oncol. 2002, 87, 1–7. [Google Scholar] [CrossRef]
- Schlaerth, A.C.; Chi, D.S.; Poynor, E.A.; Barakat, R.R.; Brown, C.L. Long-term survival after fertility-sparing surgery for epithelial ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Soeda, S.; Nishiyama, H.; Kiko, Y.; Tokunaga, H.; Shigeta, S.; Yaegashi, N.; Yamada, H.; Ohta, T.; Nagase, S.; et al. Clinical and reproductive outcomes of fertility-sparing surgery in stage I epithelial ovarian cancer. Mol. Clin. Oncol. 2020, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, Y.; Shan, Y.; Li, Y.; Jin, Y.; Pan, L. Pregnancy and oncologic outcomes of early stage low grade epithelial ovarian cancer after fertility sparing surgery: A retrospective study in one tertiary hospital of China. J. Ovarian Res. 2019, 12, 44. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kajiyama, H.; Tamauchi, S.; Suzuki, S.; Takahashi, K.; Matsui, S.; Kikkawa, F. Prognostic factors and effects of fertility-sparing surgery in women of reproductive age with ovarian clear-cell carcinoma: A propensity score analysis. J. Gynecol. Oncol. 2019, 30, e102. [Google Scholar] [CrossRef]
- Ayhan, A.; Tohma, Y.A.; Tunc, M. Fertility preservation in early-stage endometrial cancer and endometrial intraepithelial neoplasia: A single-center experience. Taiwan J. Obstet. Gynecol. 2020, 59, 415–419. [Google Scholar] [CrossRef]
- Casadio, P.; La Rosa, M.; Alletto, A.; Magnarelli, G.; Arena, A.; Fontana, E.; Fabbri, M.; Giovannico, K.; Virgilio, A.; Raimondo, D.; et al. Fertility Sparing Treatment of Endometrial Cancer with and without Initial Infiltration of Myometrium: A Single Center Experience. Cancers 2020, 12, 29. [Google Scholar] [CrossRef]
- Chae, S.H.; Shim, S.H.; Lee, S.J.; Lee, J.Y.; Kim, S.N.; Kang, S.B. Pregnancy and oncologic outcomes after fertility-sparing management for early stage endometrioid endometrial cancer. Int. J. Gynecol. Cancer 2019, 29, 77–85. [Google Scholar] [CrossRef]
- Chen, M.; Jin, Y.; Li, Y.; Bi, Y.; Shan, Y.; Pan, L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int. J. Gynaecol. Obstet. 2016, 132, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Woo, H.Y.; Lee, J.Y.; Park, E.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Mismatch repair status influences response to fertility-sparing treatment of endometrial cancer. Am. J. Obstet. Gynecol. 2020, 9, 9. [Google Scholar] [CrossRef]
- Dursun, P.; Erkanli, S.; Guzel, A.B.; Gultekin, M.; Tarhan, N.C.; Altundag, O.; Demirkiran, F.; Bese, T.; Yildirim, Y.; Bozdag, G.; et al. A Turkish Gynecologic Oncology Group study of fertility-sparing treatment for early-stage endometrial cancer. Int. J. Gynaecol. Obstet. 2012, 119, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar, Z.; Izadi-Mood, N.; Yarandi, F.; Shojaei, H.; Rezaei, Z.; Mohagheghi, S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: Our experiences with 21 patients. Int. J. Gynecol. Cancer 2009, 19, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Falcone, F.; Laurelli, G.; Losito, S.; Di Napoli, M.; Granata, V.; Greggi, S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J. Gynecol. Oncol. 2017, 28, e2. [Google Scholar] [CrossRef]
- Falcone, F.; Leone Roberti Maggiore, U.; Di Donato, V.; Perrone, A.M.; Frigerio, L.; Bifulco, G.; Polterauer, S.; Casadio, P.; Cormio, G.; Masciullo, V.; et al. Fertility-sparing treatment for intramucous, moderately differentiated, endometrioid endometrial cancer: A Gynecologic Cancer Inter-Group (GCIG) study. J. Gynecol. Oncol. 2020, 31, e74. [Google Scholar] [CrossRef]
