PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumors
2.2. Tumor Samples
2.3. Survival Endpoints
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Patients and Tumors
3.1.1. Association between Post-NAC PD-L1 Expression and Baseline Clinical and Pathologic Patterns
3.1.2. Association between Post-NAC PD-L1 Expression, KI67 and Post-NAC Pathological Patterns
3.2. Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Cancer du Sein-Les Cancers Les Plus Fréquents. Available online: https://www.e-cancer.fr/Professionnels-de-sante/Les-chiffres-du-cancer-en-France/Epidemiologie-des-cancers/Les-cancers-les-plus-frequents/Cancer-du-sein (accessed on 13 July 2019).
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Foldi, J.; Dhawan, A.; DiGiovanna, M.P.; Mamounas, E.P. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol. 2019, 20, e390–e396. [Google Scholar] [CrossRef]
- Balko, J.M.; Giltnane, J.M.; Wang, K.; Schwarz, L.J.; Young, C.D.; Cook, R.S.; Owens, P.; Sanders, M.E.; Kuba, M.G.; Sánchez, V.; et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014, 4, 232–245. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet Lond. Engl. 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Hamy, A.-S.; Darrigues, L.; Laas, E.; De Croze, D.; Topciu, L.; Lam, G.-T.; Evrevin, C.; Rozette, S.; Laot, L.; Lerebours, F.; et al. Prognostic value of the Residual Cancer Burden index according to breast cancer subtype: Validation on a cohort of BC patients treated by neoadjuvant chemotherapy. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Neoadjuvant Study of Abemaciclib, Durvalumab, and an Aromatase Inhibitor Early Stage Breast Cancer-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04088032 (accessed on 27 November 2020).
- Merck Sharp & Dohme Corp. A Randomized, Double-Blind, Phase III Study of Pembrolizumab Versus Placebo in Combination with Neoadjuvant Chemotherapy and Adjuvant Endocrine Therapy for the Treatment of High-Risk Early-Stage Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative (ER+/HER2-) Breast Cancer (KEYNOTE-756); Clinicaltrials.gov: Bethesda, MD, USA, 2020.
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Emens, L.A. Breast cancer immunotherapy: Facts and hopes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, H.; Zhao, S.; Wang, Y.; Pu, H.; Wang, Y.; Zhang, Q. Expression of PD-L1 and prognosis in breast cancer: A meta-analysis. Oncotarget 2017, 8, 31347–31354. [Google Scholar] [CrossRef]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Gonzalez-Ericsson, P.I.; Stovgaard, E.S.; Sua, L.F.; Reisenbichler, E.; Kos, Z.; Carter, J.M.; Michiels, S.; Le Quesne, J.; Nielsen, T.O.; Laenkholm, A.-V.; et al. The path to a better biomarker: Application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J. Pathol. 2020, 250, 667–684. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Nixon, M.J.; Gonzalez-Ericsson, P.I.; Bergman, R.E.; Pilkinton, M.A.; McDonnell, W.J.; Sanchez, V.; Opalenik, S.R.; Loi, S.; Zhou, J.; et al. Changes in peripheral and local tumor immunity after neoadjuvant chemotherapy reshape clinical outcomes in patients with breast cancer. Clin. Cancer Res. 2020, 26, 5668–5681. [Google Scholar] [CrossRef]
- Balaton, A.J.; Doussal, V.L.; Arnould, L.; Barlier, C.; Bellocq, J.P.; Ettore, F.; Fiche, M.; Jacquemier, J.; Grogan, G.M.; Mathieu, M.C.; et al. Recommandations pour l’évaluation immunohistochimique des récepteurs hormonaux sur coupes en paraffine dans les carcinomes mammaires Mise à jour 1999. 2008. Available online: https://www.em-consulte.com/article/88258/recommandations-pour-l-evaluation-immunohistochimi (accessed on 8 February 2020).
