Unmet Medical Needs and Future Perspectives for Leiomyosarcoma Patients—A Position Paper from the National LeioMyoSarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN)
Simple Summary
Abstract
1. Introduction
2. Pathological and Clinical Features
3. Localized Soft-Tissue (Non-Uterine) Leiomyosarcomas
4. Localized Uterine Leiomyosarcomas
5. Advanced/Metastatic Soft-Tissue (Non-Uterine) Leiomyosarcomas
6. Advanced/Metastatic Uterine Leiomyosarcomas
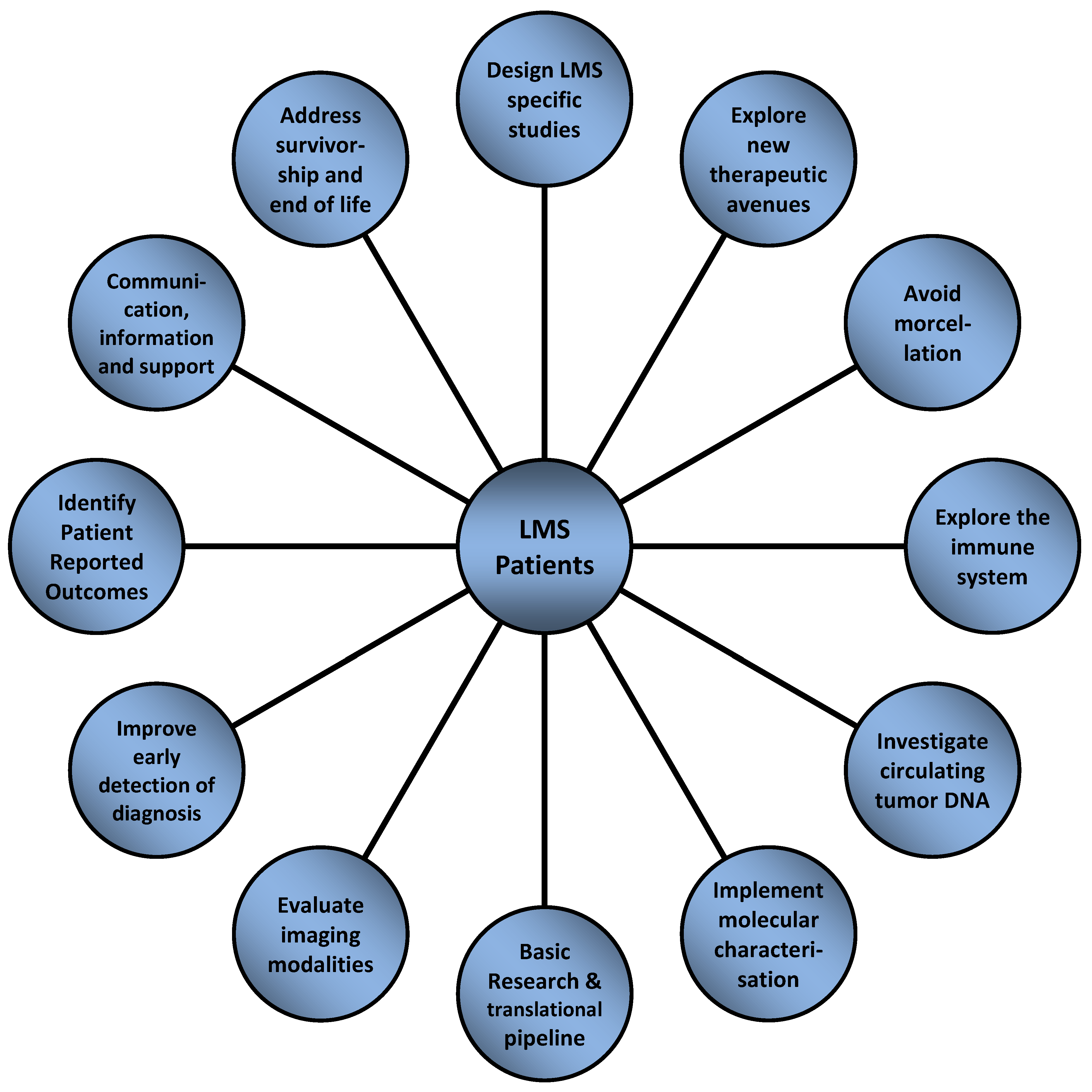
7. Unmet Medical Needs, Therapeutic Gaps and Future Perspectives
- Design LMS-specific studies for evaluating sequence and combinations of available systemic therapies: Evidence-based data for LMS mainly comes from clinical trials open for the recruitment of a variety of heterogeneous STS subtypes; there are hardly any prospective trials exclusively designed for patients with LMS. As an example, the North Eastern German Society of Gynaecological Oncology is currently evaluating the role of pazopanib versus pazopanib plus gemcitabine in the treatment of advanced or metastatic uterine LMS including carcinosarcomas in an ongoing prospective randomized controlled phase 2 trial (PazoDoble; NCT02203760). The French Sarcoma Group has conducted a randomized phase 3 study comparing the efficacy of doxorubicin plus trabectedin followed by trabectedin versus doxorubicin alone in patients with LMS from which the final results are eagerly awaited (LMS-04; NCT02997358). The EORTC/STBSG is currently setting up an open-label, randomized, phase 2 study on doxorubicin, doxorubicin plus dacarbazine, or gemcitabine plus dacarbazine for first-line treatment of advanced patients with LMS (DODECANESO) based on a published retrospective STBSG analysis [41]. There is a clear need for large, international, randomized and single-arm LMS histology specific clinical trials, with an underlying biological rationale.
