The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
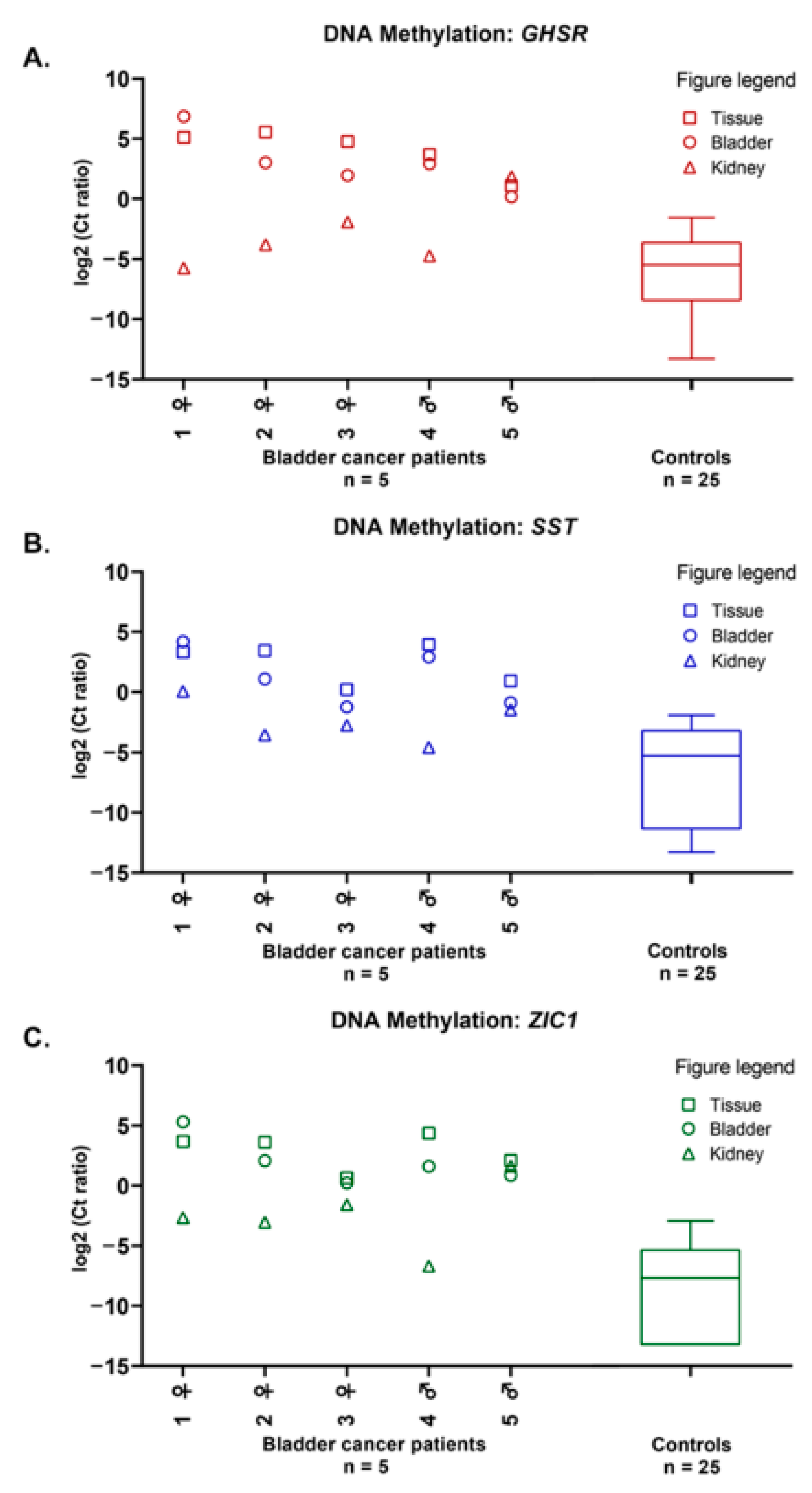
2.1. Methylation Markers in Bladder Cancer
2.2. Methylation Markers in Cervical Cancer
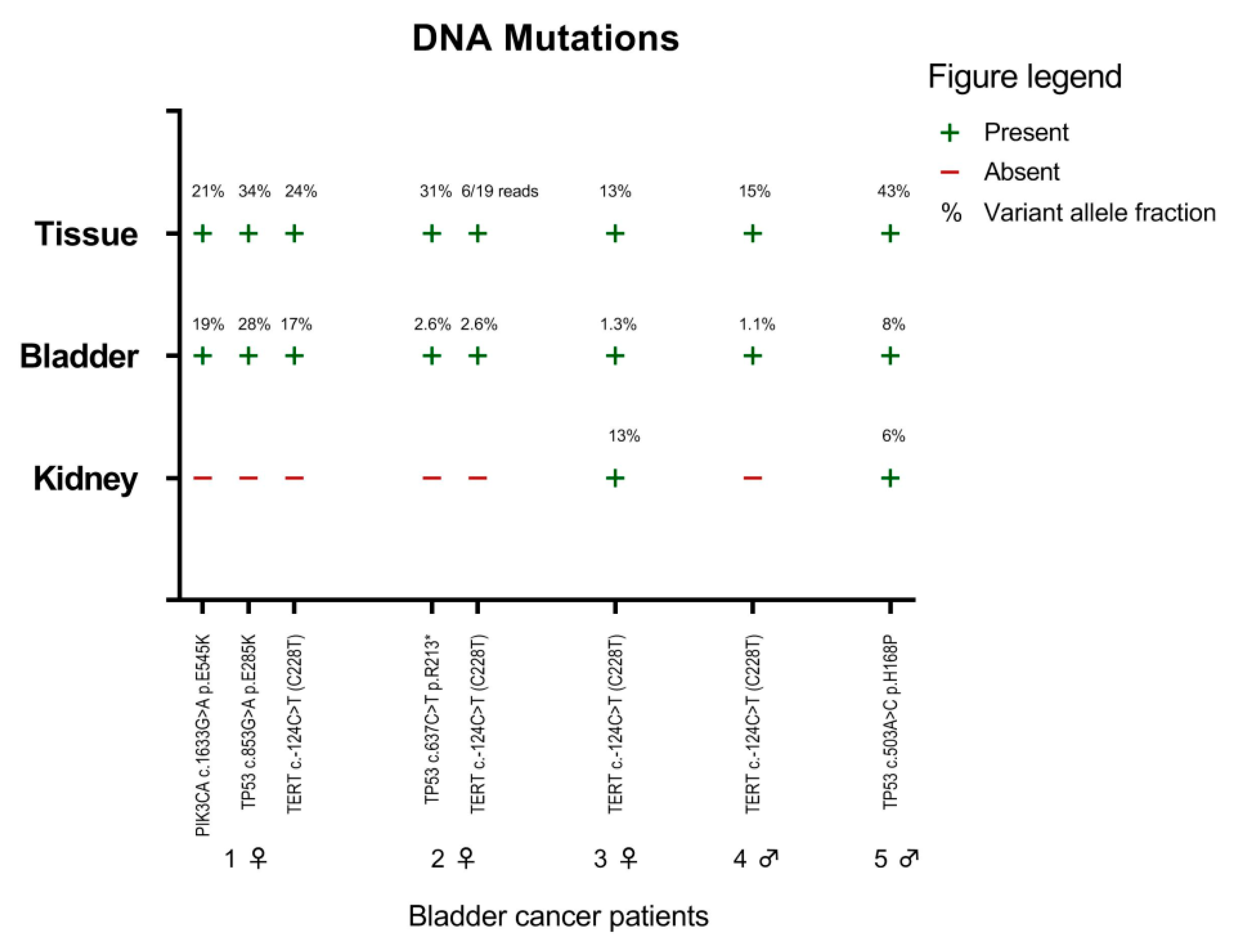
2.3. Mutation Markers in Bladder Cancer
3. Discussion
4. Materials and Methods
4.1. Sample Collection: Bladder Cancer
4.2. Sample Collection: Cervical Cancer
4.3. Urine and Tissue Samples
4.3.1. DNA Isolation
4.3.2. DNA Methylation Analysis
4.3.3. DNA Mutation Analysis
4.3.4. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, M.; Chen, C.; Hulbert, A.; Brock, M.V.; Yu, F. Non-blood circulating tumor DNA detection in cancer. Oncotarget 2017, 8, 69162–69173. [Google Scholar] [CrossRef] [PubMed]
- Bosschieter, J.; Lutz, C.; Segerink, L.I.; Vis, A.N.; Zwarthoff, E.C.; Van Moorselaar, R.J.A.; Van Rhijn, B.W.; Heymans, M.W.; Jansma, E.P.; Steenbergen, R.D.M.; et al. The diagnostic accuracy of methylation markers in urine for the detection of bladder cancer: A systematic review. Epigenomics 2018, 10, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Tan, M.-Y.; Khetrapal, P.; Dong, L.; Dewinter, P.; Feber, A.; Kelly, J.D. Novel urinary biomarkers for the detection of bladder cancer: A systematic review. Cancer Treat. Rev. 2018, 69, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Snoek, B.C.; Van Splunter, A.P.; Bleeker, M.C.G.; Van Ruiten, M.C.; Heideman, D.A.M.; Rurup, W.F.; Verlaat, W.; Schotman, H.; Van Gent, M.; Van Trommel, N.E.; et al. Cervical cancer detection by DNA methylation analysis in urine. Sci. Rep. 2019, 9, 3088. [Google Scholar] [CrossRef] [PubMed]
- Vorsters, A.; Van Damme, P.; Clifford, G. Urine testing for HPV: Rationale for using first void. BMJ 2014, 349, g6252. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, A.E.; Nieuwenhuijzen, J.; Bosschieter, J.; Van Splunter, A.P.; Lissenberg-Witte, B.I.; Van Der Voorn, J.P.; Segerink, L.I.; Van Moorselaar, R.J.A.; Steenbergen, R.D.M. Comparative Analysis of Urine Fractions for Optimal Bladder Cancer Detection Using DNA Methylation Markers. Cancers 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Helder, R.V.D.; Van Trommel, N.E.; Van Splunter, A.P.; Lissenberg-Witte, B.I.; Bleeker, M.C.; Steenbergen, R.D. Methylation analysis in urine fractions for optimal CIN3 and cervical cancer detection. Papillomavirus Res. 2020, 9, 100193. [Google Scholar] [CrossRef]
- Szarvas, T.; Kovalszky, I.; Bedi, K.; Szendroi, A.; Majoros, A.; Riesz, P.; Füle, T.; László, V.; Kiss, A.; Romics, I. Deletion analysis of tumor and urinary DNA to detect bladder cancer: Urine supernatant versus urine sediment. Oncol. Rep. 2007, 18, 405–409. [Google Scholar] [CrossRef]
- Togneri, F.S.; Ward, D.G.; Foster, J.M.; Devall, A.J.; Wojtowicz, P.; Alyas, S.; Vasques, F.R.; Oumie, A.; James, N.D.; Cheng, K.K.; et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur. J. Hum. Genet. 2016, 24, 1167–1174. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Ji, D.; Meng, Q.; Wang, C.; Chen, S.; Wang, X.; Zhu, Z.; Jiang, C.; Shi, Y.; et al. Utility of urinary circulating tumor DNA for EGFR mutation detection in different stages of non-small cell lung cancer patients. Clin. Transl. Oncol. 2017, 19, 1283–1291. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dhillon, V.; Jain, S.; Chang, T.-T.; Hu, C.-T.; Lin, Y.-J.; Chen, S.-H.; Chang, K.-C.; Song, W.; Yu, L.; et al. A Locked Nucleic Acid Clamp-Mediated PCR Assay for Detection of a p53 Codon 249 Hotspot Mutation in Urine. J. Mol. Diagn. 2011, 13, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Han, L.; Yuan, J.; Sun, Y. Circulating tumor cell free DNA from plasma and urine in the clinical management of colorectal cancer. Cancer Biomarkers 2019, 27, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Linehan, J.A.; Wilson, T.G.; Hoon, D.S.B. Emerging Utility of Urinary Cell-free Nucleic Acid Biomarkers for Prostate, Bladder, and Renal Cancers. Eur. Urol. Focus 2017, 3, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Sherman, M.E.; Schiffman, M.; Wang, S.S. Utility of methylation markers in cervical cancer early detection: Appraisal of the state-of-the-science. Gynecol. Oncol. 