- Fujiwara, H.; Jobo, T.; Takei, Y.; Saga, Y.; Imai, M.; Arai, T.; Taneichi, A.; Machida, S.; Takahashi, Y.; Suzuki, M. Fertility-sparing treatment using medroxyprogesterone acetate for endometrial carcinoma. Oncol. Lett. 2012, 3, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, Z.R.; Huang, L.N.; Wissing, M.D.; Franco, E.L.; Gotlieb, W.H. Does hormonal therapy for fertility preservation affect the survival of young women with early-stage endometrial cancer? Cancer 2017, 123, 1545–1554. [Google Scholar] [CrossRef]
- Hahn, H.S.; Yoon, S.G.; Hong, J.S.; Hong, S.R.; Park, S.J.; Lim, J.Y.; Kwon, Y.S.; Lee, I.H.; Lim, K.T.; Lee, K.H.; et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int. J. Gynecol. Cancer 2009, 19, 1068–1073. [Google Scholar] [CrossRef]
- Harrison, R.F.; He, W.; Fu, S.; Zhao, H.; Sun, C.C.; Suidan, R.S.; Woodard, T.L.; Rauh-Hain, J.A.; Westin, S.N.; Giordano, S.H.; et al. National patterns of care and fertility outcomes for reproductive-aged women with endometrial cancer or atypical hyperplasia. Am. J. Obstet. Gynecol. 2019, 221, 474.e1–474.e11. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Seong, S.J.; Kang, S.B.; Bae, D.S.; Kim, J.W.; Nam, J.H.; Lim, M.C.; Lee, T.S.; Kim, S.; Paek, J. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: A Korean Gynecologic-Oncology Group Study. J. Gynecol. Oncol. 2019, 30, e47. [Google Scholar] [CrossRef]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women With Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Habu, Y.; Kobayashi, T.; Kawarai, Y.; Ishikawa, H.; Usui, H.; Shozu, M. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J. Gynecol. Oncol. 2019, 30, e90. [Google Scholar] [CrossRef]
- Park, J.Y.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Kim, S.M.; Bae, D.S.; Nam, J.H. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet. Gynecol. 2013, 121, 136–142. [Google Scholar] [CrossRef]
- Park, J.Y.K.D.; Kim, T.J.; Kim, J.W.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Bae, D.S.; Nam, J.H. Hormonal therapy for women with stage IA endometrial cancer of all grades. Obstet. Gynecol. 2013, 122, 7–14. [Google Scholar] [CrossRef]
- Perri, T.; Korach, J.; Gotlieb, W.H.; Beiner, M.; Meirow, D.; Friedman, E.; Ferenczy, A.; Ben-Baruch, G. Prolonged conservative treatment of endometrial cancer patients: More than 1 pregnancy can be achieved. Int. J. Gynecol. Cancer 2011, 21, 72–78. [Google Scholar] [CrossRef]
- Pronin, S.M.; Novikova, O.V.; Andreeva, J.Y.; Novikova, E.G. Fertility-Sparing Treatment of Early Endometrial Cancer and Complex Atypical Hyperplasia in Young Women of Childbearing Potential. Int. J. Gynecol. Cancer 2015, 25, 1010–1014. [Google Scholar] [CrossRef]
- Sato, M.; Arimoto, T.; Kawana, K.; Miyamoto, Y.; Ikeda, Y.; Tomio, K.; Tanikawa, M.; Sone, K.; Mori-Uchino, M.; Tsuruga, T.; et al. Measurement of endometrial thickness by transvaginal ultrasonography to predict pathological response to medroxyprogesterone acetate in patients with grade 1 endometrioid adenocarcinoma. Mol. Clin. Oncol. 2016, 4, 492–496. [Google Scholar] [CrossRef]
- Son, J.; Carr, C.; Yao, M.; Radeva, M.; Priyadarshini, A.; Marquard, J.; Michener, C.M.; AlHilli, M. Endometrial cancer in young women: Prognostic factors and treatment outcomes in women aged ≤40 years. Int. J. Gynecol. Cancer 2020, 30, 631–639. [Google Scholar] [CrossRef]