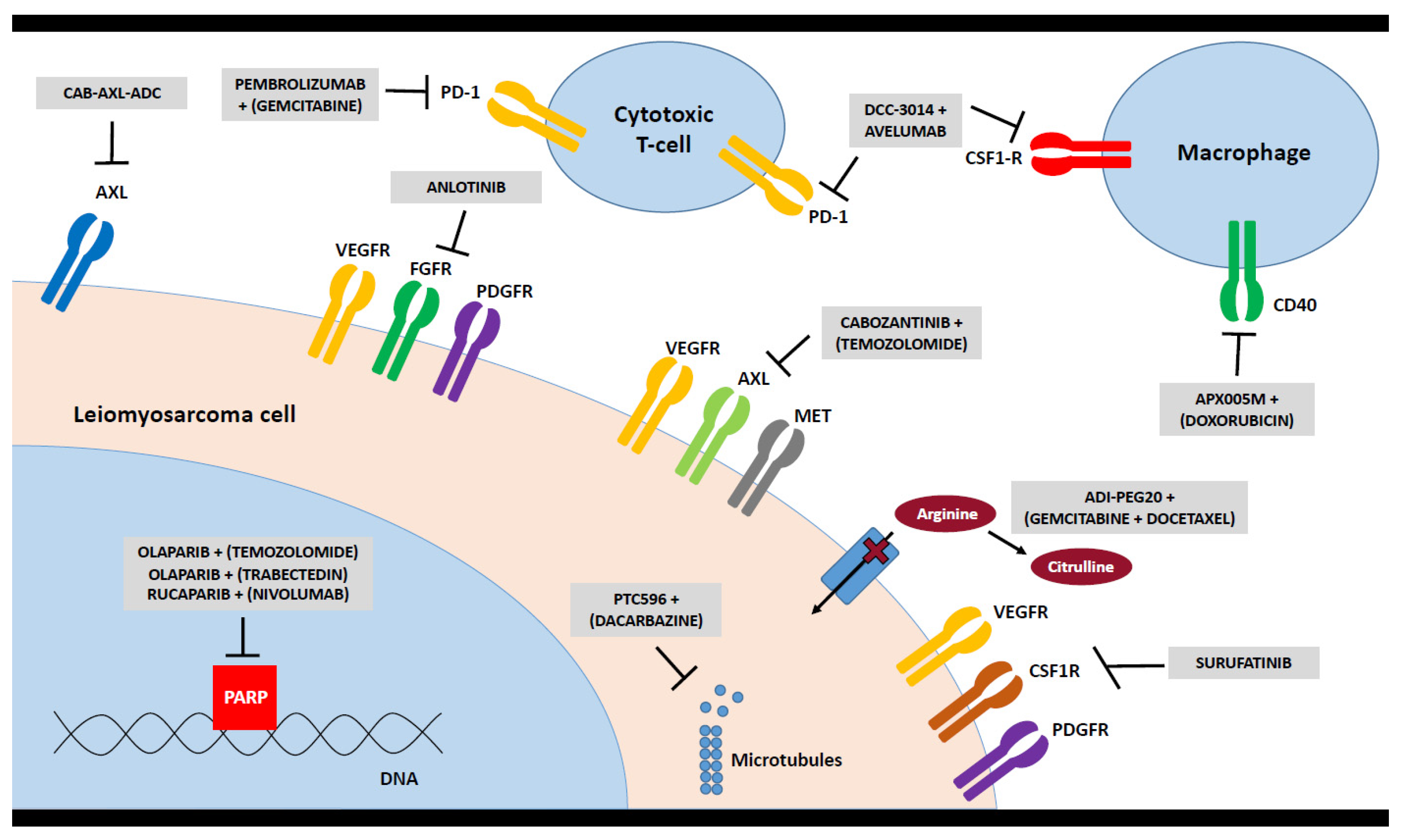
- Explore new therapeutic avenues: In addition to the evaluation of the activity of conventional chemotherapeutic agents for patients with LMS, new treatment avenues need to be explored. Currently, there are a number of ongoing trials exploring the possible value of immunotherapeutic agents in patients with LMS (see Chapter 4). Anlotinib is being evaluated in a randomized phase 3 trial with a distinct LMS cohort (APROMISS; NCT03016819). Another tyrosine kinase inhibitor (TKI), surufatinib, is also being tested in LMS. Another strategy aims to evaluate the BMI1 inhibitor PTC596 in combination with dacarbazine in participants with advanced LMS from PTC Therapeutics (NCT03761095). Based on recent basic research results for LMS [42,43], a number of trials are currently evaluating PARP inhibition combined with chemotherapy. One trial is evaluating olaparib plus trabectedin versus doctor’s choice in various solid tumors harboring deficiency in DNA repair but is not specific to sarcoma (NCT03127215). A phase 1B trial of the combination of olaparib and trabectedin in patients with previously treated advanced/metastatic STS has neared completion (NCT02398058), and a phase 2 single-arm trial of olaparib combined with trabectedin in patients with advanced sarcoma has a LMS-specific cohort (NCT04076579). Another phase 2 study is testing the combination of olaparib plus temozolomide specifically in patients with advanced, metastatic, or unresectable uterine LMS (NCT03880019). An overview on selected new treatment strategies is depicted in Figure 1.While asking patients, innovations in therapy and new treatment strategies are certainly of the utmost importance, however, it is necessary to take the whole treatment journey into account. This includes a multimodal strategy and combination of different treatment modalities such as surgery, chemotherapy, radiotherapy or potential new innovative approaches. However, it also encompasses an improved knowledge on treatment sequences depending on the risk of local recurrence and the development of metastasis. Furthermore, there is need for a more personalized approach based on molecular testing. The focus of a treatment strategy should be guided by the patients’ perspective, and a good balance of risks and benefits, and survival gain versus quality of life, respectively.
- Avoid morcellation: Morcellation of the uterus is a surgical technique which is performed to remove the uterus or leiomyomas through minimally invasive surgical approaches. It may be performed during vaginal, laparoscopic, or abdominal surgery using a scalpel, scissors, or a power morcellator. A commonly used alternative to morcellation of an enlarged uterus is a total abdominal hysterectomy associated with higher morbidity and mortality and diminished quality of life. Morcellation of a malignancy is contraindicated. Although too often not the case, women should be evaluated preoperatively to identify malignancy. Women most often do not undergo a tissue biopsy and diagnosis prior to morcellation; thus, there is a risk that a woman with a presumed leiomyoma may have a malignancy that may be spread through morcellation [44]. Morcellation bears the risk to disseminate tumor cells into the pelvis and peritoneal cavity, with a poorer prognosis as a major consequence [45,46]. The risk of an unexpected LMS diagnosis is estimated to be as frequent as 1 in 498 women. The risks associated with abdominal hysterectomy (blood loss, deep venous thrombosis, death) must be balanced against the risk of unexpected malignancy after morcellation. Existing data support a minimally invasive approach for younger women and procedures that do not involve morcellation for older women. The health care team should engage the patient in shared decision making, including informed consent, explaining the risks and benefits of each approach for presumed leiomyomas, the risks of morcellation, the rationale for a biopsy prior to surgery and alternatives to morcellation [47]. As an example, the sarcoma charity “Sarcoma UK” and the “Royal College of Obstetricians and Gynaecologists” have developed a consent advice and patient information for women offered a myomectomy or hysterectomy using morcellation, in order to enable them to make an informed choice about which surgery is right for them and to encourage discussion of the individual risks of surgery, including the risks of morcellation [48]. The question of many uterine patients with LMS remains whether or not there may be a way to make a definitive or at least more suggestive diagnosis, e.g., through imaging before any kind of invasive measure. Additional research is needed to understand the prevalence of LMS at the time of surgery for presumed leiomyomas, to better define risk factors for LMS, and to develop preoperative diagnostic tools and methods to improve the safety and efficacy of morcellation. Additionally, there is a clear need for more collaboration between gynecologic oncologists and sarcoma experts.
- Explore the immune system: Monoclonal antibodies that block suppressive functions of immune checkpoint proteins PD1/PD-L1 and CTLA4 have remarkable anti-tumor activity in a subset of patients with STS [49]. Unfortunately, both vascular and uterine LMS respond poorly to checkpoint inhibitors in published clinical trials [50], including anti-PD1/PD-L1 monotherapy [51,52], combined PD1/CTLA4 inhibition [53], or PD1 therapy combined with cyclophosphamide [54] or anti-VEGF TKI axitinib [55], although collectively the numbers of patients with LMS included in these all-comer studies are small. Multiple retrospective studies of immune-related genetic expression have suggested that LMS do have underlying immunogenicity [56,57,58], but the exact therapeutic strategy to exploit this remains elusive. Uterine LMS is being studied in a phase 2 study evaluating nivolumab alone or in combination with ipilimumab in treating patients with advanced uterine LMS from the National Cancer Institute (NCT02428192). Additionally, ongoing clinical trials are combining cytotoxic chemotherapy, including doxorubicin, gemcitabine, and trabectedin with checkpoint blockade, which may help to increase tumor immunogenicity of “cold” tumors. For example, a phase 2 study from the German Interdisciplinary Sarcoma Group (GISG) testing the combined treatment with nivolumab plus trabectedin in patients with metastatic or inoperable STS has a dedicated LMS cohort (GISG-15; NiTraSarc; NCT03590210). Additionally, studies are looking at more effective drugs to repolarize suppressive myeloid cells within tumors, suspected to be a major mechanism of resistance in LMS, including a study of DCC-3014, an anti-CSF1R TKI in combination with avelumab (anti-PD-L1) (NCT04242238). Finally, cabozantinib is being explored in a randomized study with or without dual PD1/CTLA4 checkpoint blockade, with a broader spectrum TKI potentially more impactful to the tumor microenvironment than narrow VEGF inhibitors (NCT04551430). Overall, it is critical to support preclinical and translational laboratory research with these and other ongoing studies to better understand mechanisms of response and resistance in treated patients with LMS, and to develop biomarkers for specific immune subsets of LMS to better tailor combination therapies. Additionally, further transcriptomic work to characterize potential tumor neoantigens and identify responding T cell clones may one day identify novel targets for adoptive cellular strategies.
