Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy
Simple Summary
Abstract
1. Introduction
2. TNFα Overview
3. TNFα and the Immune System
4. TNFα in Cancer
| Cancer Type | Pro-Tumorigenic | References | Anti-Tumorigenic | References |
|---|---|---|---|---|
| Breast | Promotes proliferation, progression, and metastasis | [70] | Apoptosis and inhibition of proliferation | [70] |
| Gastric | Proliferation, progression and metastasis | [104,105,106,107,108] | Apoptosis acting together with TGFβ | [109] |
| Pancreatic | Promotes tumor progression | [110,111] | Apoptosis | [112,113,114] |
| Generates a immune evasive microenvironment | [115] | - | - | |
| Ovarian | Tumor promotion through TNFR1 and IL-17 | [116] | - | - |
| Generates a immunosuppresor microenvironment | [117] | - | ||
| Contributes to the EMT process through the NF-κB pathway | [64] | - | ||
| Tumor proliferation, progression, and invasion. | [118,119,120] | - | ||
| Prostate | Survival and proliferation, progression, angiogenesis and metastasis | [121,122,123,124,125,126] | Apoptosis | [127,128] |
| Bladder | Migration and invasion through the p38 MAPK pathway | [129,130,131] | Apoptosis | [132,133] |
| Colorectal | Together with Th17-cytokines promotes immune escape, proliferation, survival, progression, and metastasis | [134,135,136] | - | - |
| Oral | Promotes immune evasion | [137] | - | - |
| Promotes cell viability | [138] | - | ||
| Promotes angiogenesis, invasion and metastasis | [139,140] | - | ||
| Liver | Induces PTTG1, which in turn upregulates c-myc | [141] | In combination with IFN-γ showed reduction of liver tumors | [142] |
| Promotes proliferation and metastasis in HCC through p38 MAPK, Erk1/2 and β-catenin | [143,144,145] | - | - | |
| Promotes resistance to the adaptive immune response through PD-L1 and PD-L2 | [146] | - | - | |
| Melanoma | Induces cell invasion and metastasis | [74,76] | Reduces tumor growth | [147,148] |
| Increases aggressiveness | [75] | Apoptosis | [149] | |
| Hematological | Cell survival | [150,151,152] | Apoptosis thorugh TNFR1, iNOS and PKC | [153,154] |
| Promotes progression through the NF-κB pathway and proliferation thorugh GM-CSF | [81,155,156] | Increases efficacy of anti-CD20 therapy | [82] | |
| Promotes cell survival in Burkitt’s lymphoma through reverse signaling | [157] | Induces maturation of AML generating specific cytotoxic CD8+ lymphocytes targeting leukemic disease | [158] | |
| - | - | Activate B cells to fight again lymphoma cells | [159] | |
| - | - | Combined with IL-1 and IFN-γ has an antiproliferative effect | [160] | |
| - | - | Participates in the crosstalk between DC and NK cells | [161] | |
| - | - | Promotes cell death in Burkitt’s lymphoma through forward signaling | [157] |
5. Immunotherapy Overview
5.1. Monoclonal Antibodies
Anti-TNFα Drugs
5.2. Monoclonal Antibodies Targeting Cancer Cells
5.2.1. HER2
5.2.2. EGFR/HER1
5.2.3. CD20
5.3. Monoclonal Antibodies against Immune Checkpoints
5.4. TNFα in Resistance to Anti-PD-1/PD-L1 and Anti-CTLA-4 Therapies
5.5. TNFα Involvement in the Adverse Effects of Immune Checkpoint Inhibitors
5.6. Adoptive Cell Therapies
6. Clinical Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coussens, L.M.L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Green, S.; Dobrjansky, A.; Carswell, E.A.; Kassel, R.L.; Old, L.J.; Fiore, N.; Schwartz, M.K. Partial Purification of a Serum Factor That Causes Necrosis of Tumors. Proc. Natl. Acad. Sci. USA 1976, 73, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Nedwin, G.E.; Hayflick, J.S.; Seeburg, P.H.; Derynck, R.; Palladino, M.A.; Kohr, W.J.; Aggarwal, B.B.; Goeddel, D.V. Human Tumour Necrosis Factor: Precursor Structure, Expression and Homology to Lymphotoxin. Nature 1984, 312, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional Control of the TNF Gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.Y.; Yie, J.; Thanos, D.; Goldfeld, A.E. Cell-Type-Specific Regulation of the Human Tumor Necrosis Factor Alpha Gene in B Cells and T Cells by NFATp and ATF-2/JUN. Mol. Cell. Biol. 1996, 16, 5232–5244. [Google Scholar] [CrossRef] [PubMed]
- Gahring, L.C.; Carlson, N.G.; Kulmar, R.A.; Rogers, S.W. Neuronal Expression of Tumor Necrosis Factor Alpha in the Murine Brain. Neuroimmunomodulation 1996, 3, 289–303. [Google Scholar] [CrossRef]
- Ranta, V.; Orpana, A.; Carpén, O.; Turpeinen, U.; Ylikorkala, O.; Viinikka, L. Human Vascular Endothelial Cells Produce Tumor Necrosis Factor-Alpha in Response to Proinflammatory Cytokine Stimulation. Crit. Care Med. 1999, 27, 2184–2187. [Google Scholar] [CrossRef]
- Rydén, M.; Arner, P. Tumour Necrosis Factor-Alpha in Human Adipose Tissue-from Signalling Mechanisms to Clinical Implications. J. Intern. Med. 2007, 262, 431–438. [Google Scholar] [CrossRef]
- Spriggs, D.R.; Deutsch, S.; Kufe, D.W. Genomic Structure, Induction, and Production of TNF-Alpha. Immunol. Ser. 1992, 56, 3–34. [Google Scholar]
- Shakhov, A.N.; Collart, M.A.; Vassalli, P.; Nedospasov, S.A.; Jongeneel, C.V. Kappa B-Type Enhancers Are Involved in Lipopolysaccharide-Mediated Transcriptional Activation of the Tumor Necrosis Factor Alpha Gene in Primary Macrophages. J. Exp. Med. 1990, 171, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhao, G.; Li, H. Forward and Reverse Signaling Mediated by Transmembrane Tumor Necrosis Factor-Alpha and TNF Receptor 2: Potential Roles in an Immunosuppressive Tumor Microenvironment. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S.D. A Novel Form of TNF/Cachectin Is a Cell Surface Cytotoxic Transmembrane Protein: Ramifications for the Complex Physiology of TNF. Cell 2018, 53, 45–53. [Google Scholar] [CrossRef]
- Perez, C.; Albert, I.; DeFay, K.; Zachariades, N.; Gooding, L.; Kriegler, M. A Nonsecretable Cell Surface Mutant of Tumor Necrosis Factor (TNF) Kills by Cell-to-Cell Contact. Cell 1990, 63, 251–258. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Weber, R.F.; Figari, I.S.; Reynolds, C.; Palladino, M.A., Jr.; Goeddel, D.V. The Two Different Receptors for Tumor Necrosis Factor Mediate Distinct Cellular Responses. Proc. Natl. Acad. Sci. USA 1991, 88, 9292–9296. [Google Scholar] [CrossRef]
- Ledgerwood, E.C.; Pober, J.S.; Bradley, J.R. Recent Advances in the Molecular Basis of TNF Signal Transduction. Lab. Invest. 1999, 79, 1041–1050. [Google Scholar]
- Aderka, D.; Engelmann, H.; Maor, Y.; Brakebusch, C.; Wallach, D. Stabilization of the Bioactivity of Tumor Necrosis Factor by Its Soluble Receptors. J. Exp. Med. 1992, 175, 323–329. [Google Scholar] [CrossRef]
- Watts, A.D.; Hunt, N.H.; Madigan, M.C.; Chaudhri, G. Soluble TNF-Alpha Receptors Bind and Neutralize over-Expressed Transmembrane TNF-Alpha on Macrophages, but Do Not Inhibit Its Processing. J. Leukoc. Biol. 1999, 66, 1005–1013. [Google Scholar] [CrossRef]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor Necrosis Factor Antagonist Mechanisms of Action: A Comprehensive Review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, K.H.; Park, H.S.; Kim, S.K.; Koh, C.M.; Park, J.Y. Differential Expression, Shedding, Cytokine Regulation and Function of TNFR1 and TNFR2 in Human Fetal Astrocytes. Yonsei Med. J. 2005, 46, 818–826. [Google Scholar] [CrossRef]
- Faustman, D.; Davis, M. TNF Receptor 2 Pathway: Drug Target for Autoimmune Diseases. Nat. Rev. Drug Discov. 2010, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Hsu, H.; Goeddel, D.V.; Karin, M. Dissection of TNF Receptor 1 Effector Functions: JNK Activation Is Not Linked to Apoptosis While NF-KappaB Activation Prevents Cell Death. Cell 1996, 87, 565–576. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M.; Tepper, C.G.; Seldin, M.F.; O’Rourke, K.; Kischkel, F.C.; Hellbardt, S.; Krammer, P.H.; Peter, M.E.; Dixit, V.M. FADD/MORT1 Is a Common Mediator of CD95 (Fas/APO-1) and Tumor Necrosis Factor Receptor-Induced Apoptosis. J. Biol. Chem. 1996, 271, 4961–4965. [Google Scholar] [CrossRef]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-Dependent APO-1 (Fas/CD95)-Associated Proteins Form a Death-Inducing Signaling Complex (DISC) with the Receptor. Embo J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Ihnatko, R.; Kubes, M. TNF Signaling: Early Events and Phosphorylation. Gen. Physiol. Biophys. 2007, 26, 159–167. [Google Scholar] [PubMed]
- Bradley, J.R. TNF-Mediated Inflammatory Disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Rothe, M.; Wong, S.C.; Henzel, W.J.; Goeddel, D.V. A Novel Family of Putative Signal Transducers Associated with the Cytoplasmic Domain of the 75 KDa Tumor Necrosis Factor Receptor. Cell 1994, 78, 681–692. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; den Boer, J.A.; Luiten, P.G.M.; Eisel, U.L.M. Tumor Necrosis Factor Receptor Cross-Talk. FEBS J. 2011, 278, 888–898. [Google Scholar] [CrossRef]
- Nunes-Alves, C.; Booty, M.G.; Carpenter, S.M.; Jayaraman, P.; Rothchild, A.C.; Behar, S.M. In Search of a New Paradigm for Protective Immunity to TB. Nat. Rev. Microbiol. 2014, 12, 289–299. [Google Scholar] [CrossRef]
- Marino, M.W.; Dunn, A.; Grail, D.; Inglese, M.; Noguchi, Y.; Richards, E.; Jungbluth, A.; Wada, H.; Moore, M.; Williamson, B.; et al. Characterization of Tumor Necrosis Factor-Deficient Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8093–8098. [Google Scholar] [CrossRef]
- Lin, P.L.; Myers, A.; Smith, L.; Bigbee, C.; Bigbee, M.; Fuhrman, C.; Grieser, H.; Chiosea, I.; Voitenek, N.N.; Capuano, S.V.; et al. Tumor Necrosis Factor Neutralization Results in Disseminated Disease in Acute and Latent Mycobacterium Tuberculosis Infection with Normal Granuloma Structure in a Cynomolgus Macaque Model. Arthritis Rheum. 2010, 62, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Meinken, C.; Schauenberg, P.; Härter, G.; Kern, P.; Modlin, R.L.; Antoni, C.; Stenger, S. Anti-TNF Immunotherapy Reduces CD8+ T Cell-Mediated Antimicrobial Activity against Mycobacterium Tuberculosis in Humans. J. Clin. Investig. 2009, 119, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Maggi, E.; Vultaggio, A. Cellular and Humoral Immune Responses during Tuberculosis Infection: Useful Knowledge in the Era of Biological Agents. J. Rheumatol. Suppl. 2014, 91, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Baseta, J.G.; Stutman, O. TNF Regulates Thymocyte Production by Apoptosis and Proliferation of the Triple Negative (CD3-CD4-CD8-) Subset. J. Immunol. 2000, 165, 5621–5630. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Luz, A.; Pfeffer, K.; Holzmann, B. Defective Peyer’s Patch Organogenesis in Mice Lacking the 55-KD Receptor for Tumor Necrosis Factor. J. Exp. Med. 1996, 184, 259–264. [Google Scholar] [CrossRef]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and Inflammatory Responses in TNF Alpha-Deficient Mice: A Critical Requirement for TNF Alpha in the Formation of Primary B Cell Follicles, Follicular Dendritic Cell Networks and Germinal Centers, and in the Maturation of the Humoral Immune Respons. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour Necrosis Factor and Cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Jung, M.K.; Lee, J.S.; Kwak, J.E.; Shin, E.C. Tumor Necrosis Factor and Regulatory T Cells. Yonsei Med. J. 2019, 60, 126–131. [Google Scholar] [CrossRef]