- Ushijima, K.; Yahata, H.; Yoshikawa, H.; Konishi, I.; Yasugi, T.; Saito, T.; Nakanishi, T.; Sasaki, H.; Saji, F.; Iwasaka, T.; et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J. Clin. Oncol. 2007, 25, 2798–2803. [Google Scholar] [CrossRef]
- Wang, C.J.; Chao, A.; Yang, L.Y.; Hsueh, S.; Huang, Y.T.; Chou, H.H.; Chang, T.C.; Lai, C.H. Fertility-preserving treatment in young women with endometrial adenocarcinoma: A long-term cohort study. Int. J. Gynecol. Cancer 2014, 24, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.T.; Bristow, R.E.; Kurman, R.J. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am. J. Surg. Pathol. 2007, 31, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tian, Y.; Fu, J.; Xu, J.; Bao, D.; Wang, G. Efficacy and prognosis of fertility-preserved hysteroscopic surgery combined with progesterone in the treatment of complex endometrial hyperplasia and early endometrial carcinoma. J. BUON 2020, 25, 1525–1533. [Google Scholar]
- Yamagami, W.; Susumu, N.; Makabe, T.; Sakai, K.; Nomura, H.; Kataoka, F.; Hirasawa, A.; Banno, K.; Aoki, D. Is repeated high-dose medroxyprogesterone acetate (MPA) therapy permissible for patients with early stage endometrial cancer or atypical endometrial hyperplasia who desire preserving fertility? J. Gynecol. Oncol. 2018, 29, e21. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, Y.; Zhu, Q.; Xie, L.; Shan, W.; Ning, C.; Xie, B.; Shi, Y.; Luo, X.; Zhang, H.; et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol. Oncol. 2019, 153, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Gulinazi, Y.; Du, Y.; Ning, C.C.; Cheng, Y.L.; Shan, W.W.; Luo, X.Z.; Zhang, H.W.; Zhu, Q.; Ma, F.H.; et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG 2020, 127, 848–857. [Google Scholar] [CrossRef]
- Yang, Y.F.; Liao, Y.Y.; Liu, X.L.; Su, S.G.; Li, L.Z.; Peng, N.F. Prognostic factors of regression and relapse of complex atypical hyperplasia and well-differentiated endometrioid carcinoma with conservative treatment. Gynecol. Oncol. 2015, 139, 419–423. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Prat, J.; Oncology, F.C.o.G. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- McAlpine, J.; Leon-Castillo, A.; Bosse, T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J. Pathol. 2018, 244, 538–549. [Google Scholar] [CrossRef]
- Hulsbosch, S.; Koskas, M.; Tomassetti, C.; De Sutter, P.; Wildiers, H.; Neven, P.; D’Hooghe, T.; Amant, F. A Real-Life Analysis of Reproductive Outcome after Fertility Preservation in Female Cancer Patients. Gynecol. Obstet. Invest. 2018, 83, 156–163. [Google Scholar] [CrossRef]
- Taarnhoj, G.A.; Christensen, I.J.; Lajer, H.; Fuglsang, K.; Jeppesen, M.M.; Kahr, H.S.; Hogdall, C. Risk of recurrence, prognosis, and follow-up for Danish women with cervical cancer in 2005-2013: A national cohort study. Cancer 2018, 124, 943–951. [Google Scholar] [CrossRef]
- Yamagami, W.A.D. Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology. J. Obstet. Gynaecol. Res 2015, 41, 167–177. [Google Scholar] [CrossRef]
- Cibula, D.P.R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; Mahantshetty, U.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef]
- Gallos, I.D.; Yap, J.; Rajkhowa, M.; Luesley, D.M.; Coomarasamy, A.; Gupta, J.K. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2012, 207, 266.e1–266.e12. [Google Scholar] [CrossRef]