- Investigate the role of circulating tumor DNA for matching therapy and as a biomarker of prognosis, response to therapy and minimal residual disease: Circulating tumor DNA (ctDNA) offers a rapid and non-invasive method of next-generation sequencing (NGS) that could be used for diagnosis, prognostic assessment, disease-response assessment to therapy, and detection of recurrence [59,60,61]. In a recent study, 59 of 73 metastatic patients with LMS were found to have >1 cancer-associated genomic alteration. A total of 45 patients were women with a median age of 63 years (range, 38–87). The most common alterations detected were in TP53 (65%), BRAF (13%), CCNE (13%), EGFR (12%), PIK3CA (12%), FGFR1 (10%), RB1 (10%), KIT (8%), and PDGFRA (8%). Additionally, alterations included RAF1, ERBB2, MET, PTEN, TERT, APC, and NOTCH1. Potentially targetable mutations were found in 40% of the 73 patients. A total of 5% were incidentally found to have germline TP53 mutations [62].NGS of ctDNA allows identification of genomic alterations in plasma from patients with LMS [63]. Other than pazopanib with its unknown mechanism of action, there is limited activity of current targeted agents for patients with LMS. These findings underscore the need to develop therapies against TP53, cell cycle, kinase signaling, and epigenetic pathways. Further validation and prospective evaluation is warranted to investigate the clinical utility of ctDNA especially for patients with LMS. A Sarcoma Alliance for Research Through Collaboration (SARC)-funded pilot study is evaluating ctDNA as a biomarker of relapse-free survival and response to therapy in patients with high-grade, high-risk, localized LMS; and a SARC-supported study of ctDNA as biomarker of sarcoma response to chemotherapy in patients with metastatic LMS is currently planned.
- Implement molecular characterization of LMS-NGS, transcriptome and exome data in order to develop prognostic and predictive markers as well as to design molecularly driven clinical trials: Over the past decade, a significant amount of work has resulted in a new molecular understanding of many sarcoma subtypes including LMS [64,65]. This includes the identification of three molecular subtypes of LMS with distinct transcriptomic profiles and clinicopathological characteristics [42,66,67]. However, unanswered questions remain. We know that LMS of different anatomic sites have different natural histories, prognosis, and responses to therapy, but the molecular characteristics which could differentiate these subtypes remain unknown. NGS of tumor specimens allows identification of specific gene alterations that can aid with tumor classification and suggest potential mutation-specific therapeutic targets or clinical trials. Recently, NGS of 21 LMS from various sites revealed 86 non-synonymous, coding region somatic variants within 151 gene targets (mean of 4.1 variants per case); the most frequently altered genes were TP53 (36%), ATM and ATRX (16%) as well as EGFR and RB1 (12%) [68]. Perhaps a molecular “signature” could serve as a better prognostic and predictive biomarker than the anatomic location. For instance, emerging data from several retrospective studies in LMS have shown that the Complexity INdex in SARComas (CINSARC) and Genomic Grade Index transcriptomic signatures have utility in predicting risk of relapse [69,70,71,72]. CINSARC is currently undergoing prospective evaluation in the peri-operative chemotherapy setting (NCT03805022, NCT02789384 and NCT04307277). Potentially, there could be a molecular marker or gene signature which could help to understand why some patients with LMS respond to ifosfamide while others do not. Moreover, which molecular characteristic could identify super-responders to temozolomide or dacarbazine, or other chemotherapies with occasional exceptional activity in patients with LMS?These questions could be answered using a large-scale genomic and transcriptomic database containing a large number of diverse LMS as well as the corresponding rich clinical data. To date, no such data set exists because current genomic databases have very few patients with LMS and sparse or completely missing clinical data.
- Essential need for basic research and translational pipeline: Valid laboratory models of LMS are urgently needed to understand LMS-specific oncogenic pathways, facilitate unbiased studies of LMS cancer dependencies, and support translational studies to identify novel therapeutic strategies for this disease. Several reports have identified a critical lack of fidelity to the human disease in epigenetic and transcriptional programs in established LMS cell lines [67,73]. This may arise from a misdiagnosis of the tumor of origin of these cells or the significant heterogeneity within this disease. Alternatively, lack of model fidelity may arise from the characteristic loss of tumor suppressors and absence of recurrent oncogene activation, leading to divergent evolution of LMS-derived cultures over time. Additional efforts at generating, validating, and distributing novel LMS cell lines is essential to future basic research efforts, including unbiased assessments of LMS-specific vulnerabilities arising from CRISPR and chemical dependency screens [74]. Mouse models of LMS have been reported, including genetically engineered mouse models that develop spontaneous LMS-like tumors [75] and LMS patient-derived xenografts (PDX) [76,77]. While there are early reports of the potential value of LMS mouse models in preclinical studies, these and other novel models need to undergo similar scrutiny as cell lines to demonstrate their fidelity to the primary disease. To evaluate new agents that exploit metabolic vulnerabilities or the immune system, consideration of more complex preclinical models (e.g., co-culture systems, syngeneic models and “humanized” mice with immune cell engraftment) would be of value. The ultimate goal of such model development and characterization is to confidently identify and prioritize therapeutic targets to translate into LMS-specific clinical trials.Despite the progress that has been achieved thus far, there remain several outstanding areas that should be the focus of future basic and translational LMS research efforts. With the limited response to current chemotherapy in LMS, there is a need for sustained efforts to define effective targeted therapies in LMS, which may include ATR inhibitors, PARP inhibitors and other DNA damage repair targets, PI3K/mTOR inhibitors [78], metabolic vulnerabilities such as exploiting arginine starvation [79,80] and directed immunotherapy. Furthermore, as omic technologies become more accessible and cost-effective, there should be concerted investigations to determine whether integration of multiomic measurements such as epigenomics, proteomics and metabolomics may yield more robust drug discovery targets and biomarkers [81]. Finally, given the rarity of the disease, there is an urgent need for international collaborations within a coordinated research strategy framework that minimizes overlap and maximizes the limited funding available in the field.
