Synergistic Impact of Alpha-Fetoprotein and Tumor Burden on Long-Term Outcomes Following Curative-Intent Resection of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Variables of Interest and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
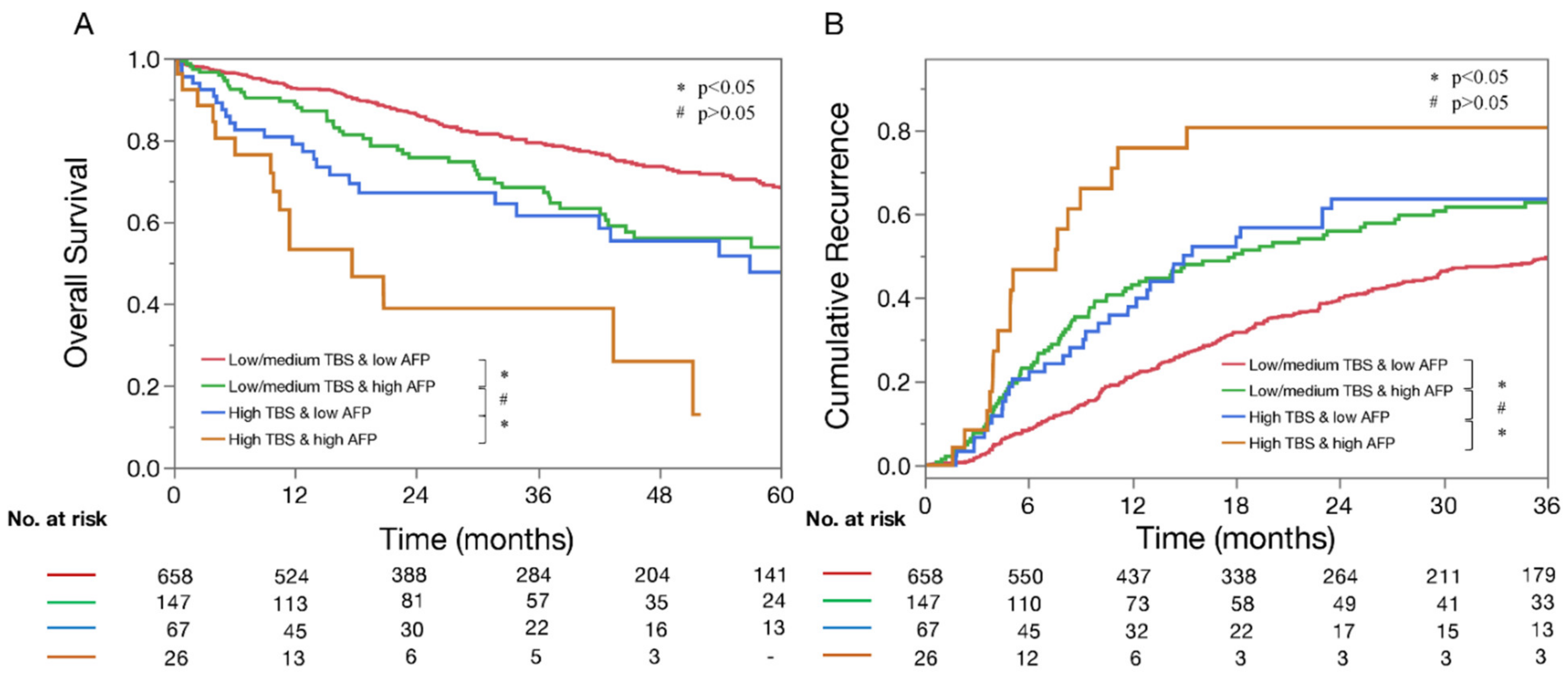
3.2. Impact of TBS and AFP on OS and Recurrence Rates
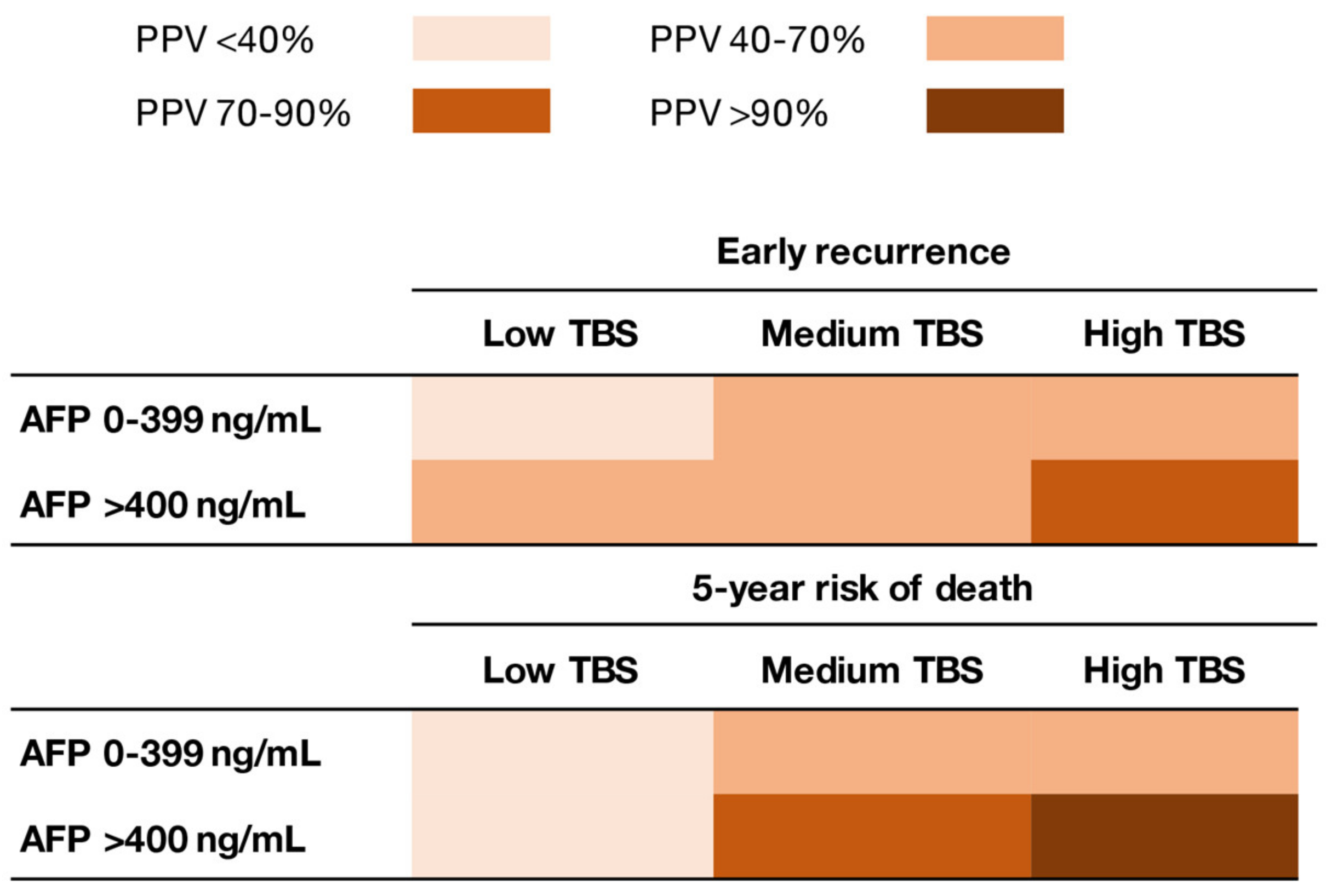
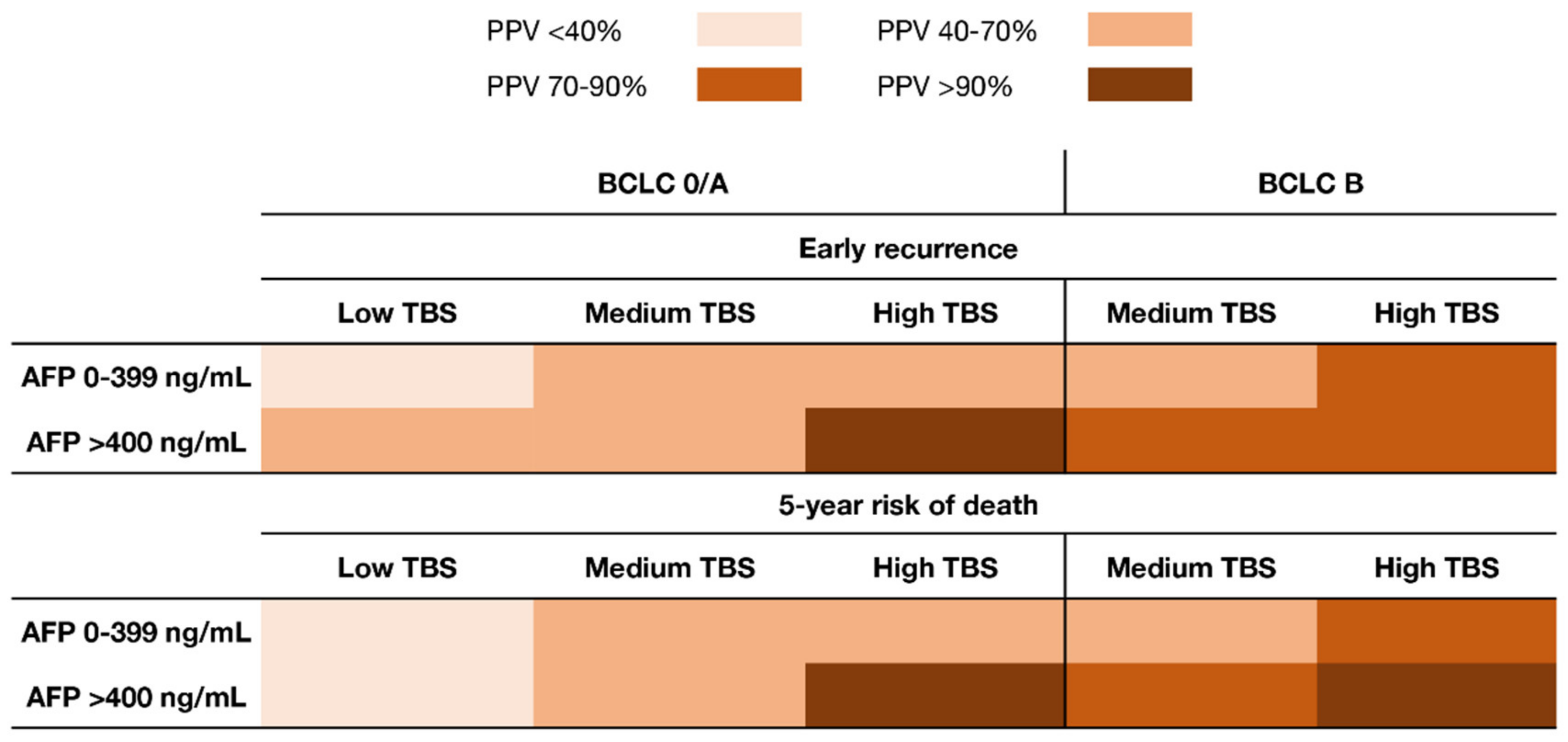
3.3. Synergistic Impact of TBS and AFP on Early Recurrence and 5-Year Death
4. Discussion
5. Conslusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Kelly, S.P.; Altekruse, S.F.; McGlynn, K.A.; Rosenberg, P.S. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J. Clin. Oncol. 2016, 34, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Bagante, F.; Moris, D.; Merath, K.; Paredes, A.Z.; Sahara, K.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: A multi-institutional analysis of 1010 patients. Surgery 2019, 166, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.D.; Yang, T.; Mazzaferro, V.; De Carlis, L.; Zhou, J.; Roayaie, S.; Shen, F.; Sposito, C.; Cescon, M.; Di Sandro, S.; et al. Liver Transplantation and Hepatic Resection can Achieve Cure for Hepatocellular Carcinoma. Ann. Surg. 2018, 268, 868–875. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Bagante, F.; Moris, D.; Hyer, J.M.; Sahara, K.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; Soubrane, O.; et al. Recurrence Patterns and Outcomes after Resection of Hepatocellular Carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann. Surg. Oncol. 2020, 27, 2321–2331. [Google Scholar] [CrossRef]