- Li, S.; Chen, L.; Jiang, J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer. Medicine 2019, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, H.; Zhou, Y.; Mao, F.; Lin, Y.; Pan, B.; Zhang, X.; Xu, Q.; Huang, X.; Sun, Q. Prognostic value of PD-L1 in breast cancer: A meta-analysis. Breast J. 2017, 23, 436–443. [Google Scholar] [CrossRef]
- Huang, W.; Ran, R.; Shao, B.; Li, H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019, 178, 17–33. [Google Scholar] [CrossRef]
- Pelekanou, V.; Barlow, W.E.; Nahleh, Z.A.; Wasserman, B.; Lo, Y.-C.; von Wahlde, M.-K.; Hayes, D.; Hortobagyi, G.N.; Gralow, J.; Tripathy, D.; et al. Tumor infiltrating lymphocytes and PD-L1 expression in pre- and post-treatment breast cancers in the SWOG S0800 Phase II neoadjuvant chemotherapy trial. Mol. Cancer Ther. 2018, 17, 1324–1331. [Google Scholar] [CrossRef]
- Li, X.; Warren, S.; Pelekanou, V.; Wali, V.; Cesano, A.; Liu, M.; Danaher, P.; Elliott, N.; Nahleh, Z.A.; Hayes, D.F.; et al. Immune profiling of pre- and post-treatment breast cancer tissues from the SWOG S0800 neoadjuvant trial. J. Immunother. Cancer 2019, 7, 88. [Google Scholar] [CrossRef]
- Noguchi, T.; Ward, J.P.; Gubin, M.M.; Arthur, C.D.; Lee, S.H.; Hundal, J.; Selby, M.J.; Graziano, R.F.; Mardis, E.R.; Korman, A.J.; et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol. Res. 2017, 5, 106–117. [Google Scholar] [CrossRef]
- Bae, S.B.; Cho, H.D.; Oh, M.-H.; Lee, J.-H.; Jang, S.-H.; Hong, S.A.; Cho, J.; Kim, S.Y.; Han, S.W.; Lee, J.E.; et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J. Breast Cancer 2016, 19, 242–251. [Google Scholar] [CrossRef]
- Beckers, R.K.; Selinger, C.I.; Vilain, R.; Madore, J.; Wilmott, J.S.; Harvey, K.; Holliday, A.; Cooper, C.L.; Robbins, E.; Gillett, D.; et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016, 69, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Lee, Y.K.; Koo, J.S. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J. Transl. Med. 2016, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Au, W.-L.; Lo, K.-Y.; Ni, Y.-B.; Hlaing, T.; Hu, J.; Chan, S.-K.; Chan, K.-F.; Cheung, S.-Y.; Tse, G.M. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res. Treat. 2017, 162, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lotfinejad, P.; Asghari Jafarabadi, M.; Abdoli Shadbad, M.; Kazemi, T.; Pashazadeh, F.; Sandoghchian Shotorbani, S.; Jadidi Niaragh, F.; Baghbanzadeh, A.; Vahed, N.; Silvestris, N.; et al. Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): A systematic review and meta-analysis study. Diagnostics 2020, 10, 704. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the PD-L1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Matikas, A.; Zerdes, I.; Lövrot, J.; Richard, F.; Sotiriou, C.; Bergh, J.; Valachis, A.; Foukakis, T. Prognostic implications of PD-L1 expression in breast cancer: Systematic review and meta-analysis of immunohistochemistry and pooled analysis of transcriptomic data. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5717–5726. [Google Scholar] [CrossRef]
- Miglietta, L.; Morabito, F.; Provinciali, N.; Canobbio, L.; Meszaros, P.; Naso, C.; Murialdo, R.; Boitano, M.; Salvi, S.; Ferrarini, M. A prognostic model based on combining estrogen receptor expression and Ki-67 value after neoadjuvant chemotherapy predicts clinical outcome in locally advanced breast cancer: Extension and analysis of a previously reported cohort of patients. Eur. J. Surg. Oncol. EJSO 2013, 39, 1046–1052. [Google Scholar] [CrossRef]
- Tokuda, E.; Horimoto, Y.; Arakawa, A.; Himuro, T.; Senuma, K.; Nakai, K.; Saito, M. Differences in Ki67 expressions between pre- and post-neoadjuvant chemotherapy specimens might predict early recurrence of breast cancer. Hum. Pathol. 2017, 63, 40–45. [Google Scholar] [CrossRef]
- Matsubara, N.; Mukai, H.; Masumoto, M.; Sasaki, M.; Naito, Y.; Fujii, S.; Wada, N. Survival outcome and reduction rate of Ki-67 between pre- and post-neoadjuvant chemotherapy in breast cancer patients with non-pCR. Breast Cancer Res. Treat. 2014, 147, 95–102. [Google Scholar] [CrossRef]
- Montagna, E.; Bagnardi, V.; Viale, G.; Rotmensz, N.; Sporchia, A.; Cancello, G.; Balduzzi, A.; Galimberti, V.; Veronesi, P.; Luini, A.; et al. Changes in PgR and Ki-67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann. Oncol. 2015, 26, 307–313. [Google Scholar] [CrossRef]
- Lee, H.-C.; Ko, H.; Seol, H.; Noh, D.-Y.; Han, W.; Kim, T.-Y.; Im, S.-A.; Park, I.A. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J. Breast Cancer 2013, 16, 395–403. [Google Scholar] [CrossRef]
- Faneyte, I.F.; Schrama, J.G.; Peterse, J.L.; Remijnse, P.L.; Rodenhuis, S.; van de Vijver, M.J. Breast cancer response to neoadjuvant chemotherapy: Predictive markers and relation with outcome. Br. J. Cancer 2003, 88, 406–412. [Google Scholar] [CrossRef]
- Enomoto, Y.; Morimoto, T.; Nishimukai, A.; Higuchi, T.; Yanai, A.; Miyagawa, Y.; Murase, K.; Imamura, M.; Takatsuka, Y.; Nomura, T.; et al. Impact of biomarker changes during neoadjuvant chemotherapy for clinical response in patients with residual breast cancers. Int. J. Clin. Oncol. 2016, 21, 254–261. [Google Scholar] [CrossRef]