2009, 112, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Herman, J.G. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000, 16, 168–174. [Google Scholar] [CrossRef]
- Bosschieter, J.; Nieuwenhuijzen, J.A.; Hentschel, A.; Van Splunter, A.P.; Segerink, L.I.; Vis, A.N.; Wilting, S.M.; Lissenberg-Witte, B.I.; A Van Moorselaar, R.J.; Steenbergen, R.D. A two-gene methylation signature for the diagnosis of bladder cancer in urine. Epigenomics 2019, 11, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Helder, R.V.D.; Wever, B.M.; Van Trommel, J.A.; Ket, J.C.; Bleeker, M.C.; Steenbergen, R.D.M.; Van Trommel, N.E. DNA methylation markers for endometrial cancer detection in minimally invasive samples: A systematic review. Epigenomics 2020, 12, 1661–1672. [Google Scholar] [CrossRef]
- Verlaat, W.; Snijders, P.J.F.; Novianti, P.W.; Wilting, S.M.; De Strooper, L.M.; Trooskens, G.; Vandersmissen, J.; Van Criekinge, W.; Wisman, G.B.A.; Meijer, C.J.L.M.; et al. Genome-wide DNA Methylation Profiling Reveals Methylation Markers Associated with 3q Gain for Detection of Cervical Precancer and Cancer. Clin. Cancer Res. 2017, 23, 3813–3822. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef]
- Hentschel, A.E.; Toom, E.E.; Vis, A.N.; Ket, J.C.; Bosschieter, J.; Heymans, M.W.; Moorselaar, R.J.A.; Steenbergen, R.D.; Nieuwenhuijzen, J. A systematic review on mutation markers for bladder cancer diagnosis in urine. BJU Int. 2021, 127, 12–27. [Google Scholar] [CrossRef]
- Lamy, A.; Gobet, F.; Laurent, M.; Blanchard, F.; Varin, C.; Moulin, C.; Andreou, A.; Frebourg, T.; Pfister, C. Molecular Profiling of Bladder Tumors Based on the Detection of FGFR3 and TP53 Mutations. J. Urol. 2006, 176, 2686–2689. [Google Scholar] [CrossRef] [PubMed]