- Xu, X.F.; Xing, H.; Han, J.; Li, Z.L.; Lau, W.Y.; Zhou, Y.H.; Gu, W.M.; Wang, H.; Chen, T.H.; Zeng, Y.Y.; et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study from China. JAMA Surg. 2019, 154, 209–217. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Ding, H.F.; Zhang, X.F.; Bagante, F.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; Alexandrescu, S.; et al. Prediction of tumor recurrence by alpha-fetoprotein model after curative resection for hepatocellular carcinoma. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Agopian, V.G.; Harlander-Locke, M.P.; Markovic, D.; Zarrinpar, A.; Kaldas, F.M.; Cheng, E.Y.; Yersiz, H.; Farmer, D.G.; Hiatt, J.R.; Busuttil, R.W. Evaluation of Patients With Hepatocellular Carcinomas That Do Not Produce alpha-Fetoprotein. JAMA Surg. 2017, 152, 55–64. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br. J. Surg. 2020, 107, 854–864. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Mehta, R.; Guglielmi, A.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; Alexandrescu, S.; et al. Recurrence beyond the Milan criteria after curative-intent resection of hepatocellular carcinoma: A novel tumor-burden based prediction model. J. Surg. Oncol. 2020, 122, 955–963. [Google Scholar] [CrossRef]
- Wang, C.C.; Iyer, S.G.; Low, J.K.; Lin, C.Y.; Wang, S.H.; Lu, S.N.; Chen, C.L. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann. Surg. Oncol. 2009, 16, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Mehta, R.; Paredes, A.Z.; Moris, D.; Sahara, K.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; et al. Overall Tumor Burden Dictates Outcomes for Patients Undergoing Resection of Multinodular Hepatocellular Carcinoma Beyond the Milan Criteria. Ann. Surg. 2020, 272, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool for Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, F.; Garuti, F.; Neri, A. Alpha-fetoprotein for Diagnosis, Prognosis, and Transplant Selection. Semin Liver Dis. 2019, 39, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Rahnemai-Azar, A.A.; Cloyd, J.M.; Weber, S.M.; Dillhoff, M.; Schmidt, C.; Winslow, E.R.; Pawlik, T.M. Update on Liver Failure Following Hepatic Resection: Strategies for Prediction and Avoidance of Post-operative Liver Insufficiency. J. Clin. Transl. Hepatol. 