- Chen, X.; He, C.; Han, D.; Zhou, M.; Wang, Q.; Tian, J.; Li, L.; Xu, F.; Zhou, E.; Yang, K. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: A systematic review and meta-analysis. Future Oncol. Lond. Engl. 2017, 13, 843–857. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, A.; Plata-Fernández, Y.M.; Fernández, M.; Jaén-Morago, A.; Fernández-Navarro, M.; de la Torre-Cabrera, C.; Ramirez-Tortosa, C.; Lomas-Garrido, M.; Llácer, C.; Navarro-Perez, V.; et al. The role of immunohistochemistry in breast cancer patients treated with neoadjuvant chemotherapy: An old tool with an enduring prognostic value. Clin. Breast Cancer 2013, 13, 146–152. [Google Scholar] [CrossRef]
- Ács, B.; Zámbó, V.; Vízkeleti, L.; Szász, A.M.; Madaras, L.; Szentmártoni, G.; Tőkés, T.; Molnár, B.Á.; Molnár, I.A.; Vári-Kakas, S.; et al. Ki-67 as a controversial predictive and prognostic marker in breast cancer patients treated with neoadjuvant chemotherapy. Diagn. Pathol. 2017, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Salter, J.; A’Hern, R.; Nerurkar, A.; Parton, M.; Reis-Filho, J.S.; Smith, I.E.; Dowsett, M. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2009, 116, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Tanei, T.; Shimomura, A.; Shimazu, K.; Nakayama, T.; Kim, S.J.; Iwamoto, T.; Tamaki, Y.; Noguchi, S. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur. J. Surg. Oncol. 2011, 37, 155–161. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Provenzano, E.; Bossuyt, V.; Viale, G.; Cameron, D.; Badve, S.; Denkert, C.; MacGrogan, G.; Penault-Llorca, F.; Boughey, J.; Curigliano, G.; et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: Recommendations from an international working group. Mod. Pathol. 2015, 28, 1185–1201. [Google Scholar] [CrossRef]
- Pinard, C.; Debled, M.; Ben Rejeb, H.; Velasco, V.; Tunon de Lara, C.; Hoppe, S.; Richard, E.; Brouste, V.; Bonnefoi, H.; MacGrogan, G. Residual cancer burden index and tumor-infiltrating lymphocyte subtypes in triple-negative breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2020, 179, 11–23. [Google Scholar] [CrossRef]
- Hamy, A.-S.; Pierga, J.-Y.; Sabaila, A.; Laas, E.; Bonsang-Kitzis, H.; Laurent, C.; Vincent-Salomon, A.; Cottu, P.; Lerebours, F.; Rouzier, R.; et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef]
- Hamy, A.-S.; Lam, G.-T.; Laas, E.; Darrigues, L.; Balezeau, T.; Guerin, J.; Livartowski, A.; Sadacca, B.; Pierga, J.-Y.; Vincent-Salomon, A.; et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res. Treat. 2018, 169, 295–304. [Google Scholar] [CrossRef]
