Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. In Vivo Cancer Model: Animal Model Selection
3. PCL Generation for In Vivo Cancer Treatment. Experimental Setup
3.1. Relevant Parameters in Plasma Treatment
3.2. Reactive Species Generated in PCL
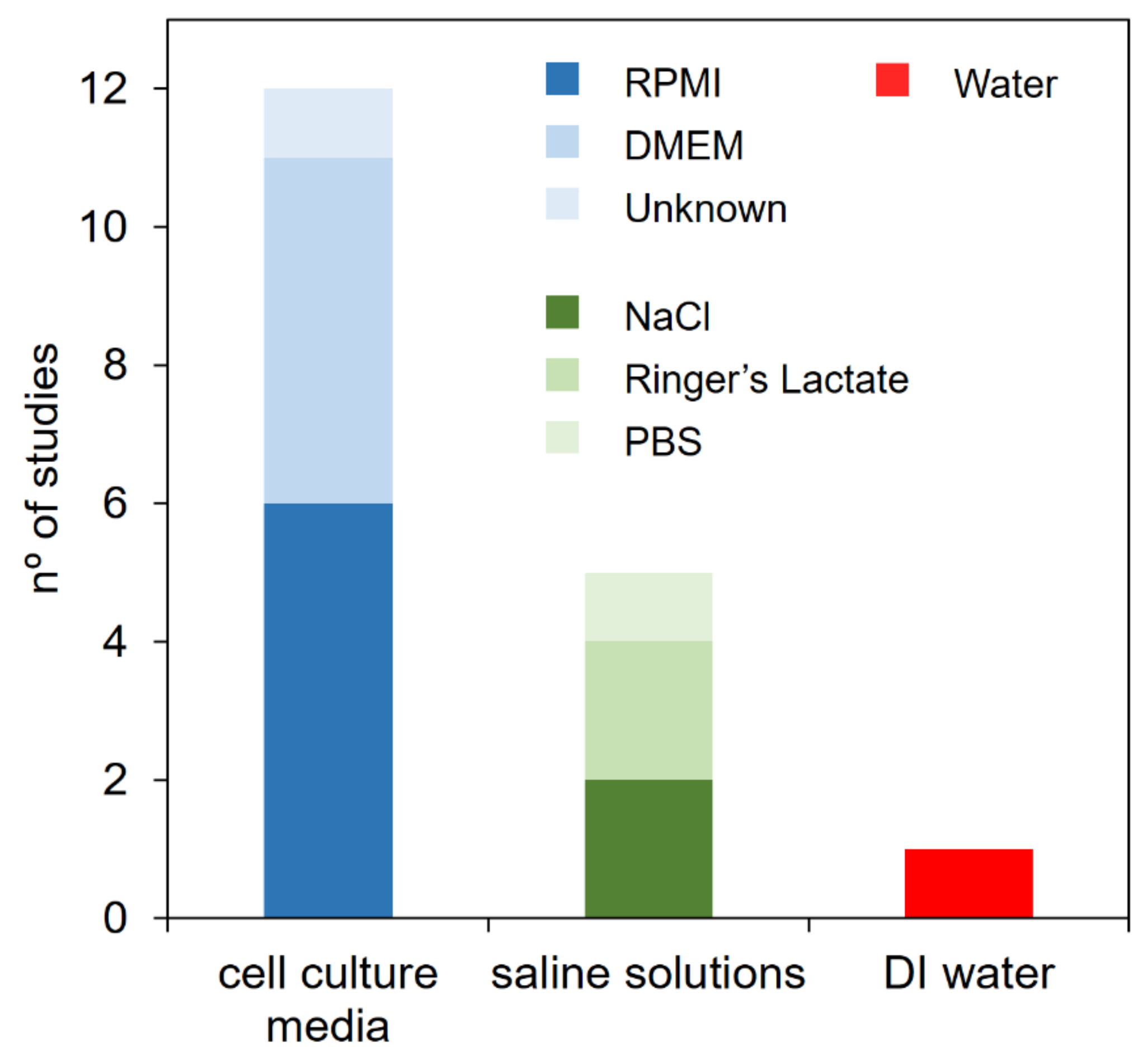
3.3. Nature of the Treated Liquid
4. In Vivo Cancer Treatment with PCL
