Antitumor Activity of Protons and Molecular Hydrogen: Underlying Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
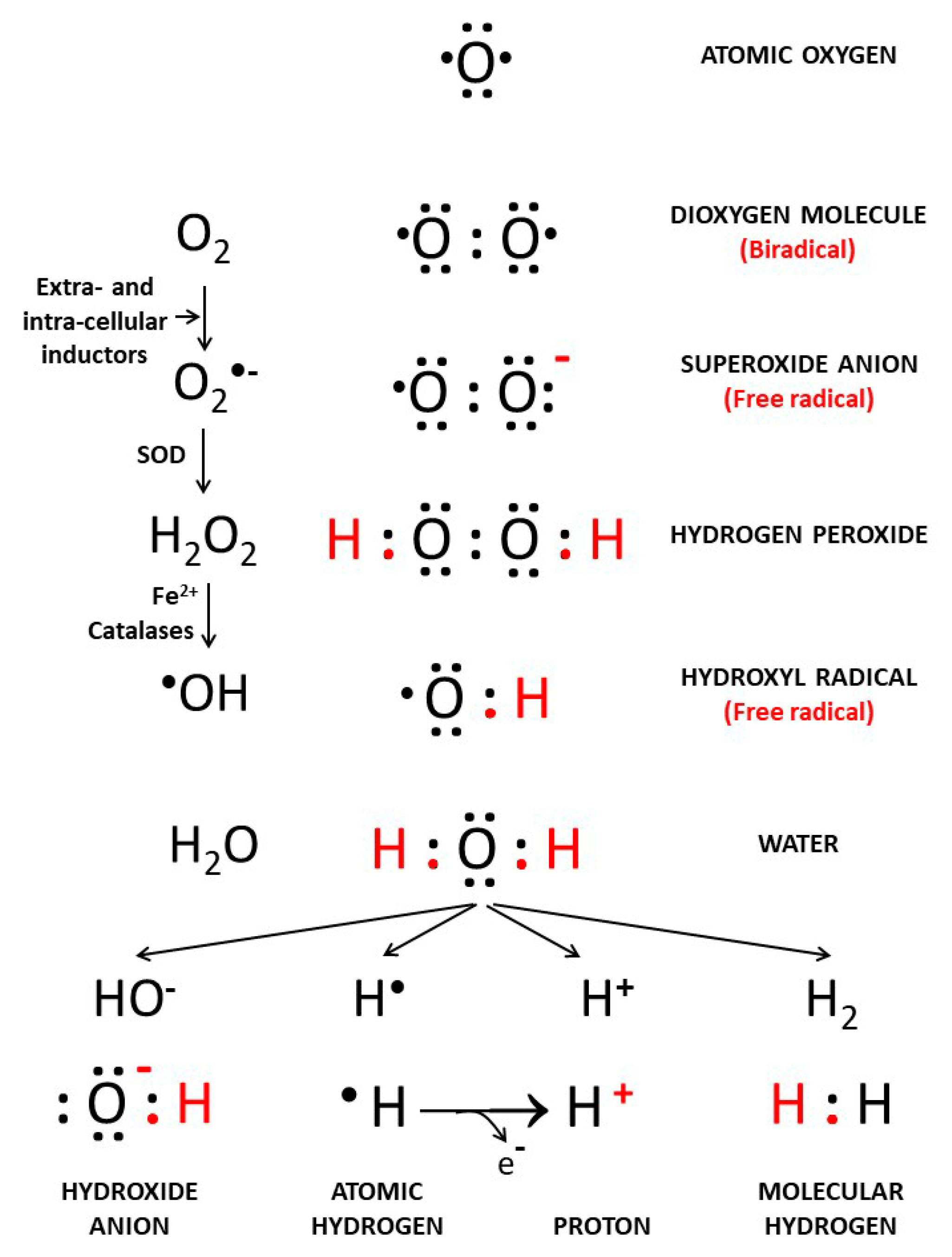
2. Background: The Different Forms of Oxygen and Hydrogen
3. Regulation of pH in Cancer Cells
4. Biochemistry of Molecular Hydrogen
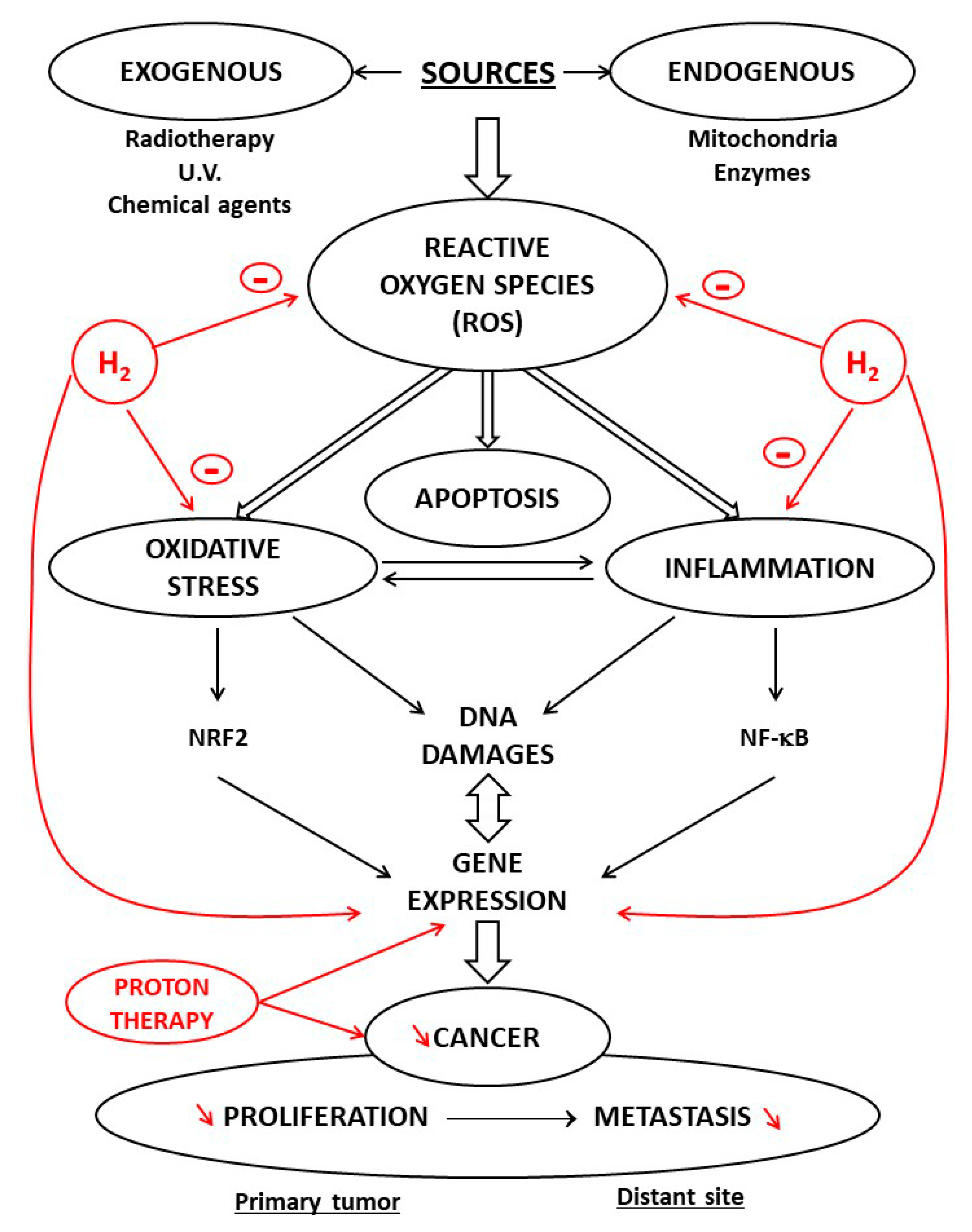
5. Antioxidant and Anti-Inflammatory Properties of H2
6. Hydrogen Paradox: Antioxidant and/or Prooxidant
7. Proton Radiotherapy and Antitumor Activity
8. Protective Properties of Molecular Hydrogen: Potential Antitumor Agent
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Vergely, C.; Maupoil, V.; Clermont, G.; Bril, A.; Rochette, L. Identification and quantification of free radicals during myocardial ischemia and reperfusion using electron paramagnetic resonance spectroscopy. Arch. Biochem. Biophys. 2003, 420, 209–216. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Aalkjaer, C. Intracellular pH in the resistance vasculature: Regulation and functional implications. J. Vasc. Res. 2012, 49, 479–496. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Et Biophys. Acta 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes. Trends Endocrinol. Metab. 2018, 29, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D.; Boron, W.F. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol. Rev. 2013, 93, 803–959. [Google Scholar] [CrossRef] [PubMed]
- Shcheynikov, N.; Son, A.; Hong, J.H.; Yamazaki, O.; Ohana, E.; Kurtz, I.; Shin, D.M.; Muallem, S. Intracellular Cl- as a signaling ion that potently regulates Na+/HCO3- transporters. Proc. Natl. Acad. Sci. USA 2015, 112, E329–E337. [Google Scholar] [CrossRef] [PubMed]
- Odunewu-Aderibigbe, A.; Fliegel, L. The Na(+) /H(+) exchanger and pH regulation in the heart. Iubmb Life 2014, 66, 679–685. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Chaiswing, L.; St Clair, W.H.; St Clair, D.K. Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer. Antioxid. Redox Signal. 2018, 29, 1237–1272. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Counillon, L. The SLC9A-C Mammalian Na(+)/H(+) Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol. Rev. 2019, 99, 2015–2113. [Google Scholar] [CrossRef]