- Miglietta, F.; Dieci, M.V.; Tsvetkova, V.; Griguolo, G.; Vernaci, G.; Menichetti, A.; Faggioni, G.; Giarratano, T.; Mioranza, E.; Genovesi, E.; et al. Validation of residual proliferative cancer burden as a predictor of long-term outcome following neoadjuvant chemotherapy in patients with hormone receptor-positive/human epidermal growth receptor 2-negative breast cancer. Oncologist 2020. [Google Scholar] [CrossRef]
- Cogswell, J.; Inzunza, H.D.; Wu, Q.; Feder, J.N.; Mintier, G.; Novotny, J.; Cardona, D.M. An analytical comparison of Dako 28-8 PharmDx Assay and an E1L3N laboratory-developed test in the immunohistochemical detection of programmed death-ligand 1. Mol. Diagn. Ther. 2017, 21, 85–93. [Google Scholar] [CrossRef]
- Schats, K.A.; Van Vré, E.A.; De Schepper, S.; Boeckx, C.; Schrijvers, D.M.; Waelput, W.; Fransen, E.; Vanden Bempt, I.; Neyns, B.; De Meester, I.; et al. Validated programmed cell death ligand 1 immunohistochemistry assays (E1L3N and SP142) reveal similar immune cell staining patterns in melanoma when using the same sensitive detection system. Histopathology 2017, 70, 253–263. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, Y.; Kim, J.-W.; Lee, J.-C.; Hwang, J.-H.; Yoon, Y.-S.; Cho, J.Y.; Han, H.-S.; Choi, Y.; Kim, H. Programmed cell death ligand-1 (PD-L1) expression in extrahepatic biliary tract cancers: A comparative study using 22C3, SP263 and E1L3N anti-PD-L1 antibodies. Histopathology 2019, 75, 526–536. [Google Scholar] [CrossRef]
- Smith, J.; Robida, M.D.; Acosta, K.; Vennapusa, B.; Mistry, A.; Martin, G.; Yates, A.; Hnatyszyn, H.J. Quantitative and qualitative characterization of two PD-L1 clones: SP263 and E1L3N. Diagn. Pathol. 2016, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Finetti, P.; Colpaert, C.; Mamessier, E.; Parizel, M.; Dirix, L.; Viens, P.; Birnbaum, D.; van Laere, S. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015, 6, 13506–13519. [Google Scholar] [CrossRef]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449–5464. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Pelekanou, V.; Carvajal-Hausdorf, D.E.; Altan, M.; Wasserman, B.; Carvajal-Hausdorf, C.; Wimberly, H.; Brown, J.; Lannin, D.; Pusztai, L.; Rimm, D.L. Effect of Neoadjuvant Chemotherapy on Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Breast Cancer and Its Clinical Significance. Breast Cancer Res. 2017, 19, 91. [Google Scholar] [CrossRef]
- Srivastava, V.; Akshay, B.R.; Kumari, S.; Meena, R.N.; Khanna, R. Effect of Neoadjuvant Chemotherapy (NAC) on Programmed Cell Death Ligand (PD-L1) in Patients of Carcinoma Breast: A Prospective Study in Indian Tertiary Care Setting. J. Fam. Med. Prim. Care 2020, 9, 4086–4091. [Google Scholar] [CrossRef]