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B Regulatory Cells and the Tumor-Promoting Actions of TNF-α during Squamous Carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667. [Google Scholar] [CrossRef]
- Mauri, C.; Menon, M. Human Regulatory B Cells in Health and Disease: Therapeutic Potential. J. Clin. Investig. 2017, 127, 772–779. [Google Scholar] [CrossRef]
- Zhao, X.X.; Rong, L.; Zhao, X.X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF Signaling Drives Myeloid-Derived Suppressor Cell Accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Krötschel, M.; Conrad, L.; Naumann, S.K.; Bachran, C.; Rolfe, A.; Umansky, V.; Helming, L.; Swee, L.K. Genetic Screen in Myeloid Cells Identifies TNF-α Autocrine Secretion as a Factor Increasing MDSC Suppressive Activity via Nos2 up-Regulation. Sci. Rep. 2018, 8, 13399. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Lu, Y.; Eisele, M.R.; Sulistijo, E.S.; Khan, N.; Fan, R.; Miller-Jensen, K. Analysis of Single-Cell Cytokine Secretion Reveals a Role for Paracrine Signaling in Coordinating Macrophage Responses to TLR4 Stimulation. Sci. Signal. 2015, 8, ra59. [Google Scholar] [CrossRef] [PubMed]
- Nagar, M.; Jacob-Hirsch, J.; Vernitsky, H.; Berkun, Y.; Ben-Horin, S.; Amariglio, N.; Bank, I.; Kloog, Y.; Rechavi, G.; Goldstein, I. TNF Activates a NF-KappaB-Regulated Cellular Program in Human CD45RA-Regulatory T Cells That Modulates Their Suppressive Function. J. Immunol. 2010, 184, 3570–3581. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2018, 357, 539–545. [Google Scholar] [CrossRef]
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour Necrosis Factor and Cancer. J. Pathol. 2013, 230, 241–248. [Google Scholar] [CrossRef]
- Bauswein, M.; Singh, A.; Ralhan, A.; Neri, D.; Fuchs, K.; Blanz, K.D.; Schäfer, I.; Hector, A.; Handgretinger, R.; Hartl, D.; et al. Human T Cells Modulate Myeloid-Derived Suppressor Cells through a TNF-α-Mediated Mechanism. Immunol. Lett. 2018, 202, 31–37. [Google Scholar] [CrossRef]
- Hu, X.; Li, B.; Li, X.; Zhao, X.; Wan, L.; Lin, G.; Yu, M.; Wang, J.; Jiang, X.; Feng, W.; et al. Transmembrane TNF-α Promotes Suppressive Activities of Myeloid-Derived Suppressor Cells via TNFR2. J. Immunol. 2014, 192, 1320–1331. [Google Scholar] [CrossRef]
- Dong, G.; You, M.; Fan, H.; Ji, J.; Ding, L.; Li, P.; Hou, Y. 17β-Estradiol Contributes to the Accumulation of Myeloid-Derived Suppressor Cells in Blood by Promoting TNF-α Secretion. Acta Biochim. Biophys. Sin. 2015, 47, 620–629. [Google Scholar] [CrossRef]
- Greish, K.; Taurin, S.; Morsy, M.A. The Effect of Adjuvant Therapy with TNF-α on Animal Model of Triple-Negative Breast Cancer. Ther. Deliv. 2018, 9, 333–342. [Google Scholar] [CrossRef]
- Rath, P.C.; Aggarwal, B.B. TNF-Induced Signaling in Apoptosis. J. Clin. Immunol. 1999, 19, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage Polarization in Bacterial Infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F.J.; Liénard, D.; Matter, M.; Rüegg, C. Efficiency of Recombinant Human TNF in Human Cancer Therapy. Cancer Immun. 2006, 6, 6. [Google Scholar] [PubMed]
- Qiao, Y.; Huang, X.; Nimmagadda, S.; Bai, R.; Staedtke, V.; Foss, C.A.; Cheong, I.; Holdhoff, M.; Kato, Y.; Pomper, M.G.; et al. A Robust Approach to Enhance Tumor-Selective Accumulation of Nanoparticles. Oncotarget 2011, 2, 59–68. [Google Scholar] [CrossRef]
- Kratochvill, F.; Neale, G.; Haverkamp, J.M.; Van de Velde, L.-A.; Smith, A.M.; Kawauchi, D.; McEvoy, J.; Roussel, M.F.; Dyer, M.A.; Qualls, J.E.; et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015, 12, 1902–1914. [Google Scholar] [CrossRef]
- Hoving, S.; Seynhaeve, A.L.B.; van Tiel, S.T.; Aan de Wiel-Ambagtsheer, G.; de Bruijn, E.A.; Eggermont, A.M.M.; ten Hagen, T.L.M. Early Destruction of Tumor Vasculature in Tumor Necrosis Factor-Alpha-Based Isolated Limb Perfusion Is Responsible for Tumor Response. Anticancer Drugs 2006, 17, 949–959. [Google Scholar] [CrossRef]
- Mackay, F.; Loetscher, H.; Stueber, D.; Gehr, G.; Lesslauer, W. Tumor Necrosis Factor Alpha (TNF-Alpha)-Induced Cell Adhesion to Human Endothelial Cells Is under Dominant Control of One TNF Receptor Type, TNF-R55. J. Exp. Med. 1993, 177, 1277–1286. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-Alpha in Promotion and Progression of Cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O. The Dual Role of Tumor Necrosis Factor-Alpha (TNF- α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell. Oncol. 2020, 1–18. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, X.; Bu, X.; Zhang, N.; Wang, W. Involvement of Tumor Necrosis Factor-Alpha in the Upregulation of CXCR4 Expression in Gastric Cancer Induced by Helicobacter Pylori. BMC Cancer 2010, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Egberts, J.-H.; Cloosters, V.; Noack, A.; Schniewind, B.; Thon, L.; Klose, S.; Kettler, B.; von Forstner, C.; Kneitz, C.; Tepel, J.; et al. Anti-Tumor Necrosis Factor Therapy Inhibits Pancreatic Tumor Growth and Metastasis. Cancer Res. 2008, 68, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-α Expression, Risk Factors, and Inflammatory Exposures in Ovarian Cancer: Evidence for an Inflammatory Pathway of Ovarian Carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.S.; Malik, S.T.; Stamp, G.W.; Jobling, T.; Balkwill, F.R. In Situ Detection of Tumour Necrosis Factor in Human Ovarian Cancer Specimens. Eur. J. Cancer 1990, 26, 1027–1030. [Google Scholar] [CrossRef]
- Morgado, M.; Sutton, M.N.; Simmons, M.; Warren, C.R.; Lu, Z.; Constantinou, P.E.; Liu, J.; Francis, L.L.W.; Steven Conlan, R.; Bast, R.C.; et al. Tumor Necrosis Factor-α and Interferon-γ Stimulate MUC16 (CA125) Expression in Breast, Endometrial and Ovarian Cancers through NFκB. Oncotarget 2016, 7, 14871–14884. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted Link between Cancer and Inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Ren, H.J.; Ma, J.; Sun, X.; Li, N.; Liu, C.; Huang, K.; Xu, M.; Ming, L. Molecular Correlates and Prognostic Value of TmTNF-α Expression in Colorectal Cancer of 5-Fluorouracil-Based Adjuvant Therapy. Cancer Biol. Ther. 2016, 17, 684–692. [Google Scholar] [CrossRef]
- Tang, D.; Tao, D.; Fang, Y.; Deng, C.; Xu, Q.; Zhou, J. TNF-Alpha Promotes Invasion and Metastasis via NF-Kappa B Pathway in Oral Squamous Cell Carcinoma. Med. Sci. Monit. Basic Res. 2017, 23, 141–149. [Google Scholar] [CrossRef]
- Roberts, R.A.; Kimber, I. Cytokines in Non-Genotoxic Hepatocarcinogenesis. Carcinogenesis 1999, 20, 1397–1401. [Google Scholar] [CrossRef]
- Burow, M.E.; Weldon, C.B.; Tang, Y.; Navar, G.L.; Krajewski, S.; Reed, J.C.; Hammond, T.G.; Clejan, S.; Beckman, B.S. Differences in Susceptibility to Tumor Necrosis Factor α-Induced Apoptosis among MCF-7 Breast Cancer Cell Variants. Cancer Res. 1998, 58, 4940–4946. [Google Scholar]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, M.F.; De Martino, M.; Venturutti, L.; Rivas, M.A.; Proietti, C.J.; Inurrigarro, G.; Frahm, I.; Allemand, D.H.; Deza, E.G.; Ares, S.; et al. TNFα-Induced Mucin 4 Expression Elicits Trastuzumab Resistance in HER2-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fernandez, S.A.; Criswell, T.; Chidiac, T.A.; Guttridge, D.; Villalona-Calero, M.; Bekaii-Saab, T.S. Disrupting Cytokine Signaling in Pancreatic Cancer: A Phase I/II Study of Etanercept in Combination with Gemcitabine in Patients with Advanced Disease. Pancreas 2013, 42, 813–818. [Google Scholar] [CrossRef]
- Katerinaki, E.; Evans, G.S.; Lorigan, P.C.; MacNeil, S. TNF-Alpha Increases Human Melanoma Cell Invasion and Migration in Vitro: The Role of Proteolytic Enzymes. Br. J. Cancer 2003, 89, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Cordella, M.; Tabolacci, C.; Nassa, G.; D’Arcangelo, D.; Senatore, C.; Pagnotto, P.; Magliozzi, R.; Salvati, A.; Weisz, A.; et al. TNF-Alpha and Metalloproteases as Key Players in Melanoma Cells Aggressiveness. J. Exp. Clin. Cancer Res. 2018, 37, 326. [Google Scholar] [CrossRef]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; van den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-Radiation-Induced Inflammation Promotes Angiotropism and Metastasis in Melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef]
- Bertrand, F.; Rochotte, J.; Colacios, C.; Montfort, A.; Tilkin-Mariamé, A.-F.; Touriol, C.; Rochaix, P.; Lajoie-Mazenc, I.; Andrieu-Abadie, N.; Levade, T.; et al. Blocking Tumor Necrosis Factor α Enhances CD8 T-Cell-Dependent Immunity in Experimental Melanoma. Cancer Res. 2015, 75, 2619–2628. [Google Scholar] [CrossRef]
- Waterston, A.M.; Salway, F.; Andreakos, E.; Butler, D.M.; Feldmann, M.; Coombes, R.C. TNF Autovaccination Induces Self Anti-TNF Antibodies and Inhibits Metastasis in a Murine Melanoma Model. Br. J. Cancer 2004, 90, 1279–1284. [Google Scholar] [CrossRef][Green Version]
- St John, M.A.R.; Li, Y.; Zhou, X.; Denny, P.; Ho, C.-M.; Montemagno, C.; Shi, W.; Qi, F.; Wu, B.; Sinha, U.; et al. Interleukin 6 and Interleukin 8 as Potential Biomarkers for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 929–935. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, H.S.; Liu, Z.X.; Kim, R.H.; Kang, M.K.; Park, N.-H.; Shin, K.-H. TNFα Enhances Cancer Stem Cell-like Phenotype via Notch-Hes1 Activation in Oral Squamous Cell Carcinoma Cells. Biochem. Biophys. Res. Commun. 2012, 424, 58–64. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Li, B.; Li, Q.; Gao, L.; Li, D.; Gong, Q.; Zhu, L.; Wang, J.; Wang, N.; et al. Transmembrane TNF-α Preferentially Expressed by Leukemia Stem Cells and Blasts Is a Potent Target for Antibody Therapy. Blood 2015, 126, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, S.; Deshpande, C.G.; Ranganathan, R.; Huang, X.; Jajeh, A.; O’Brien, T.; Huang, R.W.; Gregory, S.A.; Venugopal, P.; Preisler, H.D. Tumor Necrosis Factor Modulates CD 20 Expression on Cells from Chronic Lymphocytic Leukemia: A New Role for TNF Alpha? Microsc. Res. Tech. 2000, 50, 251–257. [Google Scholar] [CrossRef]