- Evaluate imaging modalities to better distinguish features of LMS: LMS metastasize with high frequency, and patients with advanced-stage disease have a poor prognosis. LMS may arise in various anatomic sites, but are broadly divided into uterine and extra-uterine tumors. Those found in extra-uterine soft tissues may arise within a vessel, such as the vena cava or renal vein in the retroperitoneum. Absent this association with a major vessel, imaging findings at CT or MRI are non-specific. This is especially problematic within the uterus, where benign leiomyomas may be difficult to distinguish from LMS. Unfortunately, difficulties distinguishing these entities preoperatively may lead to unplanned excisions or morcellation of uterine LMS adversely influencing patient’s outcome (see Chapter 3). It has recently been shown that the presence of at least three qualitative MR imaging features was strongly associated with a LMS: nodular borders, hemorrhage, central necrosis, and “T2 dark” area(s), with other studies emphasizing the importance of nodular contours and central necrosis [82].One advance could be the use of radiomics, which describes the extraction of large amounts of quantitative data from medical images that can be correlated with tumor histology and clinical outcomes. Radiomics entails tumor segmentation using software that subsequently analyzes various image features, yielding first-, second-, and higher-order statistics that describe image signal intensity and spatial heterogeneity. Investigators employing histogram analysis have found that LMS is marked by higher signal intensity voxels (a voxel represents a value, signal intensity in MRI or Hounsfield units in CT, in the three-dimensional image data acquired on MRI and CT scans) on T2-weighted images, specifically the mean of the bottom 10th percentile on histogram analysis [83]. Recent work has suggested that radiomics analysis of the entire uterus, and not just the tumor volume, may yield the best diagnostic performance in discriminating leiomyoma from LMS, and that optimal radiomics models perform comparably to radiologists [84]. These considerations highlight the challenge of characterizing soft-tissue tumors with conventional imaging strategies, and point toward utilization of radiomics and image texture analysis in enabling more complete and accurate uterine tumor characterization.Tumor necrosis as a CT imaging biomarker has recently been shown to improve histologic grading based on core needle biopsy alone, since biopsy may undersample areas of tumor necrosis due to intrinsic tumor heterogeneity and biopsy technique. Furthermore, radiomics features show strong correlation with histologic grade in STS. Development of a composite grading system that accounts for histology, tumor NGS, as well as novel radiomics imaging biomarkers, could lead to improved clinical decision making at the time of initial diagnosis.Because many LMS tend to be biologically aggressive tumors with high metastatic potential and local recurrence rates, systemic therapy plays an important role in the multimodality treatment strategy. The radiologic assessment of the therapeutic efficacy then becomes a crucial task so that ineffective therapies can be switched out for alternative and potentially more active regimens. In STS, it is well known that conventional size-based criteria of disease response may fail to accurately reflect treatment efficacy. This was recently highlighted in a phase 2 study (22/37 with LMS) of sorafenib and dacarbazine that showed RECIST versus Choi response rates of 14% (5/37) versus 51% (19/37), respectively [85]. Such concerns have driven the search for alternate response criteria that incorporate changes in tumor enhancement, and texture analysis of changes in T2 signal intensity may predict neoadjuvant response in STS more accurately than RECIST. Radiomics features are known to be independently associated with OS in STS, even after adjusting for patient age and tumor grade. While computationally abstruse, radiomics features may be particularly well-suited to interrogating treated LMS, where the spatial clustering of enhancing and non-enhancing voxels map histologically to viable and necrotic tumor components. Development of radiologic response criteria that may include radiomics features and are more finely tuned to the biology of STS, and those of LMS in particular, are critical in expediting assessments of drug efficacy. Future clinical trials for patients with LMS should incorporate radiomics in order to better define response to systemic therapy and diagnosis of LMS versus leiomyoma.
- Improve early detection of diagnosis: Obviously, a timely and correct diagnosis is essential to improve the prospects for survival which in general are not good for patients with LMS and have hardly improved over the past decades. According to the SPAEN research survey, most patients wish for a way to detect LMS earlier and easier. For most patients, the pathway to a correct diagnosis is a long one, with an extreme example of five years. However, early diagnosis can save lives: approximately 40% of STS are diagnosed in a locally advanced or metastatic stage [86]. While the 5 year survival rate is 81%, if the disease is caught in an early stage, it drops dramatically to 16% if diagnosed in an advanced metastatic stage. Consequently, there is a high need for (a) improved information and education for general practitioners to recognize sarcomas (symptoms and triggers for sarcomas including LMS) and transfer the patient to a specialized center in case of suspect for sarcoma, as well as (b) improved diagnostic measures to confirm the sarcoma diagnosis more easily and faster. This could be achieved through improved imaging, education and training of radiologists and new, innovative ways to detect LMS such as blood tests/ctDNA.
- Identify patient-reported outcomes (PROs): There is growing recognition in medical oncology of the potential value offered by patient-reported outcomes (PROs) partly fostered by the growth of patient involvement in research and in the provision of health care services. In a review in 1989, Maguire and Selby [87] noted an ambition: “A multidimensional scale which is specific to patients with cancer meets all the assessment criteria and provides scores which have relevance to clinical judgement remains to be developed”. Thirty-one years later, we can say that this has now been achieved. However, different tools co-exist which do not allow comparison, confusing and discouraging patients. A multidimensional scale which is specific to sarcoma remains to be developed, although that work is underway. There is some distance still to go to have one which is specific to LMS.Validated composite tools to gather the multidimensional data which enable a health-related quality of life (HRQoL) to be assessed are available, but their main weakness is that they measure a ‘moment in time’ rather than give a full picture of patient experience. The world of ‘quality-of-life’ in research has begun to shift. A fuller understanding of the domains within quality-of-life has grown and it is now possible to construct questionnaires exploring greater detail of specific aspects of the patient experience. This has opened up the importance of individual PROs. Item libraries are now available; typically, the EORTC Quality of Life Group Item Library [88] contains over 900 PRO items, each of them in many languages and all validated. A valuable development has been the PRO Common Terminology Criteria for Adverse Events (CTCAE) from NCI [89]. The CTCAE has been a mainstay of cancer clinical trial practice and reporting for many years, but the grading relied on clinician observation of patients’ experience. The PRO version calls for patients to report their experience first-hand. Gathering these data using smartphones and internet reporting opens the way for a more sensitive and often more accurate reporting of adverse events. Importantly, systems can be set up to offer ‘red-flag’ reports to clinicians, enabling a prompt intervention, sometimes even before the patient is aware a problem is developing. How do we grasp all this development and use it to create a better understanding of the needs of patients with LMS and the opportunities for treating them better? A patient might express this question somewhat differently. What have the doctors been missing about my experience as a patient and how important is it? The absence of a specific sarcoma tool is important and we should support the development work which is underway by NKI Amsterdam together with EORTC and that of UCLH in London. The developers are aware of specific aspects of LMS patient demographics (uterine, limb, RPS, etc.) and are allowing for these specific presentations.What we can do in the interim is take advantage of the existing work with the PRO CTCAE [90,91] and explore its use in our research. The development of patient input systems using smartphones, tablets and internet links would also open the way for longitudinal assessment where appropriate, allowing trends to be illustrated rather than single scores at absolute ‘moments in time’. We can also identify and share experience with use of individual PROs from Item Libraries such as EORTC. Identifying specific PROs which add value to our understanding of the LMS patient experience would be a valuable step forward.The need to identify PROs which offer value in the LMS setting calls for patients to be involved in the discussion. It would support moves to use PROs if researchers established a consultative patient group to work with them on research design issues, not necessarily exclusive to PROs, but with a focus on helping determine the experience issues which matter to patients. It can be argued that seeking PROs without taking account of patient inputs would be unethical. The patient viewpoint is that the longer-term aim should be to develop synchronous clinical and patient-reported pathway information which can inform clinicians and patients from diagnosis, through treatment, witnessing the options available and the choices which can be made at any point on that pathway [92]. Such a pathway would also have value to regulators and health care funders. In a rare disease such as LMS a well-attested pathway may also offer comparisons which can be difficult to achieve when numbers limit the practicality of traditional randomization.