- Majer, S.; Bauer, M.; Magnet, E.; Strele, A.; Giegerl, E.; Eder, M.; Lang, U.; Pertl, B. Maternal urine for prenatal diagnosis—An analysis of cell-free fetal DNA in maternal urine and plasma in the third trimester. Prenat. Diagn. 2007, 27, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, I.; Serdyuk, O.; Potapova, G.; Shelepov, V.; Alechina, R.; Molyaka, Y.; Ananév, V.; Bazin, I.; Garin, A.; Narimanov, M.; et al. Genetic analysis of DNA excreted in urine: A new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin. Chem. 2000, 46, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, Z.; Li, C.S.; Zhao, W.; Liang, Z.X.; Dai, Y.; Zhu, Q.; Miao, K.L.; Cui, D.H.; Chen, L.A. Differences in the genomic profiles of cell-free DNA between plasma, sputum, urine, and tumor tissue in advanced NSCLC. Cancer Med. 2019, 8, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, E.A.; Brenne, S.S.; Skorpen, F. DNA methylation markers detected in blood, stool, urine, and tissue in colorectal cancer: A systematic review of paired samples. Int. J. Color. Dis. 2021, 36, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, H.; Palou, J.; Van Rhijn, B.W.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 66.e25–66.e31. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Corrections to “Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Ann. Oncol. 2018, 29, iv262. [Google Scholar] [CrossRef]
- Hattori, M.; Nishimura, Y.; Toyonaga, M.; Kakinuma, H.; Matsumoto, K.; Ohbu, M. Cytological significance of abnormal squamous cells in urinary cytology. Diagn. Cytopathol. 2011, 40, 798–803. [Google Scholar] [CrossRef]
- Wang, Y.; Otis, C.N.; Florence, R.R. Atypical squamous cells in the urine revealing endometrioid adenocarcinoma of the endometrium with squamous cell differentiation: A case report. Diagn. Cytopathol. 2015, 43, 49–52. [Google Scholar] [CrossRef]
