Computed Tomography Based Radiomics as a Predictor of Survival in Ovarian Cancer Patients: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
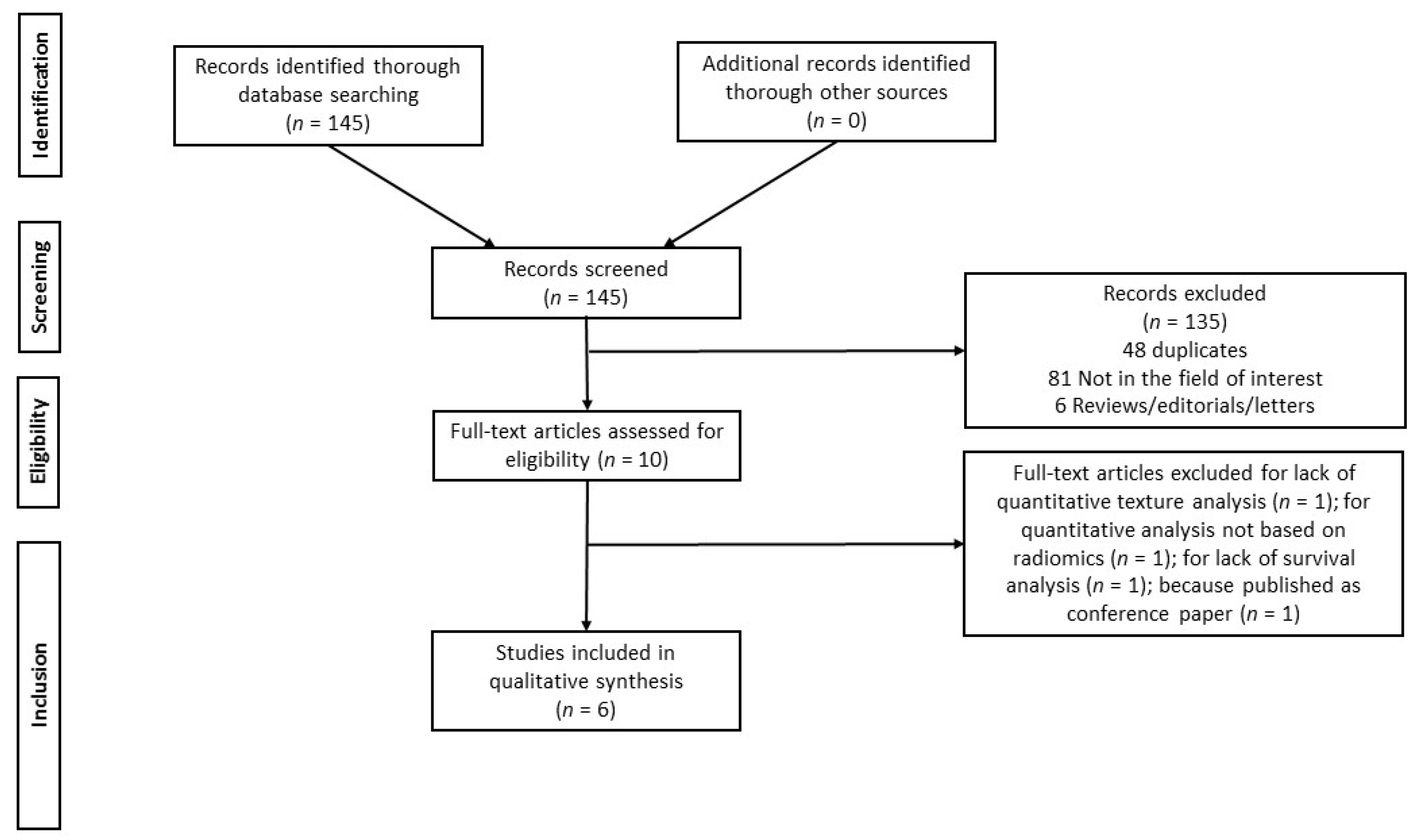
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Literature Search
3.2. Basic Study and Patient Characteristics
3.3. Methodological and Technical Aspects of the Included Studies
3.4. Main Findings
3.4.1. Overall Survival
3.4.2. Progression Free Survival
3.4.3. Radiomic Similarity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Forstner, R.; Meissnitzer, M.W.; Cunha, T.M. Update on Imaging of Ovarian Cancer. Curr. Radiol. Rep. 2016, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; McCague, C.; Tibermacine, H.; Vargas, H.A.; Rizzo, S.; Sala, E. Radiomics and radiogenomics in ovarian cancer: A literature review. Abdom. Radiol. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Hu, H.-T.; Shan, Q.-Y.; Chen, S.; Li, B.; Feng, S.-T.; Xu, E.; Li, X.; Long, J.-Y.; Xie, X.-H.; Lu, M.-D.; et al. CT-based radiomics for preoperative prediction of early recurrent hepatocellular carcinoma: Technical reproducibility of acquisition and scanners. Radiol. Med. 2020, 125, 697–705. [Google Scholar] [CrossRef]
- De Jong, E.E.; van Elmpt, W.; Rizzo, S.; Colarieti, A.; Spitaleri, G.; Leijenaar, R.T.H.; Jochems, A.; Hendriks, L.E.L.; Troost, E.G.C.; Reymen, B.; et al. Applicability of a prognostic CT-based radiomic signature model trained on stage I-III non-small cell lung cancer in stage IV non-small cell lung cancer. Lung Cancer 2018, 124, 6–11. [Google Scholar] [CrossRef]
- Botta, F.; Raimondi, S.; Rinaldi, L.; Bellerba, F.; Corso, F.; Bagnardi, V.; Origgi, D.; Minelli, R.; Pitoni, G.; Petrella, F.; et al. Association of a CT-Based Clinical and Radiomics Score of Non-Small Cell Lung Cancer (NSCLC) with Lymph Node Status and Overall Survival. Cancers 2020, 12, 1432. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.; Deist, T.M.; Peerlings, J.; De Jong, E.E.; Van Timmeren, J.; Sanduleanu, S.; LaRue, R.T.H.M.; Even, A.J.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Grassi, R.; Miele, V.; Giovagnoni, A. Artificial intelligence: A challenge for third millennium radiologist. Radiol. Med. 2019, 124, 241–242. [Google Scholar] [CrossRef]
- Neri, E.; Coppola, F.; Miele, V.; Bibbolino, C.; Grassi, R. Artificial intelligence: Who is responsible for the diagnosis? Radiol. Med. 2020, 125, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, L.; Li, G.; Zhang, X.; Ren, J.; Shi, Z.; Li, J.; Yu, S. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol. Med. 2020, 125, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Tardieu, M.; Vargas, H.; Reinhold, C.; Perre, S.V.; Bonanno, N.; Sala, E.; Thomassin-Naggara, I. Ovarian cancer: An update on imaging in the era of radiomics. Diagn. Interv. Imaging 2019, 100, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gonzalez, P.; Crispin-Ortuzar, M.; Rundo, L.; Delgado-Ortet, M.; Reinius, M.; Beer, L.; Woitek, R.; Ursprung, S.; Addley, H.; Brenton, J.D.; et al. Integrative radiogenomics for virtual biopsy and treatment monitoring in ovarian cancer. Insights Imaging 2020, 11. [Google Scholar] [CrossRef]
- De Piano, F.; Buscarino, V.; Maresca, D.; Maisonneuve, P.; Aletti, G.; Lazzari, R.; Vavassori, A.; Bellomi, M.; Rizzo, S. Do DWI and quantitative DCE perfusion MR have a prognostic value in high-grade serous ovarian cancer? Radiol. Med. 2019, 124, 1315–1323. [Google Scholar] [CrossRef]