- Chopra, V.; Dinh, T.V.; Hannigan, E.V. Serum Levels of Interleukins, Growth Factors and Angiogenin in Patients with Endometrial Cancer. J. Cancer Res. Clin. Oncol. 1997, 123, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.E.; Barron, G.A.; Bermano, G. Adipocytokines and Their Relationship to Endometrial Cancer Risk: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2020, 158, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Becker, S.; Rinaldi, S.; Lukanova, A.; Tjønneland, A.; Olsen, A.; Overvad, K.; Chabbert-Buffet, N.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; et al. Tumor Necrosis Factor (TNF)-α, Soluble TNF Receptors and Endometrial Cancer Risk: The EPIC Study. Int. J. Cancer 2011, 129, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Gray, K.P.; Harshman, L.C.; Evan, C.; Nakabayashi, M.; Fichorova, R.; Rider, J.; Mucci, L.; Kantoff, P.W.; Sweeney, C.J. Elevated IL-8, TNF-α, and MCP-1 in Men with Metastatic Prostate Cancer Starting Androgen-Deprivation Therapy (ADT) Are Associated with Shorter Time to Castration-Resistance and Overall Survival. Prostate 2014, 74, 820–828. [Google Scholar] [CrossRef]
- Banzola, I.; Mengus, C.; Wyler, S.; Hudolin, T.; Manzella, G.; Chiarugi, A.; Boldorini, R.; Sais, G.; Schmidli, T.S.; Chiffi, G.; et al. Expression of Indoleamine 2,3-Dioxygenase Induced by IFN-γ and TNF-α as Potential Biomarker of Prostate Cancer Progression. Front. Immunol. 2018, 9, 1051. [Google Scholar] [CrossRef]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef]
- Dantas, T.S.; de Barros Silva, P.G.; Lima Verde, M.E.Q.; de Ribeiro Junior, A.L.; do Cunha, M.P.S.S.; Mota, M.R.L.; Alves, A.P.N.N.; de Leitão, R.F.C.; Sousa, F.B. Role of Inflammatory Markers in Prognosis of Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 3635–3642. [Google Scholar] [CrossRef]
- Jaime-Pérez, J.C.; Gamboa-Alonso, C.M.; Jiménez-Castillo, R.A.; López-Silva, L.J.; Pinzón-Uresti, M.A.; Gómez-De León, A.; Gómez-Almaguer, D. TNF-α Increases in the CSF of Children with Acute Lymphoblastic Leukemia before CNS Relapse. Blood Cells Mol. Dis. 2017, 63, 27–31. [Google Scholar] [CrossRef]
- Potapnev, M.P.; Petyovka, N.V.; Belevtsev, M.V.; Savitskiy, V.P.; Migal, N.V. Plasma Level of Tumor Necrosis Factor-Alpha (TNF-Alpha) Correlates with Leukocytosis and Biological Features of Leukemic Cells, but Not Treatment Response of Children with Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2003, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Macia, J.; Gomez, X.; Esquerda, A.; Perez, B.; Callao, V.; Marzo, C. Value of the Determination of TNF-Alpha in the Plasma of Patients with Non-Hodgkins Lymphoma. Leuk. Lymphoma 1996, 20, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Lech-Maranda, E.; Bienvenu, J.; Broussais-Guillaumot, F.; Warzocha, K.; Michallet, A.-S.; Robak, T.; Coiffier, B.; Salles, G. Plasma TNF-Alpha and IL-10 Level-Based Prognostic Model Predicts Outcome of Patients with Diffuse Large B-Cell Lymphoma in Different Risk Groups Defined by the International Prognostic Index. Arch. Immunol. Exp. 2010, 58, 131–141. [Google Scholar] [CrossRef]
- Nakayama, S.; Yokote, T.; Tsuji, M.; Akioka, T.; Miyoshi, T.; Hirata, Y.; Hiraoka, N.; Iwaki, K.; Takayama, A.; Nishiwaki, U.; et al. TNF-α Receptor 1 Expression Predicts Poor Prognosis of Diffuse Large B-Cell Lymphoma, Not Otherwise Specified. Am. J. Surg. Pathol. 2014, 38, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Dantas, R.N.; de Souza, A.M.; Herrero, S.S.T.; Kassab, P.; Malheiros, C.A.; Lima, E.M. Association between PSCA, TNF-α, PARP1 and TP53 Gene Polymorphisms and Gastric Cancer Susceptibility in the Brazilian Population. Asian Pac. J. Cancer Prev. 2020, 21, 43–48. [Google Scholar] [CrossRef]
- Du, L.C.; Gao, R. Role of TNF-α -308G/A Gene Polymorphism in Gastric Cancer Risk: A Case Control Study and Meta-Analysis. J. Turk. Soc. Gastroenterol. 2017, 28, 272–282. [Google Scholar] [CrossRef]
- Pardo, T.; Salcedo, P.; Quintero, J.M.; Borjas, L.; Fernández-Mestre, M.; Sánchez, Y.; Carrillo, Z.; Rivera, S. Study of the association between the polymorphism of the TNF-α gene and prostate cáncer. Rev. Alerg. Mex. 2019, 66, 154–162. [Google Scholar] [CrossRef]
- Shi, H.-Z.; Ren, P.; Lu, Q.-J.; Niedrgethmnn, M.; Wu, G.-Y. Association between EGF, TGF-Β1 and TNF-α Gene Polymorphisms and Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 6217–6220. [Google Scholar] [CrossRef]
- Verma, H.K.; Merchant, N.; Bhaskar, L.V.K.S. Tumor Necrosis Factor-Alpha Gene Promoter (TNF-α G-308A) Polymorphisms Increase the Risk of Hepatocellular Carcinoma in Asians: A Meta-Analysis. Crit. Rev. Oncog. 2020, 25, 11–20. [Google Scholar] [CrossRef]
- He, Y.-Q.; Zhu, J.-H.; Huang, S.-Y.; Cui, Z.; He, J.; Jia, W.-H. The Association between the Polymorphisms of TNF-α and Non-Hodgkin Lymphoma: A Meta-Analysis. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2014, 35, 12509–12517. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Song, B.; Wang, T.; Liu, Y.; Hao, J.; Yu, J. Genetic Variations in CTLA-4, TNF-α, and LTA and Susceptibility to T-Cell Lymphoma in a Chinese Population. Cancer Epidemiol. 2013, 37, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Hellmig, S.; Fischbach, W.; Goebeler-Kolve, M.-E.; Fölsch, U.R.; Hampe, J.; Schreiber, S. A Functional Promotor Polymorphism of TNF-Alpha Is Associated with Primary Gastric B-Cell Lymphoma. Am. J. Gastroenterol. 2005, 100, 2644–2649. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.; Zidi, S.; Sghaier, I.; Ghazouani, E.; Mezlini, A.; Almawi, W.; Loueslati, B.Y. Common Variants in IL-1RN, IL-1β and TNF-α and the Risk of Ovarian Cancer: A Case Control Study. Cent. J. Immunol. 2017, 42, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Komekado, H.; Sawabu, T.; Ishizu, S.; Nakanishi, Y.; Nakatsuji, M.; Akitake-Kawano, R.; Ohno, M.; Hiraoka, Y.; Kawada, M.; et al. Nardilysin and ADAM Proteases Promote Gastric Cancer Cell Growth by Activating Intrinsic Cytokine Signalling via Enhanced Ectodomain Shedding of TNF-α. Embo Mol. Med. 2012, 4, 396–411. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Nakanishi, H.; Kodera, Y.; Ito, S.; Yamamura, Y.; Kato, T.; Hibi, K.; Akiyama, S.; Nakao, A.; Tatematsu, M. TNF-Alpha Promotes Progression of Peritoneal Metastasis as Demonstrated Using a Green Fluorescence Protein (GFP)-Tagged Human Gastric Cancer Cell Line. Clin. Exp. Metastasis 2004, 21, 39–47. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, H.; Cao, A.; Cao, L.; Hu, X. Cytokine TNF-α Promotes Invasion and Metastasis of Gastric Cancer by down-Regulating Pentraxin3. J. Cancer 2020, 11, 1800–1807. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.; Wang, X.; Yu, Z.; Pan, T.; Li, Z.; Chang, X.; Jin, Z.; Li, J.; Zhu, Z.; et al. The Reciprocal Interaction between Tumor Cells and Activated Fibroblasts Mediated by TNF-α/IL-33/ST2L Signaling Promotes Gastric Cancer Metastasis. Oncogene 2020, 39, 1414–1428. [Google Scholar] [CrossRef]
- Kim, S.; Choi, M.G.; Lee, H.S.; Lee, S.K.; Kim, S.H.; Kim, W.W.; Hur, S.M.; Kim, J.-H.; Choe, J.-H.; Nam, S.J.; et al. Silibinin Suppresses TNF-Alpha-Induced MMP-9 Expression in Gastric Cancer Cells through Inhibition of the MAPK Pathway. Molecules 2009, 14, 4300–4311. [Google Scholar] [CrossRef]
- Ha Thi, H.T.; Lim, H.-S.; Kim, J.; Kim, Y.-M.; Kim, H.-Y.; Hong, S. Transcriptional and Post-Translational Regulation of Bim Is Essential for TGF-β and TNF-α-Induced Apoptosis of Gastric Cancer Cell. Biochim. Biophys. Acta 2013, 1830, 3584–3592. [Google Scholar] [CrossRef]
- Alam, M.S.; Gaida, M.M.; Bergmann, F.; Lasitschka, F.; Giese, T.; Giese, N.A.; Hackert, T.; Hinz, U.; Hussain, S.P.; Kozlov, S.V.; et al. Selective Inhibition of the P38 Alternative Activation Pathway in Infiltrating T Cells Inhibits Pancreatic Cancer Progression. Nat. Med. 2015, 21, 1337–1343. [Google Scholar] [CrossRef]
- Aida, K.; Miyakawa, R.; Suzuki, K.; Narumi, K.; Udagawa, T.; Yamamoto, Y.; Chikaraishi, T.; Yoshida, T.; Aoki, K. Suppression of Tregs by Anti-Glucocorticoid Induced TNF Receptor Antibody Enhances the Antitumor Immunity of Interferon-α Gene Therapy for Pancreatic Cancer. Cancer Sci. 2014, 105, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin Confers Pancreatic Cancer Cell Resistance to TNF-α-Induced Apoptosis through Akt/PI3K/NF-ΚB Activation and IL-6/Mcl-1 Overexpression. Mol. Cancer 2011, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, T.; Wang, Y.; Shao, L.; Zhang, Y.; Ma, D.; Han, W. CMTM5 Induces Apoptosis of Pancreatic Cancer Cells and Has Synergistic Effects with TNF-Alpha. Biochem. Biophys. Res. Commun. 2009, 387, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.R.; King, C.R.; Osborn, R.; Fairweather, W.R.; O’Reilly, E.M.; Thornton, M.O.; Wei, L.L. Combination of Human Tumor Necrosis Factor-Alpha (HTNF-Alpha) Gene Delivery with Gemcitabine Is Effective in Models of Pancreatic Cancer. Cancer Gene Ther. 2009, 16, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Imai, K.; Ishimoto, T.; Komohara, Y.; Yamashita, Y.-I.; Nakagawa, S.; Umezaki, N.; Yamao, T.; Kitano, Y.; Miyata, T.; et al. PD-L1 Expression Enhancement by Infiltrating Macrophage-Derived Tumor Necrosis Factor-α Leads to Poor Pancreatic Cancer Prognosis. Cancer Sci. 2019, 110, 310–320. [Google Scholar] [CrossRef]
- Charles, K.A.; Kulbe, H.; Soper, R.; Escorcio-Correia, M.; Lawrence, T.; Schultheis, A.; Chakravarty, P.; Thompson, R.G.; Kollias, G.; Smyth, J.F.; et al. The Tumor-Promoting Actions of TNF-Alpha Involve TNFR1 and IL-17 in Ovarian Cancer in Mice and Humans. J. Clin. Investig. 2009, 119, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Kassim, S.K.; Saeda, L.; Laban, M.; Khalifa, A. Ovarian Cancer-Induced Immunosuppression: Relationship to Tumor Necrosis Factor-Alpha (TNF-Alpha) Release from Ovarian Tissue. Anticancer Res. 1999, 19, 5657–5662. [Google Scholar]
- Wu, S.; Rodabaugh, K.; Martinez-Maza, O.; Watson, J.M.; Silberstein, D.S.; Boyer, C.M.; Peters, W.P.; Weinberg, J.B.; Berek, J.S.; Bast, R.C.J. Stimulation of Ovarian Tumor Cell Proliferation with Monocyte Products Including Interleukin-1, Interleukin-6, and Tumor Necrosis Factor-Alpha. Am. J. Obstet. Gynecol. 1992, 166, 997–1007. [Google Scholar] [CrossRef]
- Li, H.; Chen, A.; Yuan, Q.; Chen, W.; Zhong, H.; Teng, M.; Xu, C.; Qiu, Y.; Cao, J. NF-ΚB/Twist Axis Is Involved in Chysin Inhibition of Ovarian Cancer Stem Cell Features Induced by Co-Treatment of TNF-α and TGF-β. Int. J. Clin. Exp. Pathol. 2019, 12, 101–112. [Google Scholar]
- Kim, D.S.; Jang, Y.-J.; Jeon, O.-H.; Kim, D.-S. Saxatilin, a Snake Venom Disintegrin, Suppresses TNF-Alpha-Induced Ovarian Cancer Cell Invasion. J. Biochem. Mol. Biol. 2007, 40, 290–294. [Google Scholar] [CrossRef]
- Schröder, S.K.; Asimakopoulou, A.; Tillmann, S.; Koschmieder, S.; Weiskirchen, R. TNF-α Controls Lipocalin-2 Expression in PC-3 Prostate Cancer Cells. Cytokine 2020, 135, 155214. [Google Scholar] [CrossRef] [PubMed]