- Bosschieter, J.; Bach, S.; Bijnsdorp, I.V.; Segerink, L.I.; Rurup, W.F.; Van Splunter, A.P.; Bahce, I.; Novianti, P.W.; Kazemier, G.; Van Moorselaar, R.J.A.; et al. A protocol for urine collection and storage prior to DNA methylation analysis. PLoS ONE 2018, 13, e0200906. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carunchio, L.; Soveral, I.; Steenbergen, R.D.M.; Torné, A.; Martinez, S.; Fusté, P.; Pahisa, J.; Marimon, L.; Ordi, J.; Del Pino, M. HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG Int. J. Obstet. Gynaecol. 2014, 122, 119–127. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, L.M.A.; Van Zummeren, M.; Steenbergen, R.D.M.; Bleeker, M.C.; Hesselink, A.T.; Wisman, G.B.A.; Snijders, P.J.; Heideman, D.A.M.; Meijer, C.J.L.M. CADM1, MAL and miR124-2 methylation analysis in cervical scrapes to detect cervical and endometrial cancer. J. Clin. Pathol. 2014, 67, 1067–1071. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hentschel, A.E.; van den Helder, R.; van Trommel, N.E.; van Splunter, A.P.; van Boerdonk, R.A.A.; van Gent, M.D.J.M.; Nieuwenhuijzen, J.A.; Steenbergen, R.D.M. The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion. Cancers 2021, 13, 535. https://doi.org/10.3390/cancers13030535
Hentschel AE, van den Helder R, van Trommel NE, van Splunter AP, van Boerdonk RAA, van Gent MDJM, Nieuwenhuijzen JA, Steenbergen RDM. The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion. Cancers. 2021; 13(3):535. https://doi.org/10.3390/cancers13030535
Chicago/Turabian StyleHentschel, Anouk E., Rianne van den Helder, Nienke E. van Trommel, Annina P. van Splunter, Robert A. A. van Boerdonk, Mignon D. J. M. van Gent, Jakko A. Nieuwenhuijzen, and Renske D. M. Steenbergen. 2021. "The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion" Cancers 13, no. 3: 535. https://doi.org/10.3390/cancers13030535
APA StyleHentschel, A. E., van den Helder, R., van Trommel, N. E., van Splunter, A. P., van Boerdonk, R. A. A., van Gent, M. D. J. M., Nieuwenhuijzen, J. A., & Steenbergen, R. D. M. (2021). The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion. Cancers, 13(3), 535. https://doi.org/10.3390/cancers13030535