- Ciolina, M.; Vinci, V.; Villani, L.; Gigli, S.; Saldari, M.; Panici, P.B.; Perniola, G.; Laghi, A.; Catalano, C.; Manganaro, L. Texture analysis versus conventional MRI prognostic factors in predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced cancer of the uterine cervix. Radiol. Med. 2019, 124, 955–964. [Google Scholar] [CrossRef]
- McInnes, M.D.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; PRISMA-DTA. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.M.; The QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Lu, H.; Arshad, M.; Thornton, A.; Avesani, G.; Cunnea, P.; Curry, E.; Kanavati, F.; Liang, J.; Nixon, K.; Williams, S.T.; et al. A mathematical-descriptor of tumor-mesoscopic-structure from computed-tomography images annotates prognostic- and molecular-phenotypes of epithelial ovarian cancer. Nat. Commun. 2019, 10, 764. [Google Scholar] [CrossRef]
- Meier, A.; Veeraraghavan, H.; Nougaret, S.; Lakhman, Y.; Sosa, R.; Soslow, R.; Sutton, E.J.; Hricak, H.; Sala, E.; Vargas, H.A. Association between CT-texture-derived tumor heterogeneity, outcomes, and BRCA mutation status in patients with high-grade serous ovarian cancer. Abdom. Radiol. 2019, 44, 2040–2047. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Buscarino, V.; Colarieti, A.; Tomao, F.; Aletti, G.; Zanagnolo, V.; Del Grande, M.; et al. Radiomics of high-grade serous ovarian cancer: Association between quantitative CT features, residual tumour and disease progression within 12 months. Eur. Radiol. 2018, 28, 4849–4859. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Veeraraghavan, H.; Micco, M.; Nougaret, S.; Lakhman, Y.; Meier, A.A.; Sosa, R.; Soslow, R.A.; Levine, D.A.; Weigelt, B.; et al. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur. Radiol. 2017, 27, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Z.; Rong, Y.; Zhou, B.; Bai, Y.; Wang, S.; Wang, M.; Guo, Y.; Tian, J. A Computed Tomography-Based Radiomic Prognostic Marker of Advanced High-Grade Serous Ovarian Cancer Recurrence: A Multicenter Study. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Zargari, A.; Du, Y.; Heidari, M.; Thai, T.C.; Gunderson, C.C.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Prediction of chemotherapy response in ovarian cancer patients using a new clustered quantitative image marker. Phys. Med. Biol. 2018, 63, 155020. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for ad-vanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Chi, D.; Franklin, C.C.; Levine, D.A.; Akselrod, F.; Sabbatini, P.; Jarnagin, W.R.; DeMatteo, R.; Poynor, E.A.; Abu-Rustum, N.R.; Barakat, R.R. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: A change in surgical approach. Gynecol. Oncol. 2004, 94, 650–654. [Google Scholar] [CrossRef]
- Chang, S.-J.; Bristow, R.E.; Ryu, H.-S. Impact of Complete Cytoreduction Leaving No Gross Residual Disease Associated with Radical Cytoreductive Surgical Procedures on Survival in Advanced Ovarian Cancer. Ann. Surg. Oncol. 2012, 19, 4059–4067. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Garganese, G.; Vizzielli, G.; Carone, V.; Salerno, M.G.; Scambia, G. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am. J. Obstet. Gynecol. 2008, 199, 642.e1–642.e6. [Google Scholar] [CrossRef]