- Li, N.; Rochette, L.; Wu, Y.; Rosenblatt-Velin, N. New Insights into the Role of Exosomes in the Heart After Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Alfarouk, K.; Orozco, J.P.; Hardonniere, K.; Stanciu, D.; Fais, S.; Devesa, J. A New and Integral Approach to the Etiopathogenesis and Treatment of Breast Cancer Based upon Its Hydrogen Ion Dynamics. Int. J. Mol. Sci. 2020, 21, 1110. [Google Scholar] [CrossRef] [PubMed]
- Lebelo, M.T.; Joubert, A.M.; Visagie, M.H. Warburg effect and its role in tumourigenesis. Arch. Pharm. Res. 2019, 42, 833–847. [Google Scholar] [CrossRef]
- Cardone, R.A.; Alfarouk, K.O.; Elliott, R.L.; Alqahtani, S.S.; Ahmed, S.B.M.; Aljarbou, A.N.; Greco, M.R.; Cannone, S.; Reshkin, S.J. The Role of Sodium Hydrogen Exchanger 1 in Dysregulation of Proton Dynamics and Reprogramming of Cancer Metabolism as a Sequela. Int. J. Mol. Sci. 2019, 20, 3694. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Jahng, J.; Jung, I.S.; Choi, E.J.; Conklin, J.L.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol. Motil. 2012, 24, 185–190.e192. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal gases: Influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef]
- Mohr, T.; Aliyu, H.; Kuchlin, R.; Polliack, S.; Zwick, M.; Neumann, A.; Cowan, D.; de Maayer, P. CO-dependent hydrogen production by the facultative anaerobe Parageobacillus thermoglucosidasius. Microb. Cell Fact. 2018, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.M. Miniaturizing Proton Therapy: A Technical Challenge With Unclear Clinical Impact. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Sugimoto, R.; Billiar, T.R.; McCurry, K.R. Therapeutic antioxidant medical gas. J. Clin. Biochem. Nutr. 2009, 44, 1–13. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Benfeito, S.; Oliveira, C.; Soares, P.; Fernandes, C.; Silva, T.; Teixeira, J.; Borges, F. Antioxidant therapy: Still in search of the ‘magic bullet’. Mitochondrion 2013, 13, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Sun, M.; Yin, Z.; Qian, J. High mobility group box 1-mediated autophagy promotes neuroblastoma cell chemoresistance. Oncol. Rep. 2015, 34, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Song, G.; Qin, S. Molecular hydrogen: Current knowledge on mechanism in alleviating free radical damage and diseases. Acta Biochim. Biophys. Sin. (Shanghai) 2019, 51, 1189–1197. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of alpha-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; He, Y.; Jia, L.; Yang, C.S.; Reiter, R.J.; Zhang, J. Antioxidant and Pro-Oxidant Activities of Melatonin in the Presence of Copper and Polyphenols In Vitro and In Vivo. Cells 2019, 8, 903. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, K.; Hirata, K.; Shinzawa-Itoh, K.; Yoko-o, S.; Yamashita, E.; Aoyama, H.; Tsukihara, T.; Yoshikawa, S. A histidine residue acting as a controlling site for dioxygen reduction and proton pumping by cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2007, 104, 7881–7886. [Google Scholar] [CrossRef]
- Whitehead, S.J.; Iwaki, M.; Cotton, N.P.; Rich, P.R.; Jackson, J.B. Inhibition of proton-transfer steps in transhydrogenase by transition metal ions. Biochim. Biophys. Acta 2009, 1787, 1276–1288. [Google Scholar] [CrossRef][Green Version]
- Verma, V.; Shah, C.; Rwigema, J.C.; Solberg, T.; Zhu, X.; Simone, C.B., 2nd. Cost-comparativeness of proton versus photon therapy. Chin. Clin. Oncol. 2016, 5, 56. [Google Scholar] [CrossRef]
- Wroe, A.J.; Cornelius, I.M.; Rosenfeld, A.B. The role of nonelastic reactions in absorbed dose distributions from therapeutic proton beams in different medium. Med. Phys. 2005, 32, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological effects of proton radiation: An update. Radiat. Prot. Dosim. 2015, 166, 334–338. [Google Scholar] [CrossRef]
- Weber, D.C.; Wang, H.; Cozzi, L.; Dipasquale, G.; Khan, H.G.; Ratib, O.; Rouzaud, M.; Vees, H.; Zaidi, H.; Miralbell, R. RapidArc, intensity modulated photon and proton techniques for recurrent prostate cancer in previously irradiated patients: A treatment planning comparison study. Radiat. Oncol. 2009, 4, 34. [Google Scholar] [CrossRef]
- McGowan, S.E.; Burnet, N.G.; Lomax, A.J. Treatment planning optimisation in proton therapy. Br. J. Radiol. 2013, 86, 20120288. [Google Scholar] [CrossRef]
- Dalloz, F.; Maingon, P.; Cottin, Y.; Briot, F.; Horiot, J.C.; Rochette, L. Effects of combined irradiation and doxorubicin treatment on cardiac function and antioxidant defenses in the rat. Free Radic. Biol. Med. 1999, 26, 785–800. [Google Scholar] [CrossRef]
- Liu, C.; Cui, J.; Sun, Q.; Cai, J. Hydrogen therapy may be an effective and specific novel treatment for acute radiation syndrome. Med. Hypotheses 2010, 74, 145–146. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Stepien, K.; Ostrowski, R.P.; Matyja, E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med. Oncol. 2016, 33, 101. [Google Scholar] [CrossRef] [PubMed]