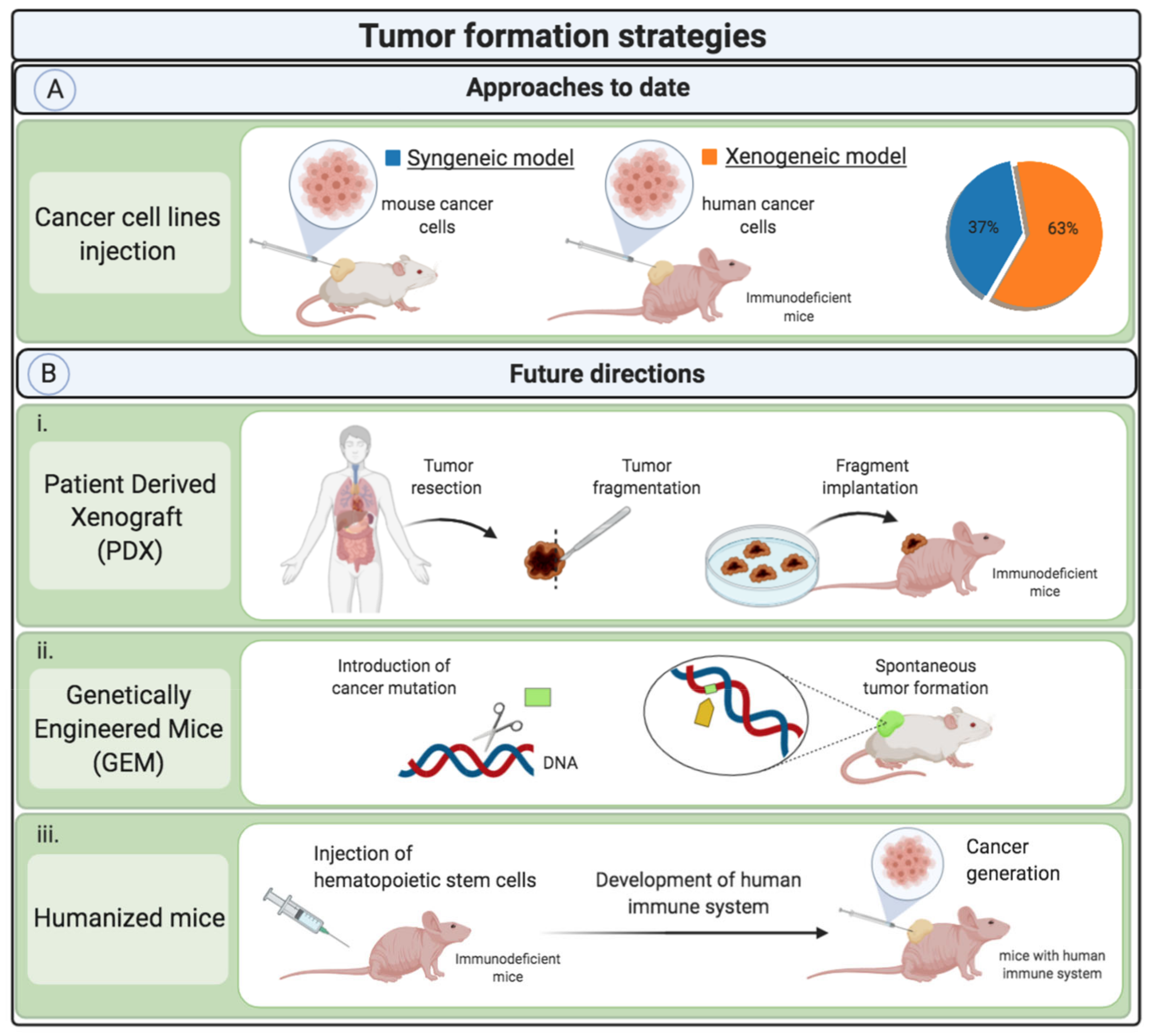
4.1. Tumor Formation
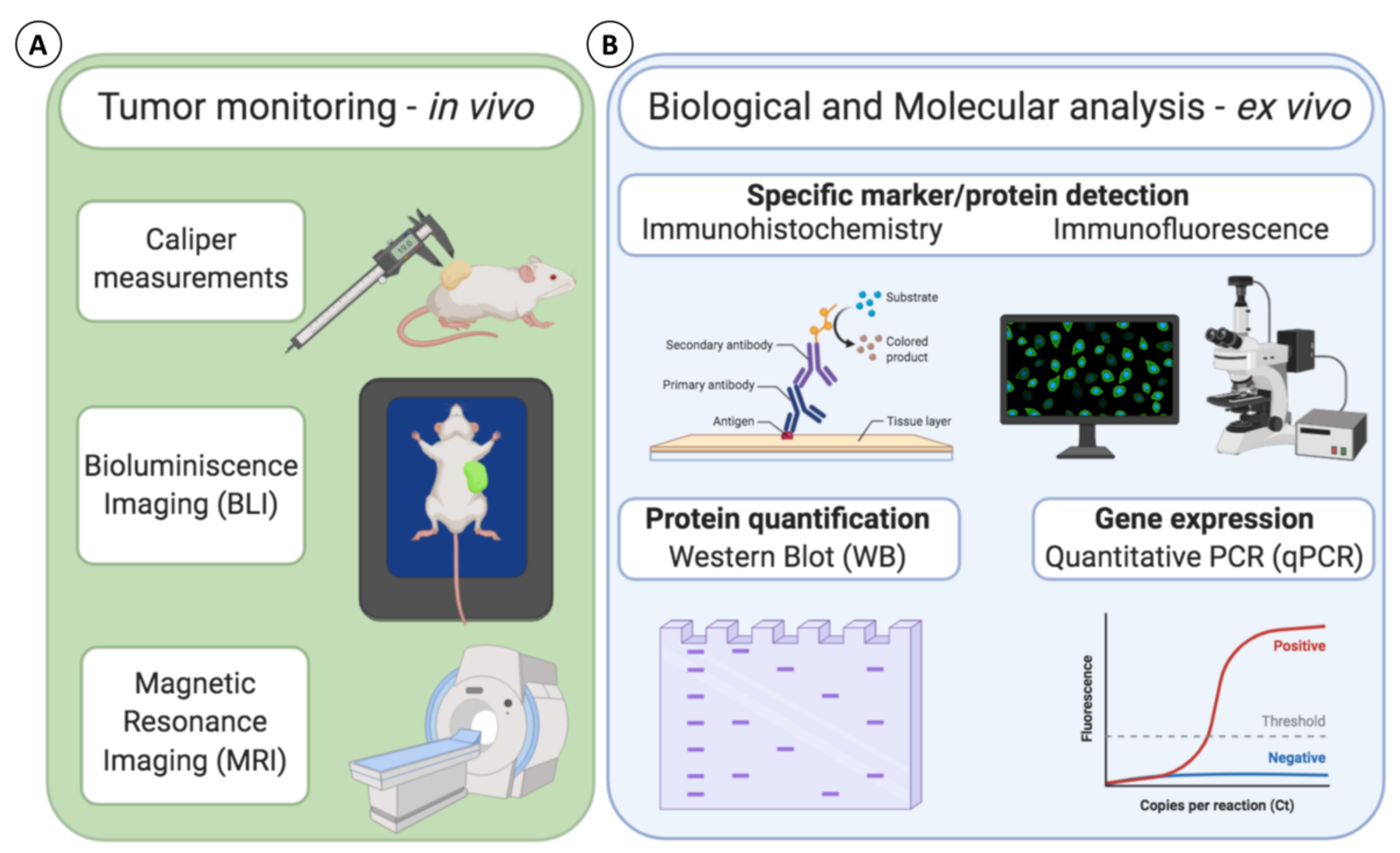
4.2. Tumor Monitoring
4.3. Biological and Molecular Evaluation: Analytical Techniques