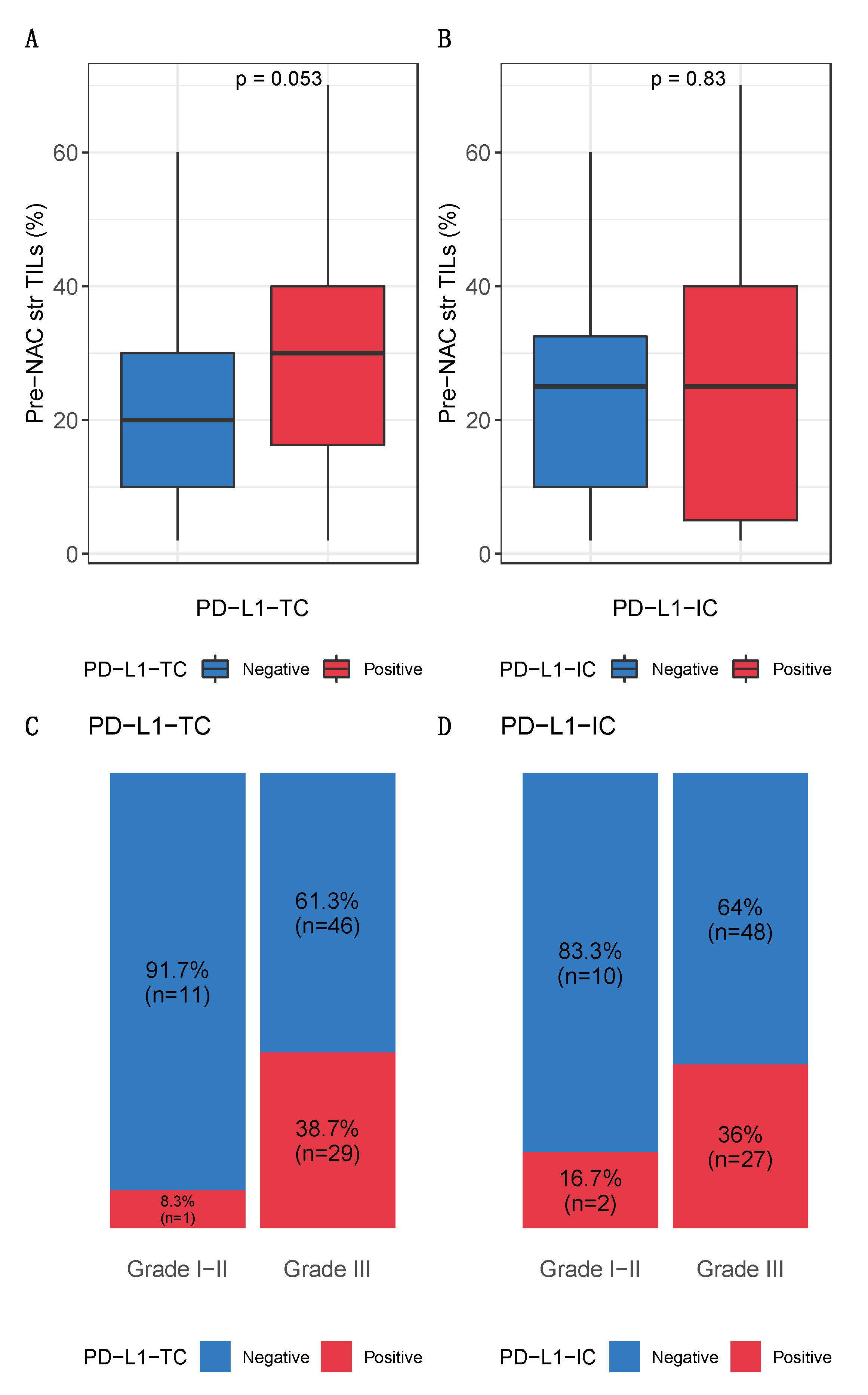


| PDL-L1-TC | PDL-L1-IC | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Class | Overall | Negative (0%) | Positive (≥1%) | p | Negative (0%) | Positive (≥1%) | p |
| n= | 89 | 58 | 31 | 59 | 30 | |||
| Baseline Characteristics | ||||||||
| Age | 50.20 [39.40, 57.90] | 49.03 (10.87) | 49.16 (11.23) | 0.960 | 48.94 (10.54) | 49.34 (11.85) | 0.871 | |
| Family history | No | 68 (76.4) | 44 (75.9) | 24 (77.4) | 1.000 | 46 (78.0) | 22 (73.3) | 0.824 |
| Yes | 21 (23.6) | 14 (24.1) | 7 (22.6) | 13 (22.0) | 8 (26.7) | |||
| Menopausal status | Premenopausal | 51 (57.3) | 33 (56.9) | 18 (58.1) | 1.000 | 34 (57.6) | 17 (56.7) | 1.000 |
| Postmenopausal | 38 (42.7) | 25 (43.1) | 13 (41.9) | 25 (42.4) | 13 (43.3) | |||
| BMI classes | 18.5–24.9 | 47 (52.8) | 34 (58.6) | 13 (41.9) | 0.266 | 34 (57.6) | 13 (43.3) | 0.221 |
| <18.5 | 2 (2.2) | 2 (3.4) | 0 (0.0) | 2 (3.4) | 0 (0.0) | |||
| 25–29.9 | 22 (24.7) | 12 (20.7) | 10 (32.3) | 11 (18.6) | 11 (36.7) | |||
| >=30 | 18 (20.2) | 10 (17.2) | 8 (25.8) | 12 (20.3) | 6 (20.0) | |||
| Smoking status | Never | 61 (71.8) | 42 (75.0) | 19 (65.5) | 0.452 | 42 (73.7) | 19 (67.9) | 0.366 |
| Current | 12 (14.1) | 8 (14.3) | 4 (13.8) | 9 (15.8) | 3 (10.7) | |||
| Former | 12 (14.1) | 6 (10.7) | 6 (20.7) | 6 (10.5) | 6 (21.4) | |||
| Comorbidity | No | 35 (45.5) | 25 (49.0) | 10 (38.5) | 0.524 | 27 (51.9) | 8 (32.0) | 0.162 |
| Yes | 42 (54.5) | 26 (51.0) | 16 (61.5) | 25 (48.1) | 17 (68.0) | |||
| Clinicar T stage | T0–T1 | 6 (6.7) | 2 (3.4) | 4 (12.9) | 0.077 | 4 (6.8) | 2 (6.7) | 0.257 |
| T2 | 62 (69.7) | 39 (67.2) | 23 (74.2) | 38 (64.4) | 24 (80.0) | |||
| T3–T4 | 21 (23.6) | 17 (29.3) | 4 (12.9) | 17 (28.8) | 4 (13.3) | |||
| Clinical N stage | N0 | 38 (42.7) | 25 (43.1) | 13 (41.9) | 1.000 | 26 (44.1) | 12 (40.0) | 0.889 |
| N1–N2–N3 | 51 (57.3) | 33 (56.9) | 18 (58.1) | 33 (55.9) | 18 (60.0) | |||
| SBR grade | Grade I-II | 12 (13.8) | 11 (19.3) | 1 (3.3) | 0.084 | 10 (17.2) | 2 (6.9) | 0.323 |
| Grade III | 75 (86.2) | 46 (80.7) | 29 (96.7) | 48 (82.8) | 27 (93.1) | |||
| Mitotic index | 32.62 (25.80) | 23.00 [11.00, 40.25] | 35.50 [14.75, 52.75] | 0.165 | 24.00 [11.00, 41.00] | 30.00 [14.00, 52.00] | 0.283 | |
| Mitotic index class | [0,20) | 34 (41.5) | 24 (44.4) | 10 (35.7) | 0.600 | 24 (43.6) | 10 (37.0) | 0.740 |
| >=20 | 48 (58.5) | 30 (55.6) | 18 (64.3) | 31 (56.4) | 17 (63.0) | |||
| Ductal carcinoma in situ | No | 83 (93.3) | 53 (91.4) | 30 (96.8) | 0.601 | 55 (93.2) | 28 (93.3) | 1.000 |