- Communication, information and support: The disease is rare and therefore unknown to most people. Reason enough for patients with LMS to feel lonely and have a great need for information about their disease and possible treatments as well as support. This need for information and support is a holistic one for patients, as this disease is not just about treatment and therapy options but a complete change in lifestyle and priorities. Thus, patients would like to see a clear case management during treatment, which pays attention to the patient as a human being, opposed to just the tumor. This also includes psycho-social support, better physio and recovery care. Additionally, this need is not only relevant at a single point of time, but it is a long-term, ongoing and changing need during the entire patient journey. This starts with better information at the time of diagnosis, including the possibility to ask for a second opinion, information on sarcoma specialists and expert centers for sarcomas, as well as information on how the disease and the treatments will impact the life of the patient (work, family and partnership) and how to cope with the burden of this disease. Furthermore, many patients ask for information on what they can do themselves to improve their quality of life and to strengthen their physical as well as mental well-being. This involves questions or changes in lifestyle, diet or exercise. While patient advocacy groups offer a wide range of practical information, the treating doctor or treatment team is considered to be a partner and an important source for information and is therefore expected to be able to support the patient. Joint decision making during treatment is important but requires adequate information on the different options for treatment. In this respect patients also mention that this should look at the entire trajectory of treatment, not just the next step. It also requires an open and honest communication between both parties.
- Address survivorship and end of life: Those two topics seem to be contrary, but they share challenges. Both might be considered to be the “forgotten” parts in sarcoma management. While sarcoma survivors must cope with having been a patient and returning to a “new normal”, they often feel left alone and insecure. Not only is the distinction between “patient” and “survivor” or “healed person” unclear. Follow-up procedures (if any) are very often not specified, in terms of frequency, intervals and extensiveness. Furthermore, dealing with the potential risk of late effects is very often burdened on the patient. “End of life”, on the other hand, is a delicate, but important question for many patients. The thought of death is scary, but for a lot of sarcoma patients it is unfortunately part of their story. Questions on how the last part of the journey will look like, if they have choice of where to die and how to tell their families are important topics and should and need to be addressed in a sensitive way.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martin-Liberal, J. Leiomyosarcoma: Principles of management. Intractable Rare Dis. Res. 2013, 2, 127–129. [Google Scholar] [CrossRef][Green Version]
- Reichardt, P. Soft tissue sarcomas, a look into the future: Different treatments for different subtypes. Future Oncol. 2014, 10 (Suppl. 8), s19–s27. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, N.; Torres, K.E.; Lin, H.Y.; Ravi, V.; Roland, C.L.; Mann, G.N.; Hunt, K.K.; Cormier, J.N.; Feig, B.W. Recurrence patterns of retroperitoneal leiomyosarcoma and impact of salvage surgery. J. Surg. Oncol. 2017, 116, 313–319. [Google Scholar] [CrossRef]
- Martin-Liberal, J.; Benson, C. Systemic chemotherapy for inoperable, locally advanced, recurrent or metastatic uterine leiomyosarcoma. Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 343–345. [Google Scholar] [CrossRef]
- Italiano, A.; Toulmonde, M.; Stoeckle, E.; Kind, M.; Kantor, G.; Coindre, J.-M.; Bui, B. Clinical outcome of leiomyosarcomas of vascular origin: Comparison with leiomyosarcomas of other origin. Ann. Oncol. 2010, 21, 1915–1921. [Google Scholar] [CrossRef]
- Fletcher, B.; Hogendoorn, P.C.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; World Health Organization: Geneva, Switzerland, 2013; p. 467. [Google Scholar]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Gladdy, R.A.; Qin, L.X.; Moraco, N.; Agaram, N.P.; Brennan, M.F.; Singer, S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann. Surg. Oncol. 2013, 20, 1851–1857. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Martin-Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.-Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Reed, N.S.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: A European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef]
- Hensley, M.L.; Ishill, N.; Soslow, R.; Larkin, J.; Abu-Rustum, N.; Sabbatini, P.; Konner, J.; Tew, W.; Spriggs, D.; Aghajanian, C.A. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol. Oncol. 2009, 112, 563–567. [Google Scholar] [CrossRef]
- Hensley, M.L.; Wathen, J.K.; Maki, R.G.; Araujo, D.M.; Sutton, G.; Priebat, D.A.; George, S.; Soslow, R.A.; Baker, L.H. Adjuvant therapy for high grade, uterus-limited leiomyosarcoma: Results of a phase 2 trial (SARC 005). Cancer 2013, 119, 1555–1561. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Romanini, A.; Genazzani, A.R. The management of patients with uterine sarcoma: A debated clinical challenge. Crit. Rev. Oncol. Hematol. 2008, 65, 129–142. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Rahal, C.; Mir, O. Doxorubicin Plus Dacarbazine (DD) in Advanced Leiomyosarcoma. A Retrospective Review of Gustave Roussy Institute; The European Cancer Congress: Amsterdam, The Netherlands, 27 September 2013. [Google Scholar]
- Penel, N.; Italiano, A.; Isambert, N.; Bompas, E.; Bousquet, G.; Duffaud, F.; French Sarcoma Group (Groupe Sarcome Français/Groupe d’Etude des Tumeurs Osseuses). Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Ann. Oncol. 2010, 21, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Sleijfer, S.; Ouali, M.; van Glabbeke, M.; Krarup-Hansen, A.; Rodenhuis, S.; Le Cesne, A.; Hogendoorn, P.C.W.; Verweij, J.; Blay, J.-Y. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur. J. Cancer 2010, 46, 72–83. [Google Scholar]
- Pautier, P.; Floquet, A.; Chevreau, C.; Penel, N.; Guillemet, C.; Delcambre, C.; Cupissol, D.; Selle, F.; Isambert, N.; Piperno-Neumann, S.; et al. Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): A non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 457–464. [Google Scholar] [CrossRef]