- Casal, M.A.; Nolin, T.D.; Beumer, J.H. Estimation of Kidney Function in Oncology: Implications for Anticancer Drug Selection and Dosing. Clin. J. Am. Soc. Nephrol. 2019, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hakiminia, B.; Goudarzi, A.; Moghaddas, A. Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J. Biochem. Mol. Toxicol. 2019, 33, e22349. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharm. 2009, 64, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.M.; Kang, Y.N.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med. Gas. Res. 2011, 1, 11. [Google Scholar] [CrossRef]
- Rochette, L.; Guenancia, C.; Gudjoncik, A.; Hachet, O.; Zeller, M.; Cottin, Y.; Vergely, C. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharm. Sci. 2015, 36, 326–348. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, H.; Fan, Y.; Li, L.; Fang, J.; Yang, W. Hydrogen-Rich Saline Attenuates Cardiac and Hepatic Injury in Doxorubicin Rat Model by Inhibiting Inflammation and Apoptosis. Mediat. Inflamm. 2016, 2016, 1320365. [Google Scholar] [CrossRef]
- Lathia, J.; Liu, H.; Matei, D. The Clinical Impact of Cancer Stem Cells. Oncology 2020, 25, 123–131. [Google Scholar] [CrossRef]
- Kawai, D.; Takaki, A.; Nakatsuka, A.; Wada, J.; Tamaki, N.; Yasunaka, T.; Koike, K.; Tsuzaki, R.; Matsumoto, K.; Miyake, Y.; et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology 2012, 56, 912–921. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xie, F.; Zhang, Y.; Wang, T.T.; Ma, S.N.; Zhao, P.X.; Zhang, X.; Lebaron, T.W.; Yan, X.L.; Ma, X.M. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mu, F.; Lu, T.; Du, D.; Xu, K. Brain Metastases Completely Disappear in Non-Small Cell Lung Cancer Using Hydrogen Gas Inhalation: A Case Report. Oncotargets Ther. 2019, 12, 11145–11151. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Polo Orozco, J.; Alfarouk, K.O.; Devesa, J. Hydrogen Ion Dynamics of Cancer and a New Molecular, Biochemical and Metabolic Approach to the Etiopathogenesis and Treatment of Brain Malignancies. Int. J. Mol. Sci. 2019, 20, 4278. [Google Scholar] [CrossRef]