2018, 6, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pericleous, M.; Khan, S.A. Epidemiology of HPB malignancy in the elderly. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Xing, H.; Liang, L.; Wang, H.; Zhou, Y.H.; Pei, Y.L.; Li, C.; Zeng, Y.Y.; Gu, W.M.; Chen, T.H.; Li, J.; et al. Multicenter analysis of long-term oncologic outcomes of hepatectomy for elderly patients with hepatocellular carcinoma. HPB 2020, 22, 1314–1323. [Google Scholar] [CrossRef]
- Nault, J.C.; Villanueva, A. Biomarkers for Hepatobiliary Cancers. Hepatology 2020. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Longo, V.; Brunetti, O.; Gnoni, A.; Licchetta, A.; Delcuratolo, S.; Memeo, R.; Solimando, A.G.; Argentiero, A. Emerging role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Medicina 2019, 55, 698. [Google Scholar] [CrossRef]
- Galon, J.; Pages, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.A.; Gajewski, T.F.; Ascierto, P.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10, 1. [Google Scholar] [CrossRef]
- Ros-Martinez, S.; Navas-Carrillo, D.; Alonso-Romero, J.L.; Orenes-Pinero, E. Immunoscore: A novel prognostic tool. Association with clinical outcome, response to treatment and survival in several malignancies. Crit. Rev. Clin. Lab. Sci. 2020, 57, 432–443. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, S.W.; Hong, S.K.; Yoon, K.C.; Kim, H.S.; Choi, Y.R.; Lee, H.W.; Yi, N.J.; Lee, K.W.; Suh, K.S.; et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1045–1052. [Google Scholar] [CrossRef]