| Yes | 6 (6.7) | 5 (8.6) | 1 (3.2) | 4 (6.8) | 2 (6.7) | |||
| % stromal lymphocytes | 25.00 [10.00, 40.00] | 20.00 [10.00, 30.00] | 30.00 [16.25, 40.00] | 0.053 | 25.00 [10.00, 32.50] | 25.00 [5.00, 40.00] | 0.823 | |
| BC surgery | Lumpectomy | 59 (66.3) | 41 (70.7) | 18 (58.1) | 0.334 | 42 (71.2) | 17 (56.7) | 0.257 |
| Mastectomy | 30 (33.7) | 17 (29.3) | 13 (41.9) | 17 (28.8) | 13 (43.3) | |||
| NAC regimen | anthra-taxans | 85 (95.5) | 55 (94.8) | 30 (96.8) | 0.761 | 56 (94.9) | 29 (96.7) | 0.773 |
| anthra | 3 (3.4) | 2 (3.4) | 1 (3.2) | 2 (3.4) | 1 (3.3) | |||
| taxanes | 1 (1.1) | 1 (1.7) | 0 (0.0) | 1 (1.7) | 0 (0.0) | |||
| Post-NAC characteristics | ||||||||
| ypN stage | 0 | 53 (59.6) | 35 (60.3) | 18 (58.1) | 0.109 | 33 (55.9) | 20 (66.7) | 0.065 |
| [1-3] | 22 (24.7) | 17 (29.3) | 5 (16.1) | 19 (32.2) | 3 (10.0) | |||
| [4-9] | 13 (14.6) | 5 (8.6) | 8 (25.8) | 6 (10.2) | 7 (23.3) | |||
| 10 and more | 1 (1.1) | 1 (1.7) | 0 (0.0) | 1 (1.7) | 0 (0.0) | |||
| RCB index | 2.12 [1.54, 2.98] | 2.03 [1.45, 2.56] | 2.27 [1.67, 3.72] | 0.035 | 2.19 [1.52, 2.76] | 2.10 [1.63, 3.45] | 0.456 | |
| RCB classes | RCB-I | 15 (17.2) | 12 (21.1) | 3 (10.0) | 0.040 | 13 (22.0) | 2 (7.1) | 0.185 |
| 53 (60.9) | 37 (64.9) | 16 (53.3) | 35 (59.3) | 18 (64.3) | ||||
| RCB-III | 19 (21.8) | 8 (14.0) | 11 (36.7) | 11 (18.6) | 8 (28.6) | |||
| KI67 status | [0,20) | 51 (57.3) | 34 (58.6) | 17 (54.8) | 0.905 | 34 (57.6) | 17 (56.7) | 1.000 |
| >=20 | 38 (42.7) | 24 (41.4) | 14 (45.2) | 25 (42.4) | 13 (43.3) | |||
| Mitotic index | 31.28 (40.93) | 15.00 [0.00, 47.00] | 25.00 [3.00, 70.00] | 0.036 | 17.00 [0.00, 50.00] | 14.00 [2.50, 51.50] | 0.541 | |
| Mitotic index class | [0,20) | 43 (55.1) | 29 (55.8) | 14 (53.8) | 1.000 | 28 (53.8) | 15 (57.7) | 0.936 |
| >=20 | 35 (44.9) | 23 (44.2) | 12 (46.2) | 24 (46.2) | 11 (42.3) | |||
| % stromal lymphocytes | 15.00 [10.00, 30.00] | 15.00 [10.00, 30.00] | 20.00 [6.25, 30.00] | 0.860 | 15.00 [10.00, 30.00] | 22.50 [5.00, 30.00] | 0.850 | |
| Lymphovascular invasion | No | 54 (70.1) | 32 (65.3) | 22 (78.6) | 0.335 | 32 (65.3) | 22 (78.6) | 0.335 |
| Yes | 23 (29.9) | 17 (34.7) | 6 (21.4) | 17 (34.7) | 6 (21.4) | |||
| First Author | Journal | Year | Country | n Patients (RD) | Detection Technique (IHC Clone) | Cut-Off | PDL-1 Prevalence in CNB | PDL1 and pCR | PDL-1 Prevalence in RD | PDL1 and DFS | PDL1 and OS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelekanou (SWOG S0800) [64] | Breast cancer research | 2017 | USA | 58 (Her2-negative) | RD: SP142 and 22C3 Dako combined CNB: E1L3N | QIF = 500 | 21/41 (51.0%) | p = 0.018 | 10 (17.2%) | - | - |