- Reverdy, T.; Sajous, C.; Péron, J.; Glehen, O.; Bakrin, N.; Gertych, W.; Lopez, J.; You, B.; Freyer, G. Front-Line Maintenance Therapy in Advanced Ovarian Cancer—Current Advances and Perspectives. Cancers 2020, 12, 2414. [Google Scholar] [CrossRef]
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125 (Suppl. S24), 4563–4572. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B.; et al. Novel Molecular Subtypes of Serous and Endometrioid Ovarian Cancer Linked to Clinical Outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Minasian, L.M.; Doong, H. New strategies in ovarian cancer treatment. Cancer 2019, 125, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Tamayo, P.; Yang, J.-Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H.; et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Investig. 2012, 123, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yan, C.; Qu, L.; Du, R.; Lin, J. The efficacy and toxicity of angiogenesis inhibitors for ovarian cancer: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Liu, Y.; Zhang, T.; He, J.; Zhao, H.; An, R.; Xue, Y. Efficacy and safety of PARP inhibitors in the treatment of advanced ovarian cancer: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2020, 157, 103145. [Google Scholar] [CrossRef]

| Authors | Year | Country | Journal Impact Factor * | Quartile * | Study Design | N Patients | Mean/Median Age | FIGO Stage I–II | FIGO Stage III–V | OS Evaluation (Median, Follow-Up Months) | PFS Evaluation (Median, Follow-Up Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu, H., et al. [19] | 2019 | UK | 12.121 | Q1 | R | 364 | 62 | 53 | 223 | Yes (53.2) | Yes (23.1) |
| Meier, A., et al. [20] | 2019 | US | 2.429 | Q2 | R | 88 | 75 | ND | ND | Yes (59) | Yes (59) |
| Rizzo, S., et al. [21] | 2018 | Italy | 4.101 | Q1 | R | 101 | 53 | 11 | 90 | No | Yes (26) |
| Vargas, H.A., et al. [22] | 2017 | US | 4.101 | Q1 | R | 38 | ND | 0 | 38 | Yes (56.4) | No |
| Wei, W., et al. [23] | 2019 | China | 4.848 | Q2 | R | 142 | 50 | 0 | 142 | No | Yes (27.7) |
| Zargari, A., et al. [24] | 2019 | US | 2.883 | Q2 | R | 120 | 67 | ND | ND | No | Yes (ND) |
| Authors | Validation Group/Groups | Extraction of Features Exclusively from Ovaries | Extraction of Features from More than One Site of Disease | Number of Features Included in the Final Model | Categories and Names of Features Included | Software Used for Segmentation | Software Used for Feature Extraction |
|---|---|---|---|---|---|---|---|
| Lu, H., et al. [19] | Yes (internal and external) | Yes | No | 4 | Shape, density, texture and wavelet (GLRLM; NGTDM; FOS; FD) | ITK-SNAP | TextLAB 2.0 |
| Meier, A., et al. [20] | No | No | Yes | 3 | Texture, Haralick (GLCM; SE; SCV; SCP) | ITK-SNAP | Matlab based |
| Rizzo, S., et al. [21] | No | Yes | No | 3 | Shape, density, texture (GLRLM; shape 3D; GLCM) | DICOM RT structure | IBEX |
| Vargas, H.A., et al. [22] | No | No | Yes | 3 | Texture, Haralick (GLCM; SE; SCS; SCP) | 3D Slicer | ND |
| Wei, W., et al. [23] | Yes (internal and external) | Yes | No | 4 | Shape, texture, histogram, wavelet (FOS; GLSZM) | ITK-SNAP | Matlab based |
| Zargari, A., et al. [24] | Yes (internal) | No | Yes | 11 | Shape, density, texture, wavelet Shape and density; DCT; GLDM; Wavelet) | ND | ND |
| Authors | CT Scan Manufacturer and Protocol (Slice Thickness; Acquisition Parameters; Contrast Bolus) | ROI Tracing (Single Slice/Volumetric; Manual/Semi-Automatic/Automatic; Single or Multi Reviewers) |
|---|---|---|