- Safari, H.; Zabihi, E.; Pouramir, M.; Morakabati, P.; Abedian, Z.; Karkhah, A.; Nouri, H.R. Decrease of Intracellular ROS by Arbutin Is Associated with Apoptosis Induction and Downregulation of IL-1β and TNF-α in LNCaP.; Prostate Cancer. J. Food Biochem. 2020, e13360. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.Q.; He, Y.H.; Wang, S.B.; Yang, S.; Wang, Y.J.; Nan, C.J.; Bao, Y.F.; Xie, Q.P.; Chen, Y.H. MiR-130b/TNF-α/NF-ΚB/VEGFA Loop Inhibits Prostate Cancer Angiogenesis. Clin. Transl. Oncol. 2020, 22, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yuan, J.; Huang, T.; Zhang, C.; Zhu, Z.; Wang, L.; Jiang, G.; Zeng, F. Stabilization of Snail by HIF-1α and TNF-α Is Required for Hypoxia-Induced Invasion in Prostate Cancer PC3 Cells. Mol. Biol. Rep. 2014, 41, 4573–4582. [Google Scholar] [CrossRef]
- Maolake, A.; Izumi, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; Shigehara, K.; Kadono, Y.; Hiratsuka, K.; et al. Tumor Necrosis Factor-α Induces Prostate Cancer Cell Migration in Lymphatic Metastasis through CCR7 Upregulation. Cancer Sci. 2018, 109, 1524–1531. [Google Scholar] [CrossRef]
- Wang, H.; Fang, R.; Wang, X.-F.; Zhang, F.; Chen, D.-Y.; Zhou, B.; Wang, H.-S.; Cai, S.-H.; Du, J. Stabilization of Snail through AKT/GSK-3β Signaling Pathway Is Required for TNF-α-Induced Epithelial-Mesenchymal Transition in Prostate Cancer PC3 Cells. Eur. J. Pharm. 2013, 714, 48–55. [Google Scholar] [CrossRef]
- Pilling, A.B.; Hwang, O.; Boudreault, A.; Laurent, A.; Hwang, C. IAP Antagonists Enhance Apoptotic Response to Enzalutamide in Castration-Resistant Prostate Cancer Cells via Autocrine TNF-α Signaling. Prostate 2017, 77, 866–877. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Serradji, N.; Xu, X.; Zhou, H.; Ma, Y.; Sun, Z.; Jiang, P.; Du, Y.; Yang, J.; et al. PMS1077 Sensitizes TNF-α Induced Apoptosis in Human Prostate Cancer Cells by Blocking NF-ΚB Signaling Pathway. PLoS ONE 2013, 8, e61132. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, W.-J.; Moon, S.-K. Cordycepin Suppresses TNF-Alpha-Induced Invasion, Migration and Matrix Metalloproteinase-9 Expression in Human Bladder Cancer Cells. Phytother. Res. 2010, 24, 1755–1761. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, S.-S.; Lee, U.-S.; Kim, W.-J.; Moon, S.-K. Signaling Pathway for TNF-Alpha-Induced MMP-9 Expression: Mediation through P38 MAP Kinase, and Inhibition by Anti-Cancer Molecule Magnolol in Human Urinary Bladder Cancer 5637 Cells. Int. Immunopharmacol. 2008, 8, 1821–1826. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, S.-S.; Cho, Y.-H.; Park, K.; Kim, E.-J.; Jung, K.-H.; Kim, S.-K.; Kim, W.-J.; Moon, S.-K. Activation of Matrix Metalloproteinase-9 by TNF-Alpha in Human Urinary Bladder Cancer HT1376 Cells: The Role of MAP Kinase Signaling Pathways. Oncol. Rep. 2008, 19, 1007–1013. [Google Scholar] [PubMed]
- Yang, T.; Shi, R.; Chang, L.; Tang, K.; Chen, K.; Yu, G.; Tian, Y.; Guo, Y.; He, W.; Song, X.; et al. Huachansu Suppresses Human Bladder Cancer Cell Growth through the Fas/Fasl and TNF- Alpha/TNFR1 Pathway in Vitro and in Vivo. J. Exp. Clin. Cancer Res. 2015, 34, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Y.; Zheng, R.; Qin, D.; Liu, G. Effect of TNF-Alpha and IFN-Alpha on the Proliferation and Cytotoxicity of Lymphokine-Activated Killer Cells in Patients with Bladder Cancer. Chin. Med. J. 1997, 110, 180–183. [Google Scholar] [PubMed]
- Grimm, M.; Lazariotou, M.; Kircher, S.; Höfelmayr, A.; Germer, C.T.; von Rahden, B.H.A.; Waaga-Gasser, A.M.; Gasser, M. Tumor Necrosis Factor-α Is Associated with Positive Lymph Node Status in Patients with Recurrence of Colorectal Cancer--Indications for Anti-TNF-α Agents in Cancer Treatment. Anal. Cell. Pathol. 2010, 33, 151–163. [Google Scholar] [CrossRef]
- Shen, Z.; Zhou, R.; Liu, C.; Wang, Y.; Zhan, W.; Shao, Z.; Liu, J.; Zhang, F.; Xu, L.; Zhou, X.; et al. MicroRNA-105 Is Involved in TNF-α-Related Tumor Microenvironment Enhanced Colorectal Cancer Progression. Cell Death Dis. 2017, 8, 3213. [Google Scholar] [CrossRef]
- Møller, T.; James, J.P.; Holmstrøm, K.; Sørensen, F.B.; Lindebjerg, J.; Nielsen, B.S. Co-Detection of MiR-21 and TNF-α MRNA in Budding Cancer Cells in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 1907. [Google Scholar] [CrossRef]
- Kassouf, N.; Thornhill, M.H. Oral Cancer Cell Lines Can Use Multiple Ligands, Including Fas-L, TRAIL and TNF-Alpha, to Induce Apoptosis in Jurkat T Cells: Possible Mechanisms for Immune Escape by Head and Neck Cancers. Oral Oncol. 2008, 44, 672–682. [Google Scholar] [CrossRef]
- Iulia Irimie, A.; Braicu, C.; Zanoaga, O.; Pileczki, V.; Soritau, O.; Berindan-Neagoe, I.; Septimiu Campian, R. Inhibition of Tumor Necrosis Factor Alpha Using RNA Interference in Oral Squamous Cell Carcinoma. J. Buon. 2015, 20, 1107–1114. [Google Scholar]
- Lai, K.-C.; Liu, C.-J.; Lin, T.-J.; Mar, A.-C.; Wang, H.-H.; Chen, C.-W.; Hong, Z.-X.; Lee, T.-C. Blocking TNF-α Inhibits Angiogenesis and Growth of IFIT2-Depleted Metastatic Oral Squamous Cell Carcinoma Cells. Cancer Lett. 2016, 370, 207–215. [Google Scholar] [CrossRef]
- Hsing, E.-W.; Shiah, S.-G.; Peng, H.-Y.; Chen, Y.-W.; Chuu, C.-P.; Hsiao, J.-R.; Lyu, P.-C.; Chang, J.-Y. TNF-α-Induced MiR-450a Mediates TMEM182 Expression to Promote Oral Squamous Cell Carcinoma Motility. PLoS ONE 2019, 14, e0213463. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Guo, Y.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. PTTG1 Is Involved in TNF-α-Related Hepatocellular Carcinoma via the Induction of c-Myc. Cancer Med. 2019, 8, 5702–5715. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, Q.; Rescorla, F.J.; Grosfeld, J.L. Experimental Liver Cancer: Improved Response after Hepatic Artery Ligation and Infusion of Tumor Necrosis Factor-Alpha and Interferon-Gamma. Surgery 1995, 118, 764–768. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yan, H.Q.; Wang, F.; Wang, Y.Y.; Jiang, Y.N.; Wang, Y.N.; Gao, F.G. TIPE2 Inhibits TNF-α-Induced Hepatocellular Carcinoma Cell Metastasis via Erk1/2 Downregulation and NF-ΚB Activation. Int. J. Oncol. 2015, 46, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-P.; Yue, X.; Li, S.-Q. Cathepsin C Interacts with TNF-α/P38 MAPK Signaling Pathway to Promote Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res. Treat. 2020, 52, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, H.; Zhou, C.; Su, Q.; Lin, Y.; Xie, Y.; Huang, Y.; Qiu, Q.; Lin, J.; Huang, X.; et al. TNF-α Derived from M2 Tumor-Associated Macrophages Promotes Epithelial-Mesenchymal Transition and Cancer Stemness through the Wnt/β-Catenin Pathway in SMMC-7721 Hepatocellular Carcinoma Cells. Exp. Cell Res. 2019, 378, 41–50. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Zhang, N.; Zhuang, M.; Zong, Z.; Zou, J.; Li, G.; Wang, X.; Zhou, H.; Zhang, L.; et al. Cross-Talk between TNF-α and IFN-γ Signaling in Induction of B7-H1 Expression in Hepatocellular Carcinoma Cells. Cancer Immunol. Immunother. 2018, 67, 271–283. [Google Scholar] [CrossRef]
- Curnis, F.; Sacchi, A.; Borgna, L.; Magni, F.; Gasparri, A.; Corti, A. Enhancement of Tumor Necrosis Factor Alpha Antitumor Immunotherapeutic Properties by Targeted Delivery to Aminopeptidase N (CD13). Nat. Biotechnol. 2000, 18, 1185–1190. [Google Scholar] [CrossRef]
- Cervera-Carrascon, V.; Siurala, M.; Santos, J.M.; Havunen, R.; Tähtinen, S.; Karell, P.; Sorsa, S.; Kanerva, A.; Hemminki, A. TNFa and IL-2 Armed Adenoviruses Enable Complete Responses by Anti-PD-1 Checkpoint Blockade. Oncoimmunology 2018, 7, e1412902. [Google Scholar] [CrossRef]
- Broussard, L.; Howland, A.; Ryu, S.; Song, K.; Norris, D.; Armstrong, C.A.; Song, P.I. Melanoma Cell Death Mechanisms. Chonnam Med. J. 2018, 54, 135–142. [Google Scholar] [CrossRef][Green Version]
- Warzocha, K.; Robak, T. Antileukemic Effects of Recombinant Human Tumor Necrosis Factor Alpha (Rh-TNF Alpha) with Cyclophosphamide or Methotrexate on Leukemia L1210 and Leukemia P388 in Mice. Acta Haematol. Pol. 1992, 23, 55–62. [Google Scholar]
- Kitajima, I.; Nakajima, T.; Imamura, T.; Takasaki, I.; Kawahara, K.; Okano, T.; Tokioka, T.; Soejima, Y.; Abeyama, K.; Maruyama, I. Induction of Apoptosis in Murine Clonal Osteoblasts Expressed by Human T-Cell Leukemia Virus Type I Tax by NF-Kappa B and TNF-Alpha. J. Bone Miner. Res. 1996, 11, 200–210. [Google Scholar] [CrossRef]
- Gautam, S.C.; Pindolia, K.R.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Freytag, S.O. Antileukemic Activity of TNF-Alpha Gene Therapy with Myeloid Progenitor Cells against Minimal Leukemia. J. Hematother. 1998, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Auh, S.; Blokh, L.; Long, C.; Gagnon, I.; Hamann, K.J. TNF-Alpha Induces Transient Resistance to Fas-Induced Apoptosis in Eosinophilic Acute Myeloid Leukemia Cells. Cell. Mol. Immunol. 2007, 4, 43–52. [Google Scholar]
- D’Alessandro, N.; Flugy, A.; Tolomeo, M.; Dusonchet, L. The Apoptotic Signaling of TNF-Alpha in Multidrug Resistant Friend Leukemia Cells. Anticancer Res. 1998, 18, 3065–3072. [Google Scholar] [PubMed]
- Kagoya, Y.; Yoshimi, A.; Kataoka, K.; Nakagawa, M.; Kumano, K.; Arai, S.; Kobayashi, H.; Saito, T.; Iwakura, Y.; Kurokawa, M. Positive Feedback between NF-ΚB and TNF-α Promotes Leukemia-Initiating Cell Capacity. J. Clin. Investig. 2014, 124, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Brailly, H.; Pebusque, M.J.; Tabilio, A.; Mannoni, P. TNF Alpha Acts in Synergy with GM-CSF to Induce Proliferation of Acute Myeloid Leukemia Cells by up-Regulating the GM-CSF Receptor and GM-CSF Gene Expression. Leukemia 1993, 7, 1557–1563. [Google Scholar]
- Zhang, H.; Yan, D.; Shi, X.; Liang, H.; Pang, Y.; Qin, N.; Chen, H.; Wang, J.; Yin, B.; Jiang, X.; et al. Transmembrane TNF-Alpha Mediates “Forward” and “Reverse” Signaling, Inducing Cell Death or Survival via the NF-KappaB Pathway in Raji Burkitt Lymphoma Cells. J. Leukoc. Biol. 2008, 84, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Saudemont, A.; Corm, S.; Wickham, T.; Hetuin, D.; Quesnel, B. Induction of Leukemia-Specific CD8+ Cytotoxic T Cells with Autologous Myeloid Leukemic Cells Maturated with a Fiber-Modified Adenovirus Encoding TNF-Alpha. Mol. Ther. 2005, 11, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Heilig, B.; Mapara, M.; Bargou, R.; Fiehn, C.; Dörken, B. TNF Alpha Therapy Activates Human B-Lymphoma Cells in Vivo and May Protect Myelopoiesis. Leuk. Res. 1992, 16, 769–773. [Google Scholar] [CrossRef]
- Di Pietro, R.; Robuffo, I.; Pucci, A.M.; Bosco, D.; Santavenere, E. Effects of TNF-Alpha/Colchicine Combined Treatment on Burkitt Lymphoma Cells: Molecular and Ultrastructural Changes. Cytokine 1999, 11, 144–150. [Google Scholar] [CrossRef]