| Pelekanou (SWOG S0800) [27] | Molecular cancer therapeutics | 2018 | USA | 43 (Her2-negative) out of which 9 TNBC | 22C3 Dako | QIF = 500 | 52/120 (43%) | higher pCR rates immune + tumor c. (p = 0.008) sole tumor c. NS (p = 0.10) | 14 (33%) | - (in CNB NS p = 0.14) | - (in CNB NS p = 0.64) |
| Wang [25] | Journal of Breast Cancer | 2018 | China | 114 TNBC | EPR19759 | H-score of 100 | Tumor and/or Immune c. 48 (32.4%) | HR = 1.34 (0.54; 3.30) p = 0.53 | 43 (37.7%) | Tumor/Immune c. NS; HR = 1.421 (0.78; 2.59) p = 0.249 | - |
| Li (SWOG S0800) [28] | Journal for Immunotherapy of Cancer | 2019 | USA | 60 (Her2-negative) | 22C3 Dako | 1% | - | NS in tumor c. (p = 0.1578) and immune c. (p = 0.0722) | - | - | - |
| Loibl (GeparNuevo study) [15] | Annals of Oncology | 2019 | Germany | 174 TNBC | SP263 Ventana | 1% | Tumor and/or Immune c. 138/158 (87.3%) | Durvalumab group 47/88 (53.4%) Placebo group 38/86 (44.2%) | - | - | - |
| Srivastava [65] | Journal of Family Medicine and Primary Care | 2020 | India | 30 | Abcam NA | H-score of 100 | 11 (36.7%) | - | 4 (13.3%) | - | - |
| Grandal | Cancers | 2020 | France | 89 TNBC | E1L3N | 1% | - | - | Tumor c. n = 17 (19.1%) Immune c. n = 14 (15.7%) | Tumor c. NS (p = 0.38) Immune c. NS (p = 0.23) | Tumor c. NS (p = 0.48) Immune c. NS (p = 0.46) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandal, B.; Mangiardi-Veltin, M.; Laas, E.; Laé, M.; Meseure, D.; Bataillon, G.; El-Alam, E.; Darrigues, L.; Dumas, E.; Daoud, E.; et al. PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy. Cancers 2021, 13, 746. https://doi.org/10.3390/cancers13040746
Grandal B, Mangiardi-Veltin M, Laas E, Laé M, Meseure D, Bataillon G, El-Alam E, Darrigues L, Dumas E, Daoud E, et al. PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy. Cancers. 2021; 13(4):746. https://doi.org/10.3390/cancers13040746
Chicago/Turabian StyleGrandal, Beatriz, Manon Mangiardi-Veltin, Enora Laas, Marick Laé, Didier Meseure, Guillaume Bataillon, Elsy El-Alam, Lauren Darrigues, Elise Dumas, Eric Daoud, and et al. 2021. "PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy" Cancers 13, no. 4: 746. https://doi.org/10.3390/cancers13040746
APA StyleGrandal, B., Mangiardi-Veltin, M., Laas, E., Laé, M., Meseure, D., Bataillon, G., El-Alam, E., Darrigues, L., Dumas, E., Daoud, E., Vincent-Salomon, A., Talagrand, L.-S., Pierga, J.-Y., Reyal, F., & Hamy, A.-S. (2021). PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy. Cancers, 13(4), 746. https://doi.org/10.3390/cancers13040746