- Demetri, G.D.; Chawla, S.P.; von Mehren, M.; Ritch, P.; Baker, L.H.; Blay, J.-Y.; Hande, K.R.; Keohan, M.L.; Samuels, B.L.; Schuetze, S.; et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J. Clin. Oncol. 2009, 27, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.J.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.L.; Chawla, S.; Patel, S.; von Mehren, M.; Hamm, J.; Kaiser, P.E.; Schuetze, S.; Li, J.; Aymes, A.; Demetri, G.D. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: Results of a worldwide expanded access program study. Ann. Oncol. 2013, 24, 1703–1709. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- García-Del-Muro, X.; López-Pousa, A.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A.; et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef]
- Tap, W.D.; Wagner, A.J.; Schöffski, P.; Martin-Broto, J.; Krarup-Hansen, A.; Ganjoo, K.N.; Yen, C.-C.; Abdul Razak, A.R.; Spira, A.; Kawai, A.; et al. Effect of Doxorubicin plus Olaratumab vs Doxorubicin plus Placebo on Survival in Patients with Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA 2020, 323, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected]. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef]
- Pautier, P.; Floquet, A.; Penel, N.; Piperno-Neumann, S.; Isambert, N.; Rey, A.; Bompas, E.; Cioffi, A.; Delcambre, C.; Cupissol, D.; et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: A Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 2012, 17, 1213–1220. [Google Scholar]
- Duffaud, F.; Pautier, P.; Bui Nguyen, B.; Hensley, M.L.; Penel, N.; Rey, A.; Le Cesne, A.; Reinke, D.; Blay, J.-Y.; Maki, R. A pooled analysis of the final results of two randomised phase II studies comparing gemcitabine vs. gemcitabine plus docetaxel in patients with metastatic/relapsed leiomyosarcoma. In Proceedings of the 16th Annual Meeting of the Connective Tissue Oncology Society (CTOS), Paris, France, 11 November 2010. [Google Scholar]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Dileo, P.; Morgan, J.A.; Zahrieh, D.; Desai, J.; Salesi, J.M.; Harmon, D.C.; Quigley, M.T.; Polson, K.; Demetri, G.D.; George, S. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: Results of a phase II trial. Cancer 2007, 109, 1863–1869. [Google Scholar] [CrossRef]
- Van der Graaf, W.T.A.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- George, S.; Feng, Y.; Manola, J.; Nucci, M.R.; Butrynski, J.E.; Morgan, J.A.; Ramaiya, N.; Quek, R.; Penson, R.T.; Wagner, A.J.; et al. Phase 2 trial of aromatase inhibition with letrozole in patients with uterine leiomyosarcomas expressing estrogen and/or progesterone receptors. Cancer 2014, 120, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Sbaraglia, M.; Fumagalli, E.; Baldi, G.G.; Fucà, G.; Morosi, C.; Barisella, M.; Dei Tos, A.P.; Casali, P.G. Activity of hormonal treatment in uterine smooth muscle tumors of uncertain malignant potential (STUMP): A mono-institutional referral center experience in advanced disease. J. Clin. Oncol. 2019, 37 (Suppl. 15), 11066. [Google Scholar] [CrossRef]
- Hensley, M.L.; Blessing, J.A.; Degeest, K.; Abulafia, O.; Rose, P.G.; Homesley, H.D. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol. Oncol. 2008, 109, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Blessing, J.A.; Mannel, R.; Rose, P.G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol. Oncol. 2008, 109, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Grosso, F.; Jones, R.L.; Banerjee, S.; Pilotti, S.; D’Incalci, M.; Dei Tos, A.P.; Raspagliesi, F.; Judson, I.; Casali, P.G. Trabectedin in advanced uterine leiomyosarcomas: A retrospective case series analysis from two reference centers. Gynecol. Oncol. 2011, 123, 553–556. [Google Scholar] [CrossRef]
- Hensley, M.L.; Patel, S.R.; von Mehren, M.; Ganjoo, K.; Jones, R.L.; Staddon, A.; Rushing, D.; Milhem, M.; Monk, B.; Wang, G.; et al. Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecol. Oncol. 2017, 146, 531–537. [Google Scholar] [CrossRef]
- Benson, C.; Ray-Coquard, I.; Sleijfer, S.; Litière, S.; Blay, J.-Y.; Le Cesne, A.; Papai, Z.; Judson, I.; Schöffski, P.; Chawla, S.; et al. Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol. Oncol. 2016, 142, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Scurr, M.; Jones, R.L.; Wood, Z.; Propert-Lewis, C.; Fisher, C.; Flanagan, A.; Sunkersing, J.; A’Hern, R.; Whelan, J.; et al. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin. Sarcoma Res. 2015, 5, 13. [Google Scholar] [CrossRef]
- Patel, S.; von Mehren, M.; Reed, D.R.; Kaiser, P.; Charlson, J.; Ryan, C.W.; Rushing, D.; Livingston, M.; Singh, A.; Seth, R.; et al. Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 2019, 125, 2610–2620. [Google Scholar] [CrossRef]
- D’Ambrosio, L.; Touati, N.; Blay, J.-Y.; Grignani, G.; Flippot, R.; Czarnecka, A.M.; Piperno-Neumann, S.; Martin-Broto, J.; Sanfilippo, R.; Katz, D.; et al. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: A propensity score matching analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer 2020, 126, 2637–2647. [Google Scholar] [PubMed]
- Chudasama, P.; Mughal, S.S.; Sanders, M.A.; Hübschmann, D.; Chung, I.; Deeg, K.I.; Wong, S.-H.; Rabe, S.; Hlevnjak, M.; Zapatka, M.; et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat. Commun. 2018, 9, 144. [Google Scholar] [CrossRef]
- Pignochino, Y.; Capozzi, F.; D’Ambrosio, L.; Dell’Aglio, C.; Basiricò, M.; Canta, M.; Lorenzato, A.; Vignolo Lutati, F.; Aliberti, S.; Palesandro, E.; et al. PARP1 expression drives the synergistic antitumor activity of trabectedin and PARP1 inhibitors in sarcoma preclinical models. Mol. Cancer 2017, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Stone, R.L.; Fader, A.N. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol. Oncol. 2017, 145, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Nemec, W.; Inwald, E.C.; Buchholz, S.; Klinkhammer Schalke, M.; Gerken, M.; Ortmann, O. Effects of morcellation on long-term outcomes in patients with uterine leiomyosarcoma. Arch. Gynecol. Obstet. 2016, 294, 825–831. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/consent-advice/consent-advice-no-13-morcellation-myomectormy-hysterectomy.pdf (accessed on 29 January 2021).
- Chalas, E. Morcellation in gynecologic oncology. Curr. Opin. Obstet. Gynecol. 2018, 30, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. Available online: https://www.rcog.org.uk/en/news/updated-patient-information-and-consent-advice-on-morcellation-published (accessed on 29 January 2021).