- Liang, L.; Xing, H.; Zhang, H.; Zhong, J.; Li, C.; Lau, W.Y.; Wu, M.; Shen, F.; Yang, T. Surgical resection versus transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma: A systematic review and meta-analysis. HPB 2018, 20, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.H.; Lee, Y.S.; Kim, J.H.; Lee, C.U.; Jung, Y.K.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Byun, K.S. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology 2018, 68, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 898) |
|---|---|
| Age † | 67 (59–74) |
| Sex | |
| Male | 680 (75.8%) |
| Female | 218 (24.2%) |
| ASA-PS | |
| ≤2 | 521 (64.4%) |
| >2 | 288 (35.6%) |
| Cirrhosis | 353 (39.4%) |
| HBV infection | |
| No | 642 (72.3%) |
| Yes | 246 (27.7%) |
| HCV infection | |
| No | 598 (67.3%) |
| Yes | 290 (32.7%) |
| AFP, ng/mL | |
| ≤400 | 725 (80.7%) |
| >400 | 173 (19.3%) |
| Minimally Invasive Surgery | 211 (23.6%) |
| Type of resection | |
| Minor | 566 (64.7%) |
| Major | 309 (35.3%) |
| Tumor size of largest nodule, cm † | 4.8 (3.0–8.5) |
| Tumor number † | 1 (1–1) |
| Tumor Burden Score † | 5.1 (3.4–8.6) |
| Low | 233 (25.9%) |
| Medium | 572 (63.7%) |
| High | 93 (10.4%) |
| BCLC stage | |
| BCLC-0 | 59 (6.6%) |
| BCLC-A | 690 (76.8%) |
| BCLC-B | 149 (16.6%) |
| Grade | |
| Well to moderate | 679 (78.6%) |
| Poor to undifferentiated | 185 (21.4%) |
| Lympho-vascular invasion | |
| No | 496 (61.5%) |
| Yes | 311 (38.5%) |
| Liver capsule involvement | |
| No | 458 (67.2%) |
| Yes | 224 (32.8%) |
| Margin Status | |
| R0 | 779 (88.9%) |
| R1 | 97 (11.1%) |
| Variables | Low/Medium TBS and AFP < 400 ng/mL (N = 658, 73.3%) | Low/Medium TBS and AFP > 400 ng/mL (N = 147, 16.4%) | High TBS and AFP < 400 ng/mL (N = 67, 7.4%) | High TBS and AFP > 400 ng/mL (N = 26, 2.9%) | p-Value |
|---|---|---|---|---|---|
| Age † | 60 (67–74) | 65 (56–71) | 69 (62–76) | 69 (62–77) | 0.03 |
| Sex | 0.27 | ||||
| Male | 500 (76.1%) | 104 (70.7%) | 55 (82.1%) | 21 (80.8%) | |
| Female | 157 (23.9%) | 43 (29.3%) | 12 (17.9%) | 5 (19.2%) | |
| ASA-PS | 0.56 | ||||
| ≤2 | 381 (63.8%) | 89 (69.0%) | 37 (63.8%) | 14 (56.0%) | |