- Tyrrell, R.M.; Reeve, V.E. Potential protection of skin by acute UVA irradiation--from cellular to animal models. Prog. Biophys. Mol. Biol. 2006, 92, 86–91. [Google Scholar] [CrossRef]
- Shin, M.H.; Park, R.; Nojima, H.; Kim, H.C.; Kim, Y.K.; Chung, J.H. Atomic hydrogen surrounded by water molecules, H(H2O)m, modulates basal and UV-induced gene expressions in human skin in vivo. PLoS ONE 2013, 8, e61696. [Google Scholar] [CrossRef]
- Kato, S.; Saitoh, Y.; Iwai, K.; Miwa, N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type-I collagen production and oxidative-stress diminishment in fibroblasts and cell-injury prevention in keratinocytes. J. Photochem. Photobiol. B 2012, 106, 24–33. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, P.Y.; Bao, W.; Chen, S.J.; Wu, F.S.; Zhu, P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer 2020, 20, 28. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, J. Saturated hydrogen saline attenuates endotoxin-induced lung dysfunction. J. Surg. Res. 2015, 198, 41–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S.; Xu, J.; Wang, T. Hydrogen Therapy in Cardiovascular and Metabolic Diseases: From Bench to Bedside. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T. Therapeutic Efficacy of Molecular Hydrogen: A New Mechanistic Insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.; de Mattos, G.; Settineri, R.; Costa, C.; Ellithorpe, R.; Rosenblatt, S.; La Valle, J.; Jimenez, A.; Ohta, S. Clinical Effects of Hydrogen Administration: From Animal and Human Diseases to Exercise Medicine. Int. J. Clin. Med. 2016, 7, 32–76. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Antitumor Activity of Protons and Molecular Hydrogen: Underlying Mechanisms. Cancers 2021, 13, 893. https://doi.org/10.3390/cancers13040893
Rochette L, Zeller M, Cottin Y, Vergely C. Antitumor Activity of Protons and Molecular Hydrogen: Underlying Mechanisms. Cancers. 2021; 13(4):893. https://doi.org/10.3390/cancers13040893
Chicago/Turabian StyleRochette, Luc, Marianne Zeller, Yves Cottin, and Catherine Vergely. 2021. "Antitumor Activity of Protons and Molecular Hydrogen: Underlying Mechanisms" Cancers 13, no. 4: 893. https://doi.org/10.3390/cancers13040893
APA StyleRochette, L., Zeller, M., Cottin, Y., & Vergely, C. (2021). Antitumor Activity of Protons and Molecular Hydrogen: Underlying Mechanisms. Cancers, 13(4), 893. https://doi.org/10.3390/cancers13040893