- Wilky, B.A. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol. Rev. 2019, 290, 6–23. [Google Scholar] [CrossRef]
- Italiano, A.; Bellera, C.; D’Angelo, S. PD1/PD-L1 targeting in advanced soft-tissue sarcomas: A pooled analysis of phase II trials. J. Hematol. Oncol. 2020, 13, 55. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Barysauskas, C.M.; Solomon, S.; Tahlil, K.; Malley, R.; Hohos, M.; Polson, K.; Loucks, M.; Severgnini, M.; Patel, T.; et al. Immunotherapy with Single Agent Nivolumab for Advanced Leiomyosarcoma of the Uterus: Results of a Phase 2 Study. Cancer 2017, 123, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.-Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Pollack, S.M.; He, Q.; Yearley, J.H.; Emerson, R.; Vignali, M.; Zhang, Y.; Redman, M.W.; Baker, K.K.; Cooper, S.; Donahue, B.; et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017, 123, 3291–3304. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Wei-Wu Chen, T.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965. [Google Scholar] [CrossRef]
- Mas, A.; Simón, C. Molecular differential diagnosis of uterine leiomyomas and leiomyosarcomas. Biol. Reprod. 2019, 101, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Przybyl, J.; Spans, L.; Lum, D.A.; Zhu, S.; Vennam, S.; Forgó, E.; Varma, S.; Ganjoo, K.; Hastie, T.; Bowen, R.; et al. Detection of Circulating Tumor DNA in Patients with Uterine Leiomyomas. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Przybyl, J.; Chabon, J.J.; Spans, L.; Ganjoo, K.N.; Vennam, S.; Newman, A.M.; Forgó, E.; Varma, S.; Zhu, S.; Debiec-Rychter, M.; et al. Combination Approach for Detecting Different Types of Alterations in Circulating Tumor DNA in Leiomyosarcoma. Clin. Cancer Res. 2018, 24, 2688–2699. [Google Scholar] [CrossRef]
- Arshad, J.; Barreto-Coelho, P.; Jonczak, E.; Espejo, A.; D’Amato, G.; Trent, J. Identification of genetic alterations by circulating tumor DNA in leiomyosarcoma: A molecular analysis of 73 patients. J. Immunother Precis Oncol 2020, 3, 64–68. [Google Scholar] [CrossRef]
- Hemming, M.L.; Klega, K.S.; Rhoades, J.; Ha, G.; Acker, K.E.; Andersen, J.L.; Thai, E.; Nag, A.; Thorner, A.R.; Raut, C.P.; et al. Detection of Circulating Tumor DNA in Patients with Leiomyosarcoma with Progressive Disease. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.H.; Lee, C.H.; Witten, D.M.; Gleason, B.C.; Edris, B.; Espinosa, I.; Zhu, S.; Li, R.; Montgomery, K.D.; Marinelli, R.J.; et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene 2010, 29, 845–854. [Google Scholar] [CrossRef]
- Davis, L.E.; Nusser, K.D.; Przybyl, J.; Pittsenbarger, J.; Hofmann, N.E.; Varma, S.; Vennam, S.; Debiec-Rychter, M.; van de Rijn, M.; Davare, M.A. Discovery and Characterization of Recurrent, Targetable ALK Fusions in Leiomyosarcoma. Mol. Cancer Res. 2019, 17, 676–685. [Google Scholar] [CrossRef]
- Guo, X.; Forgó, E.; van de Rijn, M. Molecular subtyping of leiomyosarcoma with 3′ end RNA sequencing. Genom. Data 2015, 5, 366–367. [Google Scholar] [CrossRef][Green Version]
- Hemming, M.L.; Fan, C.; Raut, C.P.; Demetri, G.D.; Armstrong, S.A.; Sicinska, E.; George, S. Oncogenic Gene-Expression Programs in Leiomyosarcoma and Characterization of Conventional, Inflammatory, and Uterogenic Subtypes. Mol. Cancer Res. 2020, 18, 1302–1314. [Google Scholar] [CrossRef]
- Lee, P.J.; Yoo, N.S.; Hagemann, I.S.; Pfeifer, J.D.; Cottrell, C.E.; Abel, H.J.; Duncavage, E.J. Spectrum of mutations in leiomyosarcomas identified by clinical targeted next-generation sequencing. Exp. Mol. Pathol. 2017, 102, 156–161. [Google Scholar] [CrossRef]
- Chibon, F.; Lagarde, P.; Salas, S.; Pérot, G.; Brouste, V.; Tirode, F.; Lucchesi, C.; de Reynies, A.; Kauffmann, A.; Bui, B.; et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat. Med. 2010, 16, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Croce, S.; Lesluyes, T.; Valle, C.; M’Hamdi, L.; Thébault, N.; Pérot, G.; Stoeckle, E.; Noël, J.C.; Fontanges, Q.; Devouassoux-Shisheboran, M.; et al. The Nanocind Signature Is an Independent Prognosticator of Recurrence and Death in Uterine Leiomyosarcomas. Clin. Cancer Res. 2020, 26, 855–861. [Google Scholar] [CrossRef]
- Italiano, A.; Lagarde, P.; Brulard, C.; Terrier, P.; Laë, M.; Marques, B.; Ranchere-Vince, D.; Michels, J.J.; Trassard, M.; Cioffi, A.; et al. Genetic profiling identifies two classes of soft-tissue leiomyosarcomas with distinct clinical characteristics. Clin. Cancer Res. 2013, 19, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; De Nonneville, A.; Finetti, P.; Perrot, D.; Nilbert, M.; Italiano, A.; Le Cesne, A.; Skubitz, K.M.; Blay, J.-Y.; Birnbaum, D. The Genomic Grade Index predicts postoperative clinical outcome in patients with soft-tissue sarcoma. Ann. Oncol. 2018, 29, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Sonoda, K.; Tomikawa, J.; Tayama, C.; Okamura, K.; Maehara, K.; Kobayashi, H.; Wake, N.; Kato, K.; Hata, K.; et al. Genomic, Epigenomic, and Transcriptomic Profiling towards Identifying Omics Features and Specific Biomarkers That Distinguish Uterine Leiomyosarcoma and Leiomyoma at Molecular Levels. Sarcoma 2015, 2015, 412068. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J.S.; Golub, T.R. An ecosystem of cancer cell line factories to support a cancer dependency map. Nat. Rev. Genet. 2015, 16, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Politi, K.; Szabolcs, M.; Fisher, P.; Kljuic, A.; Ludwig, T.; Efstratiadis, A. A mouse model of uterine leiomyosarcoma. Am. J. Pathol. 2004, 164, 325–336. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, K.; Kiyuna, T.; Miyake, K.; Kawaguchi, K.; Igarashi, K.; Nelson, S.D.; Li, Y.; Singh, S.R.; Hoffman, R.M. A patient-derived orthotopic xenograft (PDOX) nude-mouse model precisely identifies effective and ineffective therapies for recurrent leiomyosarcoma. Pharmacol. Res. 2019, 142, 169–175. [Google Scholar] [CrossRef]
- Cornillie, J.; Wozniak, A.; Li, H.; Wang, Y.; Boeckx, B.; Gebreyohannes, Y.K.; Wellens, J.; Vanleeuw, U.; Hompes, D.; Stas, M.; et al. Establishment and Characterization of Histologically and Molecularly Stable Soft-tissue Sarcoma Xenograft Models for Biological Studies and Preclinical Drug Testing. Mol. Cancer Ther. 2019, 18, 1168–1178. [Google Scholar] [CrossRef]
- Babichev, Y.; Kabaroff, L.; Datti, A.; Uehling, D.; Isaac, M.; Al-Awar, R.; Prakesch, M.; Sun, R.X.; Boutros, P.C.; Venier, R.; et al. PI3K/AKT/mTOR inhibition in combination with doxorubicin is an effective therapy for leiomyosarcoma. J. Transl. Med. 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Prudner, B.C.; Rathore, R.; Robinson, A.M.; Godec, A.; Chang, S.F.; Hawkins, W.G.; Hirbe, A.C.; Van Tine, B.A. Arginine Starvation and Docetaxel Induce c-Myc-Driven hENT1 Surface Expression to Overcome Gemcitabine Resistance in ASS1-Negative Tumors. Clin. Cancer Res. 2019, 25, 5122–5134. [Google Scholar] [CrossRef]
- Kremer, J.C.; Prudner, B.C.; Stubbs Lange, S.E.; Bean, G.R.; Schultze, M.B.; Brashears, C.B.; Radyk, M.D.; Redlich, N.; Tzeng, S.-C.; Kami, K.; et al. Arginine Deprivation Inhibits the Warburg Effect and Upregulates Glutamine Anaplerosis and Serine Biosynthesis in ASS1-Deficient Cancers. Cell Rep. 2017, 18, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Wilding, C.P.; Jones, R.L.; Huang, P.H. Proteomic research in sarcomas—Current status and future opportunities. Semin. Cancer Biol. 2020, 61, 56–70. [Google Scholar] [CrossRef]
- Lakhman, Y.; Veeraraghavan, H.; Chaim, J.; Feier, D.; Goldman, D.A.; Moskowitz, C.S.; Nougaret, S.; Sosa, R.E.; Vargas, H.A.; Soslow, R.A.; et al. Differentiation of Uterine Leiomyosarcoma from Atypical Leiomyoma: Diagnostic Accuracy of Qualitative MR Imaging Features and Feasibility of Texture Analysis. Eur. Radiol. 2017, 27, 2903–2915. [Google Scholar] [CrossRef]
- Gerges, L.; Popiolek, D.; Rosenkrantz, A.B. Explorative Investigation of Whole-Lesion Histogram MRI Metrics for Differentiating Uterine Leiomyomas and Leiomyosarcomas. AJR Am. J. Roentgenol. 2018, 210, 1172–1177. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, X.; Ma, S.; Liu, Y.; Wang, X. Preoperative Differentiation of Uterine Sarcoma from Leiomyoma: Comparison of Three Models Based on Different Segmentation Volumes Using Radiomics. Mol. Imaging Biol. 2019, 21, 1157–1164. [Google Scholar] [CrossRef]
- D’Adamo, D.R.; Dickson, M.A.; Keohan, M.L.; Carvajal, R.D.; Hensley, M.L.; Hirst, C.M.; Ezeoke, M.O.; Ahn, L.; Qin, L.-X.; Antonescu, C.R.; et al. A Phase II Trial of Sorafenib and Dacarbazine for Leiomyosarcoma, Synovial Sarcoma, and Malignant Peripheral Nerve Sheath Tumors. Oncologist 2019, 24, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Cancer.Net. Available online: https://www.cancer.net/cancer-types/sarcoma-soft-tissue/statistics (accessed on 29 January 2021).