- Gupta, U.; Hira, S.K.; Singh, R.; Paladhi, A.; Srivastava, P.; Pratim Manna, P. Essential Role of TNF-α in Gamma c Cytokine Aided Crosstalk between Dendritic Cells and Natural Killer Cells in Experimental Murine Lymphoma. Int. Immunopharmacol. 2020, 78, 106031. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.R.; De Palma, M. Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.S.; Svrivastava, A.K.; Batra, L.; Barsoumian, H.; Shirwan, H. Current Challenges for Cancer Vaccine Adjuvant Development. Expert Rev. Vaccines 2018, 17, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Aurisicchio, L.; Pallocca, M.; Ciliberto, G.; Palombo, F. The Perfect Personalized Cancer Therapy: Cancer Vaccines against Neoantigens. J. Exp. Clin. Cancer Res. 2018, 37, 86. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, R.; Bruni, S.; De Martino, M.; Mercogliano, M.F.; Inurrigarro, G.; Frahm, I.; Proietti, C.J.; Elizalde, P.V. Abstract P6-20-14: Neutralizing Soluble Tumor Necrosis Factor Alpha Overcomes Trastuzumab-Resistant Breast Cancer Immune Evasion by Downregulating Mucin 4, Improving NK Cell Function and Decreasing Myeloid-Derived Suppressor Cells in Tumor Microenvironmen. Cancer Res. 2019, 79 (Suppl. S4), P6-20-14. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Thomas, D.; O’Brien, S.; Andreeff, M.; Kurzrock, R.; Keating, M.; Albitar, M.; Kantarjian, H.; Giles, F. Recombinant Human Soluble Tumor Necrosis Factor (TNF) Receptor (P75) Fusion Protein Enbrel in Patients with Refractory Hematologic Malignancies. Cancer Chemother. Pharm. 2002, 50, 237–242. [Google Scholar] [CrossRef]
- Woyach, J.A.; Lin, T.S.; Lucas, M.S.; Heerema, N.; Moran, M.E.; Cheney, C.; Lucas, D.M.; Wei, L.; Caligiuri, M.A.; Byrd, J.C. A Phase I/II Study of Rituximab and Etanercept in Patients with Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma. Leukemia 2009, 23, 912–918. [Google Scholar] [CrossRef]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα Blockade Overcomes Resistance to Anti-PD-1 in Experimental Melanoma. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Stucci, S.; Palmirotta, R.; Passarelli, A.; Silvestris, E.; Argentiero, A.; Lanotte, L.; Acquafredda, S.; Todisco, A.; Silvestris, F. Immune-Related Adverse Events during Anticancer Immunotherapy: Pathogenesis and Management. Oncol. Lett. 2017, 14, 5671–5680. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham III, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Badran, Y.R.; Cohen, J.V.; Brastianos, P.K.; Parikh, A.R.; Hong, T.S.; Dougan, M. Concurrent Therapy with Immune Checkpoint Inhibitors and TNFα Blockade in Patients with Gastrointestinal Immune-Related Adverse Events. J. Immunother. Cancer 2019, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, E.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; de Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF Blockade Uncouples Efficacy and Toxicity in Dual CTLA-4 and PD-1 Immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Arriola, E.; Wheater, M.; Karydis, I.; Thomas, G.; Ottensmeier, C. Infliximab for IPILIMUMAB-Related Colitis-Letter. Clin. Cancer Res. 2015, 21, 5642–5643. [Google Scholar] [CrossRef] [PubMed]
- Horvat, T.Z.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Figueroa, J.A.; Reidy, A.; Mirandola, L.; Trotter, K.; Suvorava, N.; Figueroa, A.; Konala, V.; Aulakh, A.; Littlefield, L.; Grizzi, F.; et al. Chimeric Antigen Receptor Engineering: A Right Step in the Evolution of Adoptive Cellular Immunotherapy. Int. Rev. Immunol. 2015, 34, 154–187. [Google Scholar] [CrossRef]
- von Mehren, M.; Adams, G.P.; Weiner, L.M. Monoclonal Antibody Therapy for Cancer. Annu. Rev. Med. 2003, 54, 343–369. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal Antibody Therapy of Cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The Immunogenicity of Humanized and Fully Human Antibodies: Residual Immunogenicity Resides in the CDR Regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef]
- Luo, J.-L.; Maeda, S.; Hsu, L.-C.; Yagita, H.; Karin, M. Inhibition of NF-KappaB in Cancer Cells Converts Inflammation- Induced Tumor Growth Mediated by TNFalpha to TRAIL-Mediated Tumor Regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef]
- Orosz, P.; Echtenacher, B.; Falk, W.; Rüschoff, J.; Weber, D.; Männel, D.N. Enhancement of Experimental Metastasis by Tumor Necrosis Factor. J. Exp. Med. 1993, 177, 1391–1398. [Google Scholar] [CrossRef]
- Kitakata, H.; Nemoto-Sasaki, Y.; Takahashi, Y.; Kondo, T.; Mai, M.; Mukaida, N. Essential Roles of Tumor Necrosis Factor Receptor P55 in Liver Metastasis of Intrasplenic Administration of Colon 26 Cells. Cancer Res. 2002, 62, 6682–6687. [Google Scholar] [PubMed]
- Wilson, J.; Balkwill, F. The Role of Cytokines in the Epithelial Cancer Microenvironment. Semin. Cancer Biol. 2002, 12, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Toi, M.; Saji, H.; Muta, M.; Bando, H.; Kuroi, K.; Koike, M.; Inadera, H.; Matsushima, K. Significance of Macrophage Chemoattractant Protein-1 in Macrophage Recruitment, Angiogenesis, and Survival in Human Breast Cancer. Clin. Cancer Res. 2000, 6, 3282–3289. [Google Scholar] [PubMed]
- Kishimoto, T.; Akira, S.; Narazaki, M.; Taga, T. Interleukin-6 Family of Cytokines and Gp130. Blood 1995, 86, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Moore, R.J.; Arnott, C.H.; East, N.; Thompson, R.G.; Scallon, B.J.; Shealy, D.J.; Balkwill, F.R. An Anti-Tumor Necrosis Factor-Alpha Antibody Inhibits the Development of Experimental Skin Tumors. Mol. Cancer Ther. 2003, 2, 445–451. [Google Scholar]
- Sha, K.; Yeh, S.; Chang, C.; Nastiuk, K.L.; Krolewski, J.J. TNF Signaling Mediates an Enzalutamide-Induced Metastatic Phenotype of Prostate Cancer and Microenvironment Cell Co-Cultures. Oncotarget 2015, 6, 25726–25740. [Google Scholar] [CrossRef][Green Version]
- Madhusudan, S.; Foster, M.; Muthuramalingam, S.R.; Braybrooke, J.P.; Wilner, S.; Kaur, K.; Han, C.; Hoare, S.; Balkwill, F.; Talbot, D.C.; et al. A Phase II Study of Etanercept (Enbrel), a Tumor Necrosis Factor α Inhibitor in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2004, 10, 6528–6534. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.R.; Charles, K.A.; Hoare, S.A.; Rye, R.L.; Jodrell, D.I.; Aird, R.E.; Vora, R.; Prabhakar, U.; Nakada, M.; Corringham, R.E.; et al. A Clinical Study Assessing the Tolerability and Biological Effects of Infliximab, a TNF-Alpha Inhibitor, in Patients with Advanced Cancer. Ann. Oncol. 2008, 19, 1340–1346. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Van Assche, G.; Vermeire, S. Optimizing Anti-TNF Treatment in Inflammatory Bowel Disease. Gastroenterology 2004, 126, 1593–1610. [Google Scholar] [CrossRef]
- Atzeni, F.; Sarzi-Puttini, P. Autoimmunity and the Newer Biopharmaceuticals; Chapter 92; Shoenfeld, Y., Meroni, P.L., Gershwin, M.E.B.T.-A., Third, E., Eds.; Elsevier: San Diego, CA, USA, 2014; pp. 795–802. [Google Scholar] [CrossRef]
- Kulbe, H.; Chakravarty, P.; Leinster, D.A.; Charles, K.A.; Kwong, J.; Thompson, R.G.; Coward, J.I.; Schioppa, T.; Robinson, S.C.; Gallagher, W.M.; et al. A Dynamic Inflammatory Cytokine Network in the Human Ovarian Cancer Microenvironment. Cancer Res. 2012, 72, 66–75. [Google Scholar] [CrossRef]
- Larkin, J.M.G.; Ferguson, T.R.; Pickering, L.M.; Edmonds, K.; James, M.G.; Thomas, K.; Banerji, U.; Berns, B.; de Boer, C.; Gore, M.E. A Phase I/II Trial of Sorafenib and Infliximab in Advanced Renal Cell Carcinoma. Br. J. Cancer 2010, 103, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Park, Y.Y.; Shin, S.J.; Go, H.; Park, J.M.; Yoon, S.Y.; Lee, J.L.; Cho, Y.M. Involvement of the TNF-α Pathway in TKI Resistance and Suggestion of TNFR1 as a Predictive Biomarker for TKI Responsiveness in Clear Cell Renal Cell Carcinoma. J. Korean Med. Sci. 2020, 35, e31. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.L.; Obermueller, E.; Maisey, N.R.; Hoare, S.; Edmonds, K.; Li, N.F.; Chao, D.; Hall, K.; Lee, C.; Timotheadou, E.; et al. Tumor Necrosis Factor Alpha as a New Target for Renal Cell Carcinoma: Two Sequential Phase II Trials of Infliximab at Standard and High Dose. J. Clin. Oncol. 2007, 25, 4542–4549. [Google Scholar] [CrossRef]
- Maisey, N. Antitumor Necrosis Factor (TNF-a) Antibodies in the Treatment of Renal Cell Cancer. Cancer Invest. 2007, 25, 589–593. [Google Scholar] [CrossRef]
- Navarro-Sarabia, F.; Ariza-Ariza, R.; Hernández-Cruz, B.; Villanueva, I. Adalimumab for Treating Rheumatoid Arthritis. J. Rheumatol. 2006, 33, 1075–1081. [Google Scholar] [CrossRef]
- Kobelt, D.; Zhang, C.; Clayton-Lucey, I.A.; Glauben, R.; Voss, C.; Siegmund, B.; Stein, U. Pro-Inflammatory TNF-α and IFN-γ Promote Tumor Growth and Metastasis via Induction of MACC1. Front. Immunol. 2020, 11, 980. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Giuliani, A.; Recchioni, R.; Bonafè, M.; Marcheselli, F.; De Carolis, S.; Campanati, A.; Giuliodori, K.; Rippo, M.R.; Brugè, F.; et al. Anti-TNF-α Treatment Modulates SASP and SASP-Related MicroRNAs in Endothelial Cells and in Circulating Angiogenic Cells. Oncotarget 2016, 7, 11945–11958. [Google Scholar] [CrossRef]
- Steed, P.M.; Tansey, M.G.; Zalevsky, J.; Zhukovsky, E.A.; Desjarlais, J.R.; Szymkowski, D.E.; Abbott, C.; Carmichael, D.; Chan, C.; Cherry, L.; et al. Inactivation of TNF Signaling by Rationally Designed Dominant-Negative TNF Variants. Science 2003, 301, 1895–1898. [Google Scholar] [CrossRef]
- Xu, J.; Chakrabarti, A.K.; Tan, J.L.; Ge, L.; Gambotto, A.; Vujanovic, N.L. Essential Role of the TNF-TNFR2 Cognate Interaction in Mouse Dendritic Cell-Natural Killer Cell Crosstalk. Blood 2007, 109, 3333–3341. [Google Scholar] [CrossRef]
- Vujanovic, L.; Szymkowski, D.E.; Alber, S.; Watkins, S.C.; Vujanovic, N.L.; Butterfield, L.H. Virally Infected and Matured Human Dendritic Cells Activate Natural Killer Cells via Cooperative Activity of Plasma Membrane-Bound TNF and IL-15. Blood 2010, 116, 575–583. [Google Scholar] [CrossRef]
- Vujanovic, N.L. Role of TNF Superfamily Ligands in Innate Immunity. Immunol. Res. 2011, 50, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Andrea, S.V.; Lazar, V.; Albert, B.D.L.; Fernando, C.B.; Robert, L.F.; Yan, L.; Nikola, L.V. Inhibition of Soluble Tumor Necrosis Factor Prevents Chemically Induced Carcinogenesis in Mice. Cancer Immunol. Res. 2016, 4, 441–451. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Schlimok, G.; Heumos, I.; Schaller, G.; Riethdorf, L.; Riethmüller, G.; Pantel, K. ErbB2 Overexpression on Occult Metastatic Cells in Bone Marrow Predicts Poor Clinical Outcome of Stage I-III Breast Cancer Patients. Cancer Res. 2001, 61, 1890–1895. [Google Scholar] [PubMed]
- Potti, A.; Willardson, J.; Forseen, C.; Kishor Ganti, A.; Koch, M.; Hebert, B.; Levitt, R.; Mehdi, S.A. Predictive Role of HER-2/Neu Overexpression and Clinical Features at Initial Presentation in Patients with Extensive Stage Small Cell Lung Carcinoma. Lung Cancer 2002, 36, 257–261. [Google Scholar] [CrossRef]