- Colombo, N.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; Sessa, C. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Fan, Z.; Li, H.; Hu, R.; Liu, Y.; Liu, X.; Gu, L. Fertility-Preserving Treatment in Young Women With Grade 1 Presumed Stage IA Endometrial Adenocarcinoma: A Meta-Analysis. Int. J. Gynecol. Cancer 2018, 28, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S.R.; Gehlot, A.; Zorn, K.K.; Burnett, A.F. Use of Metformin in Obese Women With Type I Endometrial Cancer Is Associated With a Reduced Incidence of Cancer Recurrence. Int. J. Gynecol. Cancer 2016, 26, 313–317. [Google Scholar] [CrossRef]
- Ko, E.M.W.P.; Jackson, A.; Clark, L.; Franasiak, J.; Bolac, C.; Havrilesky, L.J.; Secord, A.A.; Moore, D.T.; Gehrig, P.A.; Bae-Jump, V. Metformin is associated with improved survival in endometrial cancer. Gynecol. Oncol. 2014, 132, 438–442. [Google Scholar] [CrossRef]
- Gerstl, B.; Sullivan, E.; Vallejo, M.; Koch, J.; Johnson, M.; Wand, H.; Webber, K.; Ives, A.; Anazodo, A. Reproductive outcomes following treatment for a gynecological cancer diagnosis: A systematic review. J. Cancer Sur. 2019, 13, 269–281. [Google Scholar] [CrossRef]
| LLETZ/CKC/ST | VRT | ART | ||
|---|---|---|---|---|
| Laparotomic | MIS | |||
| Study/patient characteristics | ||||
| n of studies | 29 | 31 | 28 | 10 |
| Total n of patients | 2540 | 2477 | 2268 | 566 |
| n of patients excluded # | 108 | 76 | 91 | 32 |
| Age (y), median (range) | 32 (22–46) | 31 (21–42) | 32 (22–40) | 31 (22–40) |
| Tumor characteristics ^ | n of patients (%) | n of patients (%) | n of patients (%) | n of patients (%) |
| FIGO stage * | ||||
| IA1 | 1551 (60.4) | 248 (9.0) | 165 (7.1) | 13 (2.4) |
| IA2 | 547 (21.3) | 311 (11.3) | 218 (9.4) | 50 (9.3) |
| IA-IB | 0 | 230 (8.4) | 143 (6.2) | 0 |
| IB1 | 452 (17.6) | 1933 (70.3) | 1723 (74.6) | 453 (84.0) |
| IB2 | 18 (0.7) | 3 (0.1) | 20 (0.9) | 3 (0.6) |
| IB nos | 0 | 0 | 17 (0.7) | 19 (3.5) |
| IIA | 2 (0.1) | 26 (0.9) | 25 (1.1) | 1 (0.2) |
| Histological subtype | ||||
| SCC | 2316 (82.2) | 1906 (67.9) | 1689 (72.4) | 339 (63.2) |
| AC | 467 (16.6) | 770 (27.4) | 518 (22.2) | 169 (31.5) |
| ASCC | 25 (0.9) | 86 (3.1) | 84 (3.6) | 12 (2.2) |
| Other | 8 (0.3) | 47 (1.7) | 43 (1.8) | 16 (3.0) |
| LVSI~ | 150/784 (19.1) | 586/1877 (31.2) | 657/1628 (40.4) | 59/341 (17.3) |
| Patient Characteristics | |
|---|---|
| Total n of patients | 3944 |
| Age (y), median (range) | 29 (23.5–36.5) |
| Tumor characteristics | n of patients (%) |
| FIGO stage | |
| IA | 912 (23.1) |
| IB | 9 (0.2) |
| IA/IB | 1154 (29.3) |
| IA/IC1 | 18 (0.5) |
| IC nos | 808 (20.5) |
| IC1 | 220 (5.6) |
| IC2 | 35 (0.9) |
| IC3 | 25 (0.6) |
| I nos | 9 (0.2) |
| ≥II | 646 (16.4) |
| na | 108 (2.7) |
| Histological subtype | |
| mucinous | 1775 (45.0) |
| endometrioid | 1059 (26.9) |
| serous | 760 (19.3) |
| clear cell | 287 (7.3) |
| mixed | 15 (0.4) |
| other/na | 48 (1.2) |
| Grade | |
| 1–2 | 3015 (76.4) |
| 2–3 | 6 (0.2) |
| 3 (+clear cell) | 855 (21.7) |
| 3 non-clear cell | 568 (14.4) |
| na | 68 (1.7) |
| Treatment modalities * | n/total (%) |
| Type of FSS | |
| staging | |
| Complete # | 776/1110 (69.9) |
| LND ^ | 617/1110 (55.6) |
| Surgical technique | |
| laparotomy | 275/426 (64.6) |
| laparoscopy | 151/426 (35.4) |
| Chemotherapy | |
| adjuvant | 1815/3586 (50.6) |
| Patient Characteristics | |
|---|---|
| Total n of patients | 1229 |
| Age (y), median (range) | 32.6 (31–36.1) |
| Tumor characteristics | n of patients (%) |
| FIGO stage | |
| IA | 1020 (83.0) |
| IB | 6 (0.5) |
| IB-IIA | 15 (1.2) |
| I nos | 188 (15.3) |
| Histological subtype | |
| endometrioid | 1110 (90.3) |
| adenosquamous | 10 (0.8) |
| mucinous | 1 (0.1) |
| mixed | 8 (0.7) |