| >2 | 216 (36.2%) | 40 (31.0%) | 21 (36.2%) | 11 (44.0%) | |
| Cirrhosis | 287 (43.8%) | 55 (37.4%) | 9 (13.4%) | 2 (7.7%) | <0.001 |
| HBV infection | <0.001 | ||||
| No | 472 (72.7%) | 91 (61.9%) | 59 (89.4%) | 20 (76.9%) | |
| Yes | 177 (27.3%) | 56 (38.1%) | 7 (10.6%) | 6 (23.1%) | |
| HCV infection | <0.001 | ||||
| No | 414 (63.6%) | 96 (66.2%) | 64 (97.0%) | 24 (92.3%) | |
| Yes | 237 (36.4%) | 49 (33.8%) | 2 (3.0%) | 2 (7.7%) | |
| Minimally Invasive Surgery | 174 (26.4%) | 30 (20.8%) | 6 (9.1%) | 1 (3.8%) | 0.001 |
| Type of resection | <0.001 | ||||
| Minor | 472 (73.9%) | 74 (51.4%) | 15 (22.4%) | 5 (20.0%) | |
| Major | 167 (26.1%) | 70 (48.6%) | 52 (77.6%) | 20 (80.0%) | |
| Grade | <0.001 | ||||
| Well to moderate | 536 (85.1%) | 83 (58.5%) | 46 (69.7%) | 14 (53.8%) | |
| Poor to undifferentiated | 94 (14.9%) | 59 (41.5%) | 20 (30.3%) | 12 (46.2%) | |
| Lympho-vascular invasion | 172 (29.2%) | 80 (62.5%) | 37 (58.7%) | 22 (84.6%) | <0.001 |
| Liver capsule involvement | 151 (30.4%) | 47 (43.5%) | 18 (32.7%) | 8 (36.4%) | 0.07 |
| Margin Status | 0.03 | ||||
| R0 | 578 (90.3%) | 118 (81.9%) | 60 (90.9%) | 23 (88.5%) | |
| R1 | 62 (9.7%) | 26 (18.1%) | 6 (9.1%) | 3 (11.5%) |
| Variable | Bivariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.01 (0.99–1.02) | 0.14 | ||
| Gender (male) | 1.05 (0.79–1.40) | 0.74 | ||
| ASA-PS (III–IV) | 1.25 (0.94–1.66) | 0.12 | ||
| Liver cirrhosis | 1.15 (0.89–1.48) | 0.27 | ||
| MIS | 0.55 (0.38–0.81) | 0.002 | 0.66 (0.45–0.98) | 0.04 |
| Major resection | 1.55 (1.20–2.00) | 0.001 | 1.09 (0.81–1.45) | 0.58 |
| Grade (poor/undifferentiated) | 1.70 (1.28–2.24) | <0.001 | 1.49 (1.11–1.99) | 0.007 |
| Lympho-vascular invasion | 1.90 (1.45–2.48) | <0.001 | 1.33 (0.97–1.83) | 0.08 |
| Liver capsule involvement | 1.37 (1.04–1.82) | 0.02 | 1.31 (0.94–1.82) | 0.11 |
| R1 margins | 1.51 (1.04–2.20) | 0.03 | 1.31 (0.89–1.92) | 0.18 |