- Canoz, O.; Ozkan, M.; Arsav, V.; Er, O.; Coskun, H.S.; Soyuer, S.; Altinbas, M. The Role of C-ErbB-2 Expression on the Survival of Patients with Small-Cell Lung Cancer. Lung 2006, 184, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Kijima, T.; Kohmo, S.; Arase, H.; Otani, Y.; Nagatomo, I.; Takahashi, R.; Miyake, K.; Higashiguchi, M.; Morimura, O.; et al. Overcoming Chemoresistance of Small-Cell Lung Cancer through Stepwise HER2-Targeted Antibody-Dependent Cell-Mediated Cytotoxicity and VEGF-Targeted Antiangiogenesis. Sci. Rep. 2013, 3, 2669. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Kim, S.-B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.-M.; Smitt, M.; Yu, R.; Leung, A.C.F.; Wildiers, H. Trastuzumab Emtansine versus Treatment of Physician’s Choice for Pretreated HER2-Positive Advanced Breast Cancer (TH3RESA): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W.J.; Singh, S. Chemokines: Key Players in Cancer Progression and Metastasis. Front. Biosci. 2011, 3, 1569–1582. [Google Scholar] [CrossRef][Green Version]
- Balkwill, F.R. The Chemokine System and Cancer. J. Pathol. 2012, 226, 148–157. [Google Scholar] [CrossRef]
- Hobbs, S.S.; Goettel, J.A.; Liang, D.; Yan, F.; Edelblum, K.L.; Frey, M.R.; Mullane, M.T.; Polk, D.B. TNF Transactivation of EGFR Stimulates Cytoprotective COX-2 Expression in Gastrointestinal Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G220–G229. [Google Scholar] [CrossRef] [PubMed]
- Argast, G.M.; Campbell, J.S.; Brooling, J.T.; Fausto, N. Epidermal Growth Factor Receptor Transactivation Mediates Tumor Necrosis Factor-Induced Hepatocyte Replication. J. Biol. Chem. 2004, 279, 34530–34536. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Sakurai, H.; Matsuo, M.; Choo, M.K.; Koizumi, K.; Saiki, I. Selective Inhibition of TNF-Alpha-Induced Activation of Mitogen-Activated Protein Kinases and Metastatic Activities by Gefitinib. Br. J. Cancer 2005, 92, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling Networks Regulating Cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef]
- Chun, K.-S.; Surh, Y.-J. Signal Transduction Pathways Regulating Cyclooxygenase-2 Expression: Potential Molecular Targets for Chemoprevention. Biochem. Pharmacol. 2004, 68, 1089–1100. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Willett, W.C. Aspirin Use and the Risk for Colorectal Cancer and Adenoma in Male Health Professionals. Ann. Intern. Med. 1994, 121, 241–246. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Freeman, D.H.J.; Mandel, J.S.; Haile, R. Reduced Risk of Large-Bowel Adenomas among Aspirin Users. The Polyp Prevention Study Group. J. Natl. Cancer Inst. 1993, 85, 912–916. [Google Scholar] [CrossRef]
- Son, D.-S.; Kabir, S.M.; Dong, Y.; Lee, E.; Adunyah, S.E. Characteristics of Chemokine Signatures Elicited by EGF and TNF in Ovarian Cancer Cells. J. Inflamm. 2013, 10, 25. [Google Scholar] [CrossRef]
- Freedman, M.H.; Cohen, A.; Grunberger, T.; Bunin, N.; Luddy, R.E.; Saunders, E.F.; Shahidi, N.; Lau, A.; Estrov, Z. Central Role of Tumour Necrosis Factor, GM-CSF, and Interleukin 1 in the Pathogenesis of Juvenile Chronic Myelogenous Leukaemia. Br. J. Haematol. 1992, 80, 40–48. [Google Scholar] [CrossRef]
- Shan, D.; Ledbetter, J.A.; Press, O.W. Signaling Events Involved in Anti-CD20-Induced Apoptosis of Malignant Human B Cells. Cancer Immunol. Immunother. 2000, 48, 673–683. [Google Scholar] [CrossRef]
- Adami, F.; Guarini, A.; Pini, M.; Siviero, F.; Sancetta, R.; Massaia, M.; Trentin, L.; Foà, R.; Semenzato, G. Serum Levels of Tumour Necrosis Factor-Alpha in Patients with B-Cell Chronic Lymphocytic Leukaemia. Eur. J. Cancer 1994, 30A, 1259–1263. [Google Scholar] [CrossRef]
- Saulep-Easton, D.; Vincent, F.B.; Le Page, M.; Wei, A.; Ting, S.B.; Croce, C.M.; Tam, C.; Mackay, F. Cytokine-Driven Loss of Plasmacytoid Dendritic Cell Function in Chronic Lymphocytic Leukemia. Leukemia 2014, 28, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Guleria, I.; Khosroshahi, A.; Ansari, M.J.; Habicht, A.; Azuma, M.; Yagita, H.; Noelle, R.J.; Coyle, A.; Mellor, A.L.; Khoury, S.J.; et al. A Critical Role for the Programmed Death Ligand 1 in Fetomaternal Tolerance. J. Exp. Med. 2005, 202, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 Improves Myeloid Dendritic Cell-Mediated Antitumor Immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef]
- Latchman, Y.E.; Liang, S.C.; Wu, Y.; Chernova, T.; Sobel, R.A.; Klemm, M.; Kuchroo, V.K.; Freeman, G.J.; Sharpe, A.H. PD-L1-Deficient Mice Show That PD-L1 on T Cells, Antigen-Presenting Cells, and Host Tissues Negatively Regulates T Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10691–10696. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory Cytokines IL-17 and TNF-α up-Regulate PD-L1 Expression in Human Prostate and Colon Cancer Cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.-X.; Xie, F.; Huang, Q.; Zhang, X.-G. Membranous and Cytoplasmic Expression of PD-L1 in Ovarian Cancer Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharm. 2017, 43, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.; Regan, D.; Guth, A.; Dow, S. Regulation of PD-L1 Expression on Murine Tumor-Associated Monocytes and Macrophages by Locally Produced TNF-α. Cancer Immunol. Immunother. 2017, 66, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Du, J.-X.; Zhao, J.; Shi, M.-T.; Jin, M.-W.; Liu, H. Adipocytes Promote Tumor Progression and Induce PD-L1 Expression via TNF-α/IL-6 Signaling. Cancer Cell Int. 2020, 20, 179. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, Y.; Wang, X.; Chen, N.; Mao, F.; Teng, Y.; Wang, T.; Peng, L.; Zhang, J.; Cheng, P.; et al. Increased Intratumoral Mast Cells Foster Immune Suppression and Gastric Cancer Progression through TNF-α-PD-L1 Pathway. J. Immunother. Cancer 2019, 7, 54. [Google Scholar] [CrossRef]
- Ju, X.; Zhang, H.; Zhou, Z.; Chen, M.; Wang, Q. Tumor-Associated Macrophages Induce PD-L1 Expression in Gastric Cancer Cells through IL-6 and TNF-ɑ Signaling. Exp. Cell Res. 2020, 396, 112315. [Google Scholar] [CrossRef]
- Lim, S.-O.; Li, C.-W.; Xia, W.; Cha, J.-H.; Chan, L.-C.; Wu, Y.; Chang, S.-S.; Lin, W.-C.; Hsu, J.-M.; Hsu, Y.-H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef]
- Rotte, A.; D’Orazi, G.; Bhandaru, M. Nobel Committee Honors Tumor Immunologists. J. Exp. Clin. Cancer Res. 2018, 37, 262. [Google Scholar] [CrossRef]
- Zingg, D.; Arenas-Ramirez, N.; Sahin, D.; Rosalia, R.A.; Antunes, A.T.; Haeusel, J.; Sommer, L.; Boyman, O. The Histone Methyltransferase Ezh2 Controls Mechanisms of Adaptive Resistance to Tumor Immunotherapy. Cell Rep. 2017, 20, 854–867. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Hong, B.-J.; Lee, C.-J.; Kim, Y.-E.; Bok, S.; Oh, J.-M.; Gwak, S.-H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef]
- Boutsikou, E.; Domvri, K.; Hardavella, G.; Tsiouda, D.; Zarogoulidis, K.; Kontakiotis, T. Tumour Necrosis Factor, Interferon-Gamma and Interleukins as Predictive Markers of Antiprogrammed Cell-Death Protein-1 Treatment in Advanced Non-Small Cell Lung Cancer: A Pragmatic Approach in Clinical Practice. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Blidner, A.G.; Choi, J.; Cooksley, T.; Dougan, M.; Glezerman, I.; Ginex, P.; Girotra, M.; Gupta, D.; Johnson, D.; Shannon, V.R.; et al. Cancer Immunotherapy-Related Adverse Events: Causes and Challenges. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 6111–6117. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety Profiles of Anti-CTLA-4 and Anti-PD-1 Antibodies Alone and in Combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Jacoberger-Foissac, C.; Blake, S.J.; Liu, J.; McDonald, E.; Triscott, H.; Nakamura, K.; Smyth, M.J.; Teng, M.W. Concomitant or Delayed Anti-TNF Differentially Impact on Immune-Related Adverse Events and Antitumor Efficacy after Anti-CD40 Therapy. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]
- Montfort, A.; Dufau, C.; Colacios, C.; Andrieu-Abadie, N.; Levade, T.; Filleron, T.; Delord, J.-P.; Ayyoub, M.; Meyer, N.; Ségui, B. Anti-TNF, a Magic Bullet in Cancer Immunotherapy? J. Immunother. Cancer 2019, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.; Hubbard-Lucey, V.M.; Yu, J.X. Immuno-Oncology Drug Development Forges on despite COVID-19. Nat. Rev. Drug Discov. 2020, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Phelan, K.W.; Advani, A.S. Novel Therapies in Acute Lymphoblastic Leukemia. Curr. Hematol. Malig. Rep. 2018, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Frey, N. The What, When and How of CAR T Cell Therapy for ALL. Best Pr. Res. Clin. Haematol. 2017, 30, 275–281. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Raponi, S.; De Propris, M.S.; Intoppa, S.; Milani, M.L.; Vitale, A.; Elia, L.; Perbellini, O.; Pizzolo, G.; Foá, R.; Guarini, A. Flow Cytometric Study of Potential Target Antigens (CD19, CD20, CD22, CD33) for Antibody-Based Immunotherapy in Acute Lymphoblastic Leukemia: Analysis of 552 Cases. Leuk. Lymphoma 2011, 52, 1098–1107. [Google Scholar] [CrossRef]
- Scheuermann, R.H.; Racila, E. CD19 Antigen in Leukemia and Lymphoma Diagnosis and Immunotherapy. Leuk. Lymphoma 1995, 18, 385–397. [Google Scholar] [CrossRef]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing Cytokine Release Syndrome Associated with Novel T Cell-Engaging Therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T Cells Expressing Chimeric Antigen Receptors Can Cause Anaphylaxis in Humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.R.; Grioni, M.; Basso, V.; Curnis, F.; Freschi, M.; Corti, A.; Mondino, A.; Bellone, M. Targeting Tumor Vasculature with TNF Leads Effector T Cells to the Tumor and Enhances Therapeutic Efficacy of Immune Checkpoint Blockers in Combination with Adoptive Cell Therapy. Clin. Cancer Res. 2018, 24, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Piali, L.; Fichtel, A.; Terpe, H.J.; Imhof, B.A.; Gisler, R.H. Endothelial Vascular Cell Adhesion Molecule 1 Expression Is Suppressed by Melanoma and Carcinoma. J. Exp. Med. 1995, 181, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Bellone, M.; Calcinotto, A. Ways to Enhance Lymphocyte Trafficking into Tumors and Fitness of Tumor Infiltrating Lymphocytes. Front. Oncol. 2013, 3, 231. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Bellone, M.; Elia, A.R. Constitutive and Acquired Mechanisms of Resistance to Immune Checkpoint Blockade in Human Cancer. Cytokine Growth Factor Rev. 2017, 36, 17–24. [Google Scholar] [CrossRef]
- Calcinotto, A.; Grioni, M.; Jachetti, E.; Curnis, F.; Mondino, A.; Parmiani, G.; Corti, A.; Bellone, M. Targeting TNF-α to Neoangiogenic Vessels Enhances Lymphocyte Infiltration in Tumors and Increases the Therapeutic Potential of Immunotherapy. J. Immunol. 2012, 188, 2687–2694. [Google Scholar] [CrossRef]
- Manzo, T.; Sturmheit, T.; Basso, V.; Petrozziello, E.; Hess Michelini, R.; Riba, M.; Freschi, M.; Elia, A.R.; Grioni, M.; Curnis, F.; et al. T Cells Redirected to a Minor Histocompatibility Antigen Instruct Intratumoral TNFα Expression and Empower Adoptive Cell Therapy for Solid Tumors. Cancer Res. 2017, 77, 658–671. [Google Scholar] [CrossRef]