| adenocarcinoma nos | 35 (2.8) |
| na | 65 (5.3) |
| Grade | |
| 1 | 1136 (92.4) |
| 2 | 86 (7.0) |
| 3 | 4 (0.3) |
| na | 3 (0.2) |
| Treatment modalities * | n of patients (%) |
| Type of FSS | |
| D&C | 245 (19.9) |
| HR | 155 (12.6) |
| HR + D&C | 57 (4.6) |
| NR | 772 (62.8) |
| Hormonal therapy | |
| MPA | 458 (37.3) |
| MA | 337 (27.4) |
| LNG-IUD | 91 (7.4) |
| MPA + MA | 7 (0.6) |
| MPA/MA + LNG-IUD | 26 (2.1) |
| MPA/MA + metformin | 57 (4.6) |
| MPA/MA nos | 198 (16.1) |
| Progestin nos | 51 (4.1) |
| Other ^ | 4 (0.3) |
| Cervical Cancer | Ovarian Cancer | Endometrial Cancer | ||||
|---|---|---|---|---|---|---|
| LLETZ/CKC/ST | VRT | ART | ||||
| Laparotomic | MIS | |||||
| Oncological outcome * | ||||||
| Recurrence | 28/785 (3.6) | 82/1973 (4.2) | 47/1520 (3.1) | 15/335 (4.5) | 171/1084 (15.7) | 297/855 (34.7) |
| Died of disease | 6/785 (0.8) | 34/1973 (1.7) | 23/1520 (1.5) | 7/479 (1.5) | 489/3318 (14.7) | 5/648 (0.8) |
| Complete response | – | – | – | – | – | 736/918 (80.2) |
| Persistent disease | – | – | – | – | – | 182/918 (19.8) |
| PR | – | – | – | – | – | 16/504 (3.2) |
| SD | – | – | – | – | – | 52/504 (10.3) |
| PrD | – | – | – | – | – | 28/504 (5.6) |
| Follow-up in months, | 53 (9–137) | 52 (9–131) | 47 (12–120) | 44 (10–98) | 66 (38–143) | 56 (17–92) |
| median (range) | ||||||
| Fertility outcome * | ||||||
| Pregnancy wish ^ | 148/305 (48.5) | 599/1089 (55.0) | 510/1062 (48.0) | 71/199 (35.7) | 148/335 (44.2) | 279/446 (62.6) |
| Pregnancies ^ | 241/612 (39.4) | 707/1539 (45.9) | 353/1635 (21.6) | 81/344 (23.5) | 280/750 (37.3) | 256/505 (50.7) |
| Pregnant patients ^ | 196/665 (29.5) | 512/1392 (36.8) | 255/1427 (17.9) | 58/317 (18.3) | 207/701 (29.5) | 261/708 (36.9) |
| Pregnancy rate # | 82/138 (59.4) | 361/546 (66.1) | 212/471 (45.0) | 42/71 (59.2) | 110/148 (74.3) | 161/241 (66.8) |
| Fetal loss ~ | 36/234 (15.4) | 147/686 (21.4) | 101/407 (24.8) | 19/81 (23.5) | 17/191 (8.9) | 67/214 (31.3) |
| Termination ~ | 7/234 (3.0) | 26/686 (3.8) | 20/407 (4.9) | 0/81 (0) | 3/191 (1.6) | 0/214 (0) |
| Live birth ~ | 181/234 (77.4) | 484/686 (70.6) | 236/407 (58.0) | 58/81 (71.6) | 236/264 (89.4) | 172/239 (72.0) |
| Term delivery $ | 111/181 (61.3) | 262/484 (54.1) | 80/236 (33.9) | 26/58 (44.8) | 161/164 (98.2) | 82/93 (88.2) |
| Preterm delivery $ | 27/181 (14.9) | 146/484 (30.2) | 109/236 (46.2) | 32/58 (55.2) | 3/164 (1.8) | 9/93 (9.7) |
| Not specified | 43 | 76 | 47 | 0 | 72 | 79 |
| Pregnancy ongoing | 10 | 20 | 25 | 4 | 7 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuurman, T.; Zilver, S.; Samuels, S.; Schats, W.; Amant, F.; van Trommel, N.; Lok, C. Fertility-Sparing Surgery in Gynecologic Cancer: A Systematic Review. Cancers 2021, 13, 1008. https://doi.org/10.3390/cancers13051008
Schuurman T, Zilver S, Samuels S, Schats W, Amant F, van Trommel N, Lok C. Fertility-Sparing Surgery in Gynecologic Cancer: A Systematic Review. Cancers. 2021; 13(5):1008. https://doi.org/10.3390/cancers13051008
Chicago/Turabian StyleSchuurman, Teska, Sanne Zilver, Sanne Samuels, Winnie Schats, Frédéric Amant, Nienke van Trommel, and Christianne Lok. 2021. "Fertility-Sparing Surgery in Gynecologic Cancer: A Systematic Review" Cancers 13, no. 5: 1008. https://doi.org/10.3390/cancers13051008
APA StyleSchuurman, T., Zilver, S., Samuels, S., Schats, W., Amant, F., van Trommel, N., & Lok, C. (2021). Fertility-Sparing Surgery in Gynecologic Cancer: A Systematic Review. Cancers, 13(5), 1008. https://doi.org/10.3390/cancers13051008