- Maguire, P.; Selby, P. Assessing quality of life in cancer patients. Br. J. Cancer 1989, 60, 437–440. [Google Scholar] [CrossRef] [PubMed]
- EORTC Quality of Life Item Library. Available online: https://qol.eortc.org/item-library (accessed on 29 January 2021).
- Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™). Available online: https://healthcaredelivery.cancer.gov/pro-ctcae (accessed on 29 January 2021).
- Basch, E.; Dueck, A.C.; Rogak, L.J.; Mitchell, S.A.; Minasian, L.M.; Denicoff, A.M.; Wind, J.K.; Shaw, M.C.; Heon, N.; Shi, Q.; et al. Feasibility of Implementing the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events in a Multicenter Trial: NCCTG N1048. J. Clin. Oncol. 2018, 36, JCO2018788620. [Google Scholar] [CrossRef]
- Dueck, A.C.; Scher, H.I.; Bennett, A.V.; Mazza, G.L.; Thanarajasingam, G.; Schwab, G.; Weitzman, A.L.; Rogak, L.J.; Basch, E. Assessment of Adverse Events from the Patient Perspective in a Phase 3 Metastatic Castration-Resistant Prostate Cancer Clinical Trial. JAMA Oncol. 2020, 6, e193332. [Google Scholar] [CrossRef]
- Wilson, R. Patient led PROMs must take centre stage in cancer research. Res. Involv. Engag. 2018, 4, 7. [Google Scholar] [CrossRef]
- Gutierrez, J.C.; Perez, E.A.; Moffat, F.L.; Livingstone, A.S.; Franceschi, D.; Koniaris, L.G. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann. Surg. 2007, 245, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Soibinet, P.; Penel, N.; Bompas, E.; Duffaud, F.; Stoeckle, E.; Mir, O.; Adam, J.; Chevreau, C.; Bonvalot, S.; et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann. Oncol. 2017, 28, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Chiang, Y.J.; Cormier, J.N.; Torres, K.E.; Hunt, K.K.; Feig, B.W.; Roland, C.L. Treatment at low-volume hospitals is associated with reduced short-term and long-term outcomes for patients with retroperitoneal sarcoma. Cancer 2018, 124, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
| Agent(s) | Phase | n | Line | ORR | PFS (Months) | OS (Months) | |||
|---|---|---|---|---|---|---|---|---|---|
| All STS | |||||||||
| Doxorubicin vs. Doxorubicin + Ifosfamide [15] | III | 455 | 1st | 14% | 26% | 4.6 | 7.4 | 12.8 | 14.3 |
| Doxorubicin vs. Gemcitabine + Docetaxel [28] | III | 257 | 1st | 19% | 20% | 5.4 | 5.5 | 17.6 | 15.5 |
| Gemcitabine vs. Gemcitabine + Docetaxel [25] | II | 122 | 1st–3rd | 8% | 16% | 3.0 | 6.2 | 11.5 | 17.9 |
| Dacarbazine vs. Gemcitabine + Dacarbazine [29] | II | 113 | 2nd+ | 25% a | 49% a | 2 | 4.2 | 8.2 | 16.8 |
| Pazopanib vs. Placebo [31] | III | 372 | 2nd+ | 6% | 0% | 4.6 | 1.6 | 12.5 | 10.7 |
| LMS | |||||||||
| Doxorubicin + Dacarbazine [16] | retro | 22 | 1st | 15.1 | 33.9 | ||||
| Gemcitabine + Docetaxel [39] | II | 45 | 1st | 25% | 7.1 | 17.9 | |||
| Trabectedin vs.Dacarbazine [40] | III | 403 | 3rd+ | 10% | 7% | 4.8 | 1.5 | 14.1 | 13.6 |
| uLMS | |||||||||
| Gemcitabine + Docetaxel [35] | II | 42 | 1st | 36% | 4.4 | 16.1 | |||
| Gemcitabine + Docetaxel [34] | II | 51 | 2nd+ | 27% | 6.7 | 14.7 | |||
| Trabectedin vs. Dacarbazine [37] b | III | 232 | 3rd+ | 11% | 9% | 4.0 | 1.5 | 13.4 | 12.9 |
| Pazopanib vs. placebo [38] b | III | 44 | 2nd+ | 11% | 0% | 2.9 | 0.8 | 17.5 | 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasper, B.; Achee, A.; Schuster, K.; Wilson, R.; van Oortmerssen, G.; Gladdy, R.A.; Hemming, M.L.; Huang, P.; Ingham, M.; Jones, R.L.; et al. Unmet Medical Needs and Future Perspectives for Leiomyosarcoma Patients—A Position Paper from the National LeioMyoSarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN). Cancers 2021, 13, 886. https://doi.org/10.3390/cancers13040886
Kasper B, Achee A, Schuster K, Wilson R, van Oortmerssen G, Gladdy RA, Hemming ML, Huang P, Ingham M, Jones RL, et al. Unmet Medical Needs and Future Perspectives for Leiomyosarcoma Patients—A Position Paper from the National LeioMyoSarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN). Cancers. 2021; 13(4):886. https://doi.org/10.3390/cancers13040886
Chicago/Turabian StyleKasper, Bernd, Annie Achee, Kathrin Schuster, Roger Wilson, Gerard van Oortmerssen, Rebecca A. Gladdy, Matthew L. Hemming, Paul Huang, Matthew Ingham, Robin L. Jones, and et al. 2021. "Unmet Medical Needs and Future Perspectives for Leiomyosarcoma Patients—A Position Paper from the National LeioMyoSarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN)" Cancers 13, no. 4: 886. https://doi.org/10.3390/cancers13040886
APA StyleKasper, B., Achee, A., Schuster, K., Wilson, R., van Oortmerssen, G., Gladdy, R. A., Hemming, M. L., Huang, P., Ingham, M., Jones, R. L., Pollack, S. M., Reinke, D., Sanfilippo, R., Schuetze, S. M., Somaiah, N., Van Tine, B. A., Wilky, B., Okuno, S., & Trent, J. (2021). Unmet Medical Needs and Future Perspectives for Leiomyosarcoma Patients—A Position Paper from the National LeioMyoSarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN). Cancers, 13(4), 886. https://doi.org/10.3390/cancers13040886