- Plautz, G.E.; Touhalisky, J.E.; Shu, S. Treatment of Murine Gliomas by Adoptive Transfer of Ex Vivo Activated Tumor-Draining Lymph Node Cells. Cell. Immunol. 1997, 178, 101–107. [Google Scholar] [CrossRef]
- Peng, L.; Shu, S.; Krauss, J.C. Treatment of Subcutaneous Tumor with Adoptively Transferred T Cells. Cell. Immunol. 1997, 178, 24–32. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshizawa, H.; Yamaguchi, Y.; Ito, K.; Kagamu, H.; Suzuki, E.; Gejyo, F.; Hamada, H.; Arakawa, M. Successful Adoptive Immunotherapy of Murine Poorly Immunogenic Tumor with Specific Effector Cells Generated from Gene-Modified Tumor-Primed Lymph Node Cells. J. Immunol. 1999, 162, 3574–3582. [Google Scholar] [PubMed]
- Ye, Z.; Shi, M.; Chan, T.; Sas, S.; Xu, S.; Xiang, J. Engineered CD8+ Cytotoxic T Cells with Fiber-Modified Adenovirus-Mediated TNF-Alpha Gene Transfection Counteract Immunosuppressive Interleukin-10-Secreting Lung Metastasis and Solid Tumors. Cancer Gene 2007, 14, 661–675. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedersen, A.E.; Thorn, M.; Gad, M.; Walter, M.R.; Johnsen, H.E.; Gaarsdal, E.; Nikolajsen, K.; Buus, S.; Claesson, M.H.; Svane, I.M. Phenotypic and Functional Characterization of Clinical Grade Dendritic Cells Generated from Patients with Advanced Breast Cancer for Therapeutic Vaccination. Scand. J. Immunol. 2005, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Seiderer, J.; Schlamp, A.; Bidlingmaier, M.; Eigler, A.; Haimerl, W.; Lehr, H.A.; Krieg, A.M.; Hartmann, G.; Endres, S. Enhanced Dendritic Cell Maturation by TNF-Alpha or Cytidine-Phosphate-Guanosine DNA Drives T Cell Activation in Vitro and Therapeutic Anti-Tumor Immune Responses in Vivo. J. Immunol. 2000, 165, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.; Kimber, I. Tumour Necrosis Factor-Alpha Is Required for Accumulation of Dendritic Cells in Draining Lymph Nodes and for Optimal Contact Sensitization. Immunology 1995, 84, 31–35. [Google Scholar]
- Hogquist, K.A.; Jameson, S.C.; Heath, W.R.; Howard, J.L.; Bevan, M.J.; Carbone, F.R. T Cell Receptor Antagonist Peptides Induce Positive Selection. Cell 1994, 76, 17–27. [Google Scholar] [CrossRef]
- Liu, Y.; Saxena, A.; Zheng, C.; Carlsen, S.; Xiang, J. Combined Alpha Tumor Necrosis Factor Gene Therapy and Engineered Dendritic Cell Vaccine in Combating Well-Established Tumors. J. Gene Med. 2004, 6, 857–868. [Google Scholar] [CrossRef]
- Klebanoff, C.A.; Khong, H.T.; Antony, P.A.; Palmer, D.C.; Restifo, N.P. Sinks, Suppressors and Antigen Presenters: How Lymphodepletion Enhances T Cell-Mediated Tumor Immunotherapy. Trends Immunol. 2005, 26, 111–117. [Google Scholar] [CrossRef]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Restifo, N.P.; Royal, R.E.; Kammula, U.; White, D.E.; Mavroukakis, S.A.; et al. Adoptive Cell Transfer Therapy Following Non-Myeloablative but Lymphodepleting Chemotherapy for the Treatment of Patients with Refractory Metastatic Melanoma. J. Clin. Oncol. 2005, 23, 2346–2357. [Google Scholar] [CrossRef]
- Dudley, M.E.; Gross, C.A.; Langhan, M.M.; Garcia, M.R.; Sherry, R.M.; Yang, J.C.; Phan, G.Q.; Kammula, U.S.; Hughes, M.S.; Citrin, D.E.; et al. CD8+ Enriched “Young” Tumor Infiltrating Lymphocytes Can Mediate Regression of Metastatic Melanoma. Clin. Cancer Res. 2010, 16, 6122–6131. [Google Scholar] [CrossRef]
- Santos, J.M.; Cervera-carrascon, V.; Havunen, R.; Zafar, S.; Siurala, M.; Sorsa, S.; Anttila, M.; Kanerva, A.; Hemminki, A. Adenovirus Coding for Interleukin-2 and Tumor Necrosis Factor Alpha Replaces Lymphodepleting Chemotherapy in Adoptive T Cell Therapy. Mol. Ther. 2018, 26, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Siurala, M.; Havunen, R.; Saha, D.; Lumen, D.; Airaksinen, A.J.; Tähtinen, S.; Cervera-Carrascon, V.; Bramante, S.; Parviainen, S.; Vähä-Koskela, M.; et al. Adenoviral Delivery of Tumor Necrosis Factor-α and Interleukin-2 Enables Successful Adoptive Cell Therapy of Immunosuppressive Melanoma. Mol. Ther. 2016, 24, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Immunotherapy for Cancer: Harnessing the T Cell Response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef]
- Landsberg, J.; Kohlmeyer, J.; Renn, M.; Bald, T.; Rogava, M.; Cron, M.; Fatho, M.; Lennerz, V.; Wölfel, T.; Hölzel, M.; et al. Melanomas Resist T-Cell Therapy through Inflammation-Induced Reversible Dedifferentiation. Nature 2012, 490, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Kim, Y.J.; Robert, L.; Tsoi, J.; Comin-Anduix, B.; Berent-Maoz, B.; Cochran, A.J.; Economou, J.S.; Tumeh, P.C.; Puig-Saus, C.; et al. Immunotherapy Resistance by Inflammation-Induced Dedifferentiation. Cancer Discov. 2018, 8, 935–943. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2017, 168, 542. [Google Scholar] [CrossRef]
- Willrich, M.A.V.; Murray, D.L.; Snyder, M.R. Tumor Necrosis Factor Inhibitors: Clinical Utility in Autoimmune Diseases. Transl. Res. 2015, 165, 270–282. [Google Scholar] [CrossRef]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.-F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Atzeni, F.; Masala, I.F.; Caporali, R.; Montecucco, C.; Sarzi-Puttini, P. TNF Inhibitors in Rheumatoid Arthritis and Spondyloarthritis: Are They the Same? Autoimmun. Rev. 2018, 17, 24–28. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Z.; Zhu, Q.; He, D.; Ma, Y.; Du, A.; He, F.; Zhao, D.; Xu, X.; Zhang, H.; et al. TNF-α Promoter Polymorphisms Predict the Response to Etanercept More Powerfully than That to Infliximab/Adalimumab in Spondyloarthritis. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Gulli, R.; Spanò, F.; Lantieri, F.; Burlando, M.; Parodi, A.; Mandich, P.; Puppo, F. TNF-α Gene Polymorphisms: Association with Disease Susceptibility and Response to Anti-TNF-α Treatment in Psoriatic Arthritis. J. Invest. Derm. 2014, 134, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Lebwohl, M.G.; Plevy, S.E.; Hobbs, K.F.; Yocum, D.E. The Benefit/Risk Profile of TNF-Blocking Agents: Findings of a Consensus Panel. Semin. Arthritis Rheum. 2005, 34, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C. Antibody Therapy for Rheumatoid Arthritis. Curr. Opin. Pharm. 2003, 3, 323–328. [Google Scholar] [CrossRef]
- Chang, J.; Girgis, L. Clinical Use of Anti-TNF-Alpha Biological Agents—A Guide for GPs. Aust. Fam. Physician 2007, 36, 1035–1038. [Google Scholar]
- Roach, D.R.; Bean, A.G.D.; Demangel, C.; France, M.P.; Briscoe, H.; Britton, W.J. TNF Regulates Chemokine Induction Essential for Cell Recruitment, Granuloma Formation, and Clearance of Mycobacterial Infection. J. Immunol. 2002, 168, 4620–4627. [Google Scholar] [CrossRef]
- Antoni, C.; Braun, J. Side Effects of Anti-TNF Therapy: Current Knowledge. Clin. Exp. Rheumatol. 2002, 20 (Suppl. S28), S152–S157. [Google Scholar]
- Bean, A.G.; Roach, D.R.; Briscoe, H.; France, M.P.; Korner, H.; Sedgwick, J.D.; Britton, W.J. Structural Deficiencies in Granuloma Formation in TNF Gene-Targeted Mice Underlie the Heightened Susceptibility to Aerosol Mycobacterium Tuberculosis Infection, Which Is Not Compensated for by Lymphotoxin. J. Immunol. 1999, 162, 3504–3511. [Google Scholar]
- Her, M.; Kavanaugh, A. Alterations in Immune Function with Biologic Therapies for Autoimmune Disease. J. Allergy Clin. Immunol. 2016, 137, 19–27. [Google Scholar] [CrossRef]
- Leombruno, J.P.; Einarson, T.R.; Keystone, E.C. The Safety of Anti-Tumour Necrosis Factor Treatments in Rheumatoid Arthritis: Meta and Exposure-Adjusted Pooled Analyses of Serious Adverse Events. Ann. Rheum. Dis. 2009, 68, 1136–1145. [Google Scholar] [CrossRef]
- Thompson, A.E.; Rieder, S.W.; Pope, J.E. Tumor Necrosis Factor Therapy and the Risk of Serious Infection and Malignancy in Patients with Early Rheumatoid Arthritis: A Meta-Analysis of Randomized Controlled Trials. Arthritis Rheum. 2011, 63, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, C.G.; Chen, L.; Delzell, E.; Baddley, J.W.; Beukelman, T.; Winthrop, K.L.; Griffin, M.R.; Herrinton, L.J.; Liu, L.; Ouellet-Hellstrom, R.; et al. Initiation of Tumor Necrosis Factor-α Antagonists and the Risk of Hospitalization for Infection in Patients with Autoimmune Diseases. JAMA 2011, 306, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Negrini, S.; Pellecchio, M.; Greco, M.; Schiavi, C.; Giusti, F.; Puppo, F. Update upon the Infection Risk in Patients Receiving TNF Alpha Inhibitors. Expert Opin. Drug Saf. 2019, 18, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, W.; Yang, G.; Xu, Z.; Wang, J.; Cheng, Q.; Yu, M. Risk of Tuberculosis in Patients Treated with TNF-α Antagonists: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ Open 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Ramiro, S.; Sepriano, A.; Chatzidionysiou, K.; Nam, J.L.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Bijlsma, J.W.; Burmester, G.R.; et al. Safety of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2016 Update of the EULAR Recommendations for Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2017, 76, 1101–1136. [Google Scholar] [CrossRef]
- Cao, B.L.; Qasem, A.; Sharp, R.C.; Abdelli, L.S.; Naser, S.A. Systematic Review and Meta-Analysis on the Association of Tuberculosis in Crohn’s Disease Patients Treated with Tumor Necrosis Factor-α Inhibitors (Anti-TNFα). World J. Gastroenterol. 2018, 24, 2764–2775. [Google Scholar] [CrossRef]
- McDonald, J.R.; Zeringue, A.L.; Caplan, L.; Ranganathan, P.; Xian, H.; Burroughs, T.E.; Fraser, V.J.; Cunningham, F.; Eisen, S.A. Herpes Zoster Risk Factors in a National Cohort of Veterans with Rheumatoid Arthritis. Clin. Infect. Dis. 2009, 48, 1364–1371. [Google Scholar] [CrossRef]
- Che, H.; Lukas, C.; Morel, J.; Combe, B. Risk of Herpes/Herpes Zoster during Anti-Tumor Necrosis Factor Therapy in Patients with Rheumatoid Arthritis. Systematic Review and Meta-Analysis. Jt. Bone Spine 2014, 81, 215–221. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Baddley, J.W.; Chen, L.; Liu, L.; Grijalva, C.G.; Delzell, E.; Beukelman, T.; Patkar, N.M.; Xie, F.; Saag, K.G.; et al. Association between the Initiation of Anti-Tumor Necrosis Factor Therapy and the Risk of Herpes Zoster. JAMA 2013, 309, 887–895. [Google Scholar] [CrossRef]
- Deepak, P.; Stobaugh, D.J.; Sherid, M.; Sifuentes, H.; Ehrenpreis, E.D. Neurological Events with Tumour Necrosis Factor Alpha Inhibitors Reported to the Food and Drug Administration Adverse Event Reporting System. Aliment. Pharm. 2013, 38, 388–396. [Google Scholar] [CrossRef]
- Kaltsonoudis, E.; Zikou, A.K.; Voulgari, P.V.; Konitsiotis, S.; Argyropoulou, M.I.; Drosos, A.A. Neurological Adverse Events in Patients Receiving Anti-TNF Therapy: A Prospective Imaging and Electrophysiological Study. Arthritis Res. 2014, 16, R125. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Genovese, M.C.; Moreland, L.W. Demyelinating and Neurologic Events Reported in Association with Tumor Necrosis Factor Alpha Antagonism: By What Mechanisms Could Tumor Necrosis Factor Alpha Antagonists Improve Rheumatoid Arthritis but Exacerbate Multiple Sclerosis? Arthritis Rheum. 2001, 44, 1977–1983. [Google Scholar] [CrossRef]