| Group | ||||
| Low/Medium TBS and low AFP | Ref | Ref | ||
| Low/Medium TBS and high AFP | 1.59 (1.16–2.18) | 0.004 | 1.23 (0.87–1.73) | 0.24 |
| High TBS and low AFP | 2.14 (1.42–3.22) | <0.001 | 1.63 (1.03–2.56) | 0.03 |
| High TBS and high AFP | 5.28 (3.09–9.03) | <0.001 | 3.66 (2.03–6.58) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsilimigras, D.I.; Hyer, J.M.; Diaz, A.; Bagante, F.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; et al. Synergistic Impact of Alpha-Fetoprotein and Tumor Burden on Long-Term Outcomes Following Curative-Intent Resection of Hepatocellular Carcinoma. Cancers 2021, 13, 747. https://doi.org/10.3390/cancers13040747
Tsilimigras DI, Hyer JM, Diaz A, Bagante F, Ratti F, Marques HP, Soubrane O, Lam V, Poultsides GA, Popescu I, et al. Synergistic Impact of Alpha-Fetoprotein and Tumor Burden on Long-Term Outcomes Following Curative-Intent Resection of Hepatocellular Carcinoma. Cancers. 2021; 13(4):747. https://doi.org/10.3390/cancers13040747
Chicago/Turabian StyleTsilimigras, Diamantis I., J. Madison Hyer, Adrian Diaz, Fabio Bagante, Francesca Ratti, Hugo P. Marques, Olivier Soubrane, Vincent Lam, George A. Poultsides, Irinel Popescu, and et al. 2021. "Synergistic Impact of Alpha-Fetoprotein and Tumor Burden on Long-Term Outcomes Following Curative-Intent Resection of Hepatocellular Carcinoma" Cancers 13, no. 4: 747. https://doi.org/10.3390/cancers13040747
APA StyleTsilimigras, D. I., Hyer, J. M., Diaz, A., Bagante, F., Ratti, F., Marques, H. P., Soubrane, O., Lam, V., Poultsides, G. A., Popescu, I., Alexandrescu, S., Martel, G., Workneh, A., Guglielmi, A., Hugh, T., Aldrighetti, L., Endo, I., & Pawlik, T. M. (2021). Synergistic Impact of Alpha-Fetoprotein and Tumor Burden on Long-Term Outcomes Following Curative-Intent Resection of Hepatocellular Carcinoma. Cancers, 13(4), 747. https://doi.org/10.3390/cancers13040747