| Lu, H., et al. [19] | Several CT manufacturers and protocols | Volumetric; ND; 3 reviewers |
| Meier, A., et al. [20] | GE Medical Systems; tube voltage 120 kVp; tube current 240–400 mA; section thickness 5–7.5 mm; pitch < 1; kernel Bf40; iodinated contrast medium yes | Volumetric; manual; ND |
| Rizzo, S., et al. [21] | Several CT manufacturers; slice thickness 1–5 mm; tube current x rotation 58–419 mAS, reconstruction algorithm filtered back projection and iterative; iodinated contrast medium yes | Volumetric; manual; single reviewer |
| Vargas, H.A., et al. [22] | GE Medical Systems; tube voltage 120 kVp; tube current 240–400 mA; section thickness 5–7.5 mm; pitch < 1; iodinated contrast medium yes | Volumetric; manual; ND |
| Wei, W., et al. [23] | Philips Medical System, GE Medical Systems; tube voltage 120 kVp; tube current 100–500 mA; section thickness 2–5 mm; pitch < 1; iodinated contrast medium yes | Volumetric; manual; 2 reviewers |
| Zargari, A., et al. [24] | GE Medical Systems; tube voltage 120 kVp; tube current 100–600 mA; section thickness 5 mm; pitch 1.25; iodinated contrast medium yes | Volumetric, semi-automatic; single reviewer |
| Authors | Significant Associations with OS | Significant Associations with PFS | Significant Associations with Radiomic Similarity |
|---|---|---|---|
| Lu, H., et al. [19] | Association between 4 features (RPV) and OS. RPV improved the clinical prognostic methods | Association between RPV and PFS | NP |
| Meier, A., et al. [20] | Association between SE and OS | Association between SCV and SCP with PFS | Association between SE, SCV, SCP and surgical resection status in BRCA- |
| Rizzo, S., et al. [21] | NP | Association between 3 features and 12-months recurrence. The clinical-radiomics model outperformed the clinical model. | NP |
| Vargas, H.A., et al. [22] | Association between SE, SCS and SCP and OS | NP | Association between heterogeneity and surgical resection status. |
| Wei, W., et al. [23] | NP | 4 features associated with prediction of 3-year recurrence. Better performance of the radiomic model than the clinical prognostic model | NP |
| Zargari, A., et al. [24] | NP | Association between 11 features and PFS. Greater weights for the shape and density features | NP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, S.; Manganaro, L.; Dolciami, M.; Gasparri, M.L.; Papadia, A.; Del Grande, F. Computed Tomography Based Radiomics as a Predictor of Survival in Ovarian Cancer Patients: A Systematic Review. Cancers 2021, 13, 573. https://doi.org/10.3390/cancers13030573
Rizzo S, Manganaro L, Dolciami M, Gasparri ML, Papadia A, Del Grande F. Computed Tomography Based Radiomics as a Predictor of Survival in Ovarian Cancer Patients: A Systematic Review. Cancers. 2021; 13(3):573. https://doi.org/10.3390/cancers13030573
Chicago/Turabian StyleRizzo, Stefania, Lucia Manganaro, Miriam Dolciami, Maria Luisa Gasparri, Andrea Papadia, and Filippo Del Grande. 2021. "Computed Tomography Based Radiomics as a Predictor of Survival in Ovarian Cancer Patients: A Systematic Review" Cancers 13, no. 3: 573. https://doi.org/10.3390/cancers13030573
APA StyleRizzo, S., Manganaro, L., Dolciami, M., Gasparri, M. L., Papadia, A., & Del Grande, F. (2021). Computed Tomography Based Radiomics as a Predictor of Survival in Ovarian Cancer Patients: A Systematic Review. Cancers, 13(3), 573. https://doi.org/10.3390/cancers13030573