- Solomon, A.J.; Spain, R.I.; Kruer, M.C.; Bourdette, D. Inflammatory Neurological Disease in Patients Treated with Tumor Necrosis Factor Alpha Inhibitors. Mult. Scler. 2011, 17, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Saffra, N.; Astafurov, K. Visual Loss Induced by Adalimumab Used for Plaque Psoriasis. Case Rep. Dermatol. 2017, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Nakamura, I.; Kobayashi, S.; Kurihara, K.; Ito, K. New-Onset Demyelination Induced by Infliximab Therapy in Two Rheumatoid Arthritis Patients. Clin. Rheumatol. 2006, 25, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Singh, M. Anti-Tumour Necrosis Factor-Alpha Therapy in Heart Failure: Future Directions. Basic Clin. Pharm. Toxicol. 2006, 99, 391–397. [Google Scholar] [CrossRef]
- Bucalo, A.; Rega, F.; Zangrilli, A.; Silvestri, V.; Valentini, V.; Scafetta, G.; Marraffa, F.; Grassi, S.; Rogante, E.; Piccolo, A.; et al. Paradoxical Psoriasis Induced by Anti-TNFα Treatment: Evaluation of Disease-Specific Clinical and Genetic Markers. Int. J. Mol. Sci. 2020, 21, 7873. [Google Scholar] [CrossRef]
- Bonovas, S.; Minozzi, S.; Lytras, T.; González-Lorenzo, M.; Pecoraro, V.; Colombo, S.; Polloni, I.; Moja, L.; Cinquini, M.; Marino, V.; et al. Risk of Malignancies Using Anti-TNF Agents in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 2016, 15, 35–54. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Yang, Y.; Huang, Y.; Liu, G. Malignancy Risk of Anti-Tumor Necrosis Factor Alpha Blockers: An Overview of Systematic Reviews and Meta-Analyses. Clin. Rheumatol. 2016, 35, 1–18. [Google Scholar] [CrossRef]
- Shelton, E.; Laharie, D.; Scott, F.I.; Mamtani, R.; Lewis, J.D.; Colombel, J.-F.F.; Ananthakrishnan, A.N. Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 151, 97–109.e4. [Google Scholar] [CrossRef]
- Bongartz, T.; Sutton, A.J.; Sweeting, M.J.; Buchan, I.; Matteson, E.L.; Montori, V. Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and Meta-Analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA 2006, 295, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Fored, C.M.; Baecklund, E.; Brandt, L.; Backlin, C.; Ekbom, A.; Sundström, C.; Bertilsson, L.; Cöster, L.; Geborek, P.; et al. Haematopoietic Malignancies in Rheumatoid Arthritis: Lymphoma Risk and Characteristics after Exposure to Tumour Necrosis Factor Antagonists. Ann. Rheum. Dis. 2005, 64, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of Infections Using Anti-TNF Agents in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 2016, 15 (Suppl. S1), 11–34. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Van Assche, G.; Lindsay, J.O.; Lémann, M.; Söderholm, J.; Colombel, J.F.; Danese, S.; D’Hoore, A.; Gassull, M.; Gomollón, F.; et al. The Second European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease: Current Management. J. Crohns. Colitis 2010, 4, 28–62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Zhu, H.; Liu, L.S.; Huang, Y.; Guo, J.; Li, J.; Sun, X.P.; Chang, C.X.; Wang, Z.H.; Zhai, K. Tumour Necrosis Factor-α Gene Polymorphism Is Associated with Metastasis in Patients with Triple Negative Breast Cancer. Sci. Rep. 2015, 5, 10244. [Google Scholar] [CrossRef]
- Azmy, I.A.F.; Balasubramanian, S.P.; Wilson, A.G.; Stephenson, T.J.; Cox, A.; Brown, N.J.; Reed, M.W.R. Role of Tumour Necrosis Factor Gene Polymorphisms (-308 and -238) in Breast Cancer Susceptibility and Severity. Breast Cancer Res. 2004, 6, 395–400. [Google Scholar] [CrossRef]
- Yi, F.; Shi, X.; Pei, X.; Wu, X. Tumor Necrosis Factor-Alpha-308 Gene Promoter Polymorphism Associates with Survival of Cancer Patients: A Meta-Analysis. Medicine (Baltimore) 2018, 97, e13160. [Google Scholar] [CrossRef]
- Balkwill, F. Mantovani, a. Cancer and Inflammation: Implications for Pharmacology and Therapeutics. Clin. Pharm. 2010, 87, 401–406. [Google Scholar] [CrossRef]
- Lentz, M.; Kumar, K. Reduction of Plasma Levels of Soluble Tumor Necrosis Factor and Interleukin-2 Receptors by Means of a Novel Immunoadsorption Column. Ther. Apher. Dial. 2008, 12, 491–499. [Google Scholar] [CrossRef]
- Krippner-Heidenreich, A.; Grunwald, I.; Zimmermann, G.; Kühnle, M.; Gerspach, J.; Sterns, T.; Shnyder, S.D.; Gill, J.H.; Männel, D.N.; Pfizenmaier, K.; et al. Single-Chain TNF, a TNF Derivative with Enhanced Stability and Antitumoral Activity. J. Immunol. 2008, 180, 8176–8183. [Google Scholar] [CrossRef]
- Josephs, S.F.; Ichim, T.E.; Prince, S.M.; Kesari, S.; Marincola, F.M.; Escobedo, A.R.; Jafri, A. Unleashing Endogenous TNF-Alpha as a Cancer Immunotherapeutic. J. Transl. Med. 2018, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Subleski, J.J.; Kopf, H.; Howard, O.M.Z.; Männel, D.N.; Oppenheim, J.J. Cutting Edge: Expression of TNFR2 Defines a Maximally Suppressive Subset of Mouse CD4+CD25+FoxP3+ T Regulatory Cells: Applicability to Tumor-Infiltrating T Regulatory Cells. J. Immunol. 2008, 180, 6467–6471. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Smyth, M.J.; Ngiow, S.F.; Teng, M.W.L. Targeting Regulatory T Cells in Tumor Immunotherapy. Immunol. Cell Biol. 2014, 92, 473–474. [Google Scholar] [CrossRef]
- Okubo, Y.; Mera, T.; Wang, L.; Faustman, D.L. Homogeneous Expansion of Human T-Regulatory Cells via Tumor Necrosis Factor Receptor 2. Sci. Rep. 2013, 3, 3153. [Google Scholar] [CrossRef]
- Ham, B.; Wang, N.; D’Costa, Z.; Fernandez, M.C.; Bourdeau, F.; Auguste, P.; Illemann, M.; Eefsen, R.L.; Høyer-Hansen, G.; Vainer, B.; et al. TNF Receptor-2 Facilitates an Immunosuppressive Microenvironment in the Liver to Promote the Colonization and Growth of Hepatic Metastases. Cancer Res. 2015, 75, 5235–5247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Al-Lamki, R.S. Tumor Necrosis Factor Receptor 2: Its Contribution to Acute Cellular Rejection and Clear Cell Renal Carcinoma. Biomed Res. Int. 2013, 2013, 821310. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.E.; Simmons, J.G.; Ding, S.; Van Landeghem, L.; Lund, P.K. Cytokine Induction of Tumor Necrosis Factor Receptor 2 Is Mediated by STAT3 in Colon Cancer Cells. Mol. Cancer Res. 2011, 9, 1718–1731. [Google Scholar] [CrossRef]
- Nakayama, S.; Yokote, T.; Tsuji, M.; Akioka, T.; Miyoshi, T.; Hirata, Y.; Hiraoka, N.; Iwaki, K.; Takayama, A.; Nishiwaki, U.; et al. Expression of Tumour Necrosis Factor-α and Its Receptors in Hodgkin Lymphoma. Br. J. Haematol. 2014, 574–577. [Google Scholar] [CrossRef]
- Rauert, H.; Stühmer, T.; Bargou, R.; Wajant, H.; Siegmund, D. TNFR1 and TNFR2 Regulate the Extrinsic Apoptotic Pathway in Myeloma Cells by Multiple Mechanisms. Cell Death Dis. 2011, 2, e194. [Google Scholar] [CrossRef]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.-K.; Amini, B.; Andersen, E.; Andersson, A.-C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A Human Protein Atlas for Normal and Cancer Tissues Based on Antibody Proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S.; Mistry, B.; Guillard, S.; Ulrichsen, J.C.; Sandercock, A.M.; Wang, J.; González-Muñoz, A.; Parmentier, J.; Black, C.; Soden, J.; et al. Phenotypic Screening Reveals TNFR2 as a Promising Target for Cancer Immunotherapy. Oncotarget 2016, 7, 68278–68291. [Google Scholar] [CrossRef] [PubMed]
- Goukassian, D.A.; Qin, G.; Dolan, C.; Murayama, T.; Silver, M.; Curry, C.; Eaton, E.; Luedemann, C.; Ma, H.; Asahara, T.; et al. Tumor Necrosis Factor-Alpha Receptor P75 Is Required in Ischemia-Induced Neovascularization. Circulation 2007, 115, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Naserian, S.; Abdelgawad, M.E.; Afshar Bakshloo, M.; Ha, G.; Arouche, N.; Cohen, J.L.; Salomon, B.L.; Uzan, G. The TNF/TNFR2 Signaling Pathway Is a Key Regulatory Factor in Endothelial Progenitor Cell Immunosuppressive Effect. Cell Commun. Signal. 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Vanamee, É.S.; Faustman, D.L. TNFR2: A Novel Target for Cancer Immunotherapy. Trends Mol. Med. 2017, 23, 1037–1046. [Google Scholar] [CrossRef]
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D.; et al. Targeting TNFR2 with Antagonistic Antibodies Inhibits Proliferation of Ovarian Cancer Cells and Tumor-Associated Tregs. Sci. Signal. 2017, 10, eaaf8608. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tran, L.; Torrey, H.; Song, Y.; Perkins, H.; Case, K.; Zheng, H.; Takahashi, H.; Kuhtreiber, W.M.; Faustman, D.L. Optimizing TNFR2 Antagonism for Immunotherapy with Tumor Microenvironment Specificity. J. Leukoc. Biol. 2020, 107, 971–980. [Google Scholar] [CrossRef]
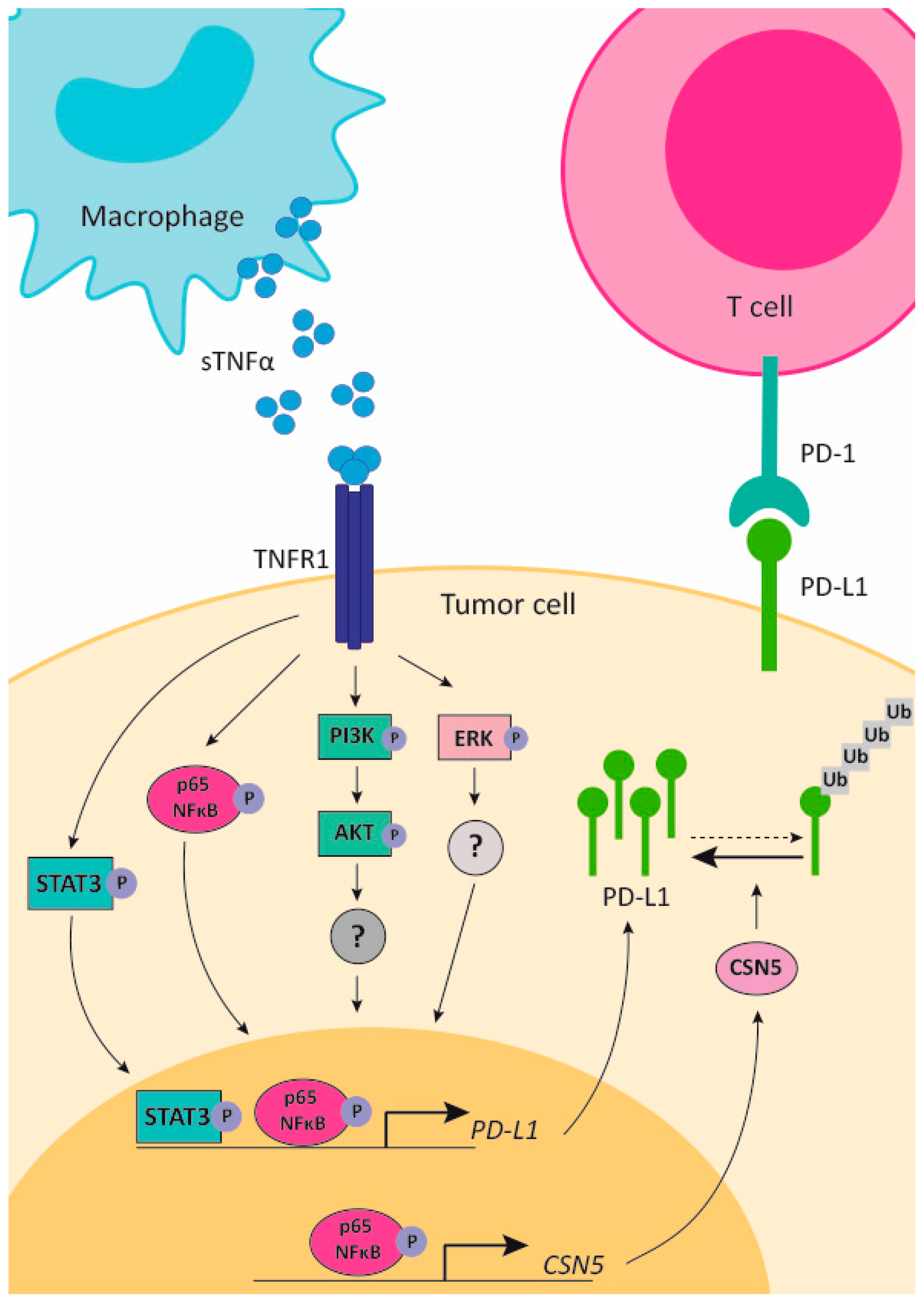
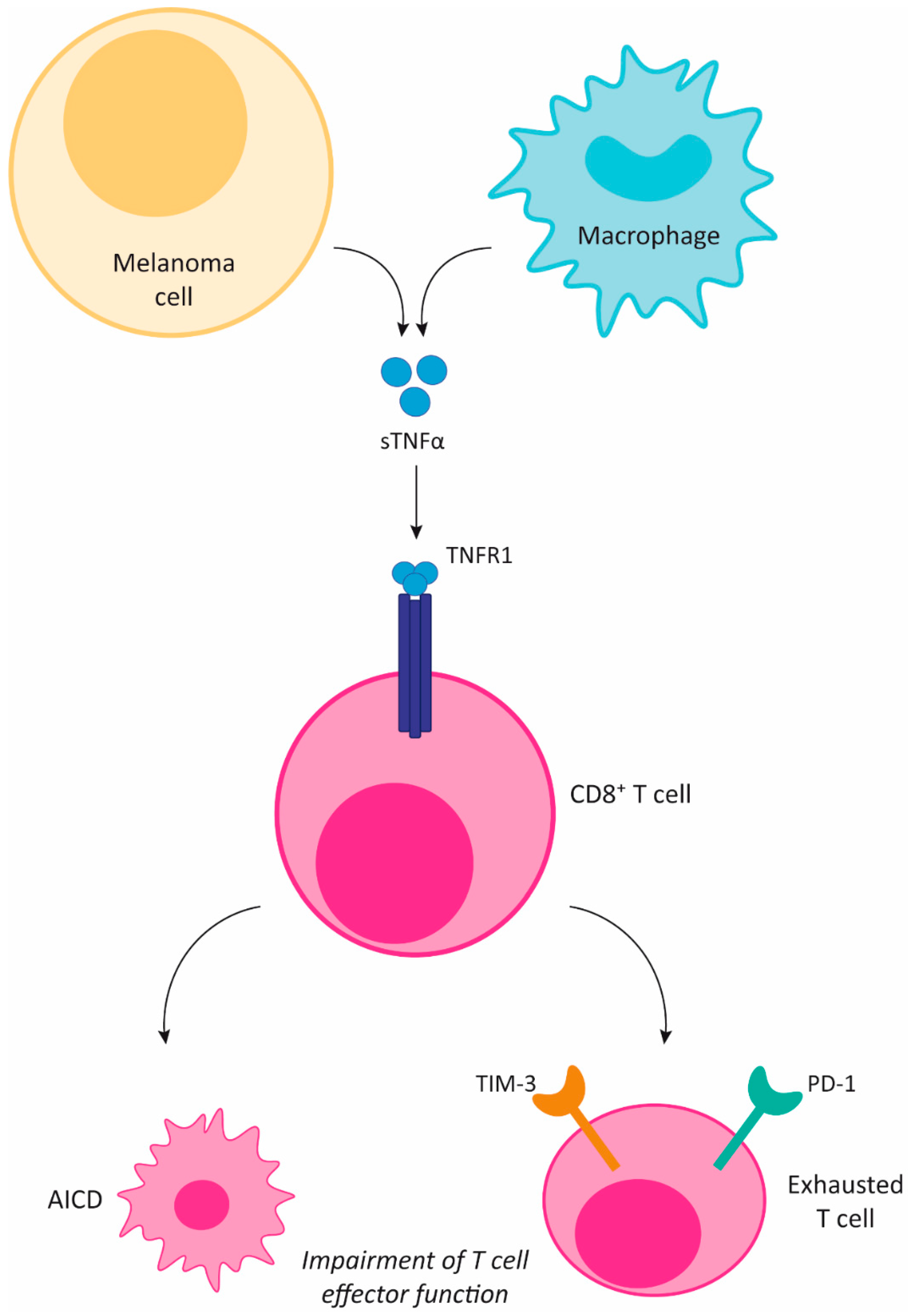
| IT | Target/Cell Type | Drug Name | Anti-TNFa | Effect on Cancer | Side Effects of IT | Ref. |
|---|---|---|---|---|---|---|
| Monoclonal antibodies | HER2 | Trastuzumab | Etanercept | Overcomes trastuzumab resistance in HER2+ breast cancer | NT | [72] |
| INB03 | Overcomes trastuzumab resistance in HER2+ breast cancer | - | [165] | |||
| CD20 | Rituximab | Etanercept | Improves disease-related symptoms and increases OS in chronic lymphocytic leukemia patients | NT | [166,167] | |
| PD-1 | anti-PD-1 | Anti-TNFR1 or anti-TNFa | Prevents T lymphocytes exhaustion and death by anti PD-1 treatment in melanoma | Prevents immune-related adverse effect | [168] | |
| Pembrolizumab | Infliximab | NT | Treatement of immune-related adverse effects | [169,170,171] | ||
| PD-1+ CTLA-4 | anti-PD-1 + anti CTL-4 | Etanercept | Improves antitumor effect of anti PD-1+ anti CTL-4 antibodies in colon cancer | Prevents immune-related adverse effect | [172] | |
| CTLA-4 | Ipilimumab | Infliximab | NT | Treatement of immune-related adverse effects | [169,170,173,174] | |
| PD-L1 | Atezolizumab, duvalumab and avelumab | Infliximab | NT | Treatement of immune-related adverse effects | [170] | |
| CAR-T cells | CD19 | - | Etanercept | NT | Treatment of systemic inflammatory response syndrome | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. https://doi.org/10.3390/cancers13030564
Mercogliano MF, Bruni S, Mauro F, Elizalde PV, Schillaci R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers. 2021; 13(3):564. https://doi.org/10.3390/cancers13030564
Chicago/Turabian StyleMercogliano, María Florencia, Sofía Bruni, Florencia Mauro, Patricia Virginia Elizalde, and Roxana Schillaci. 2021. "Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy" Cancers 13, no. 3: 564. https://doi.org/10.3390/cancers13030564
APA StyleMercogliano, M. F., Bruni, S., Mauro, F., Elizalde, P. V., & Schillaci, R. (2021). Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers, 13(3), 564. https://doi.org/10.3390/cancers13030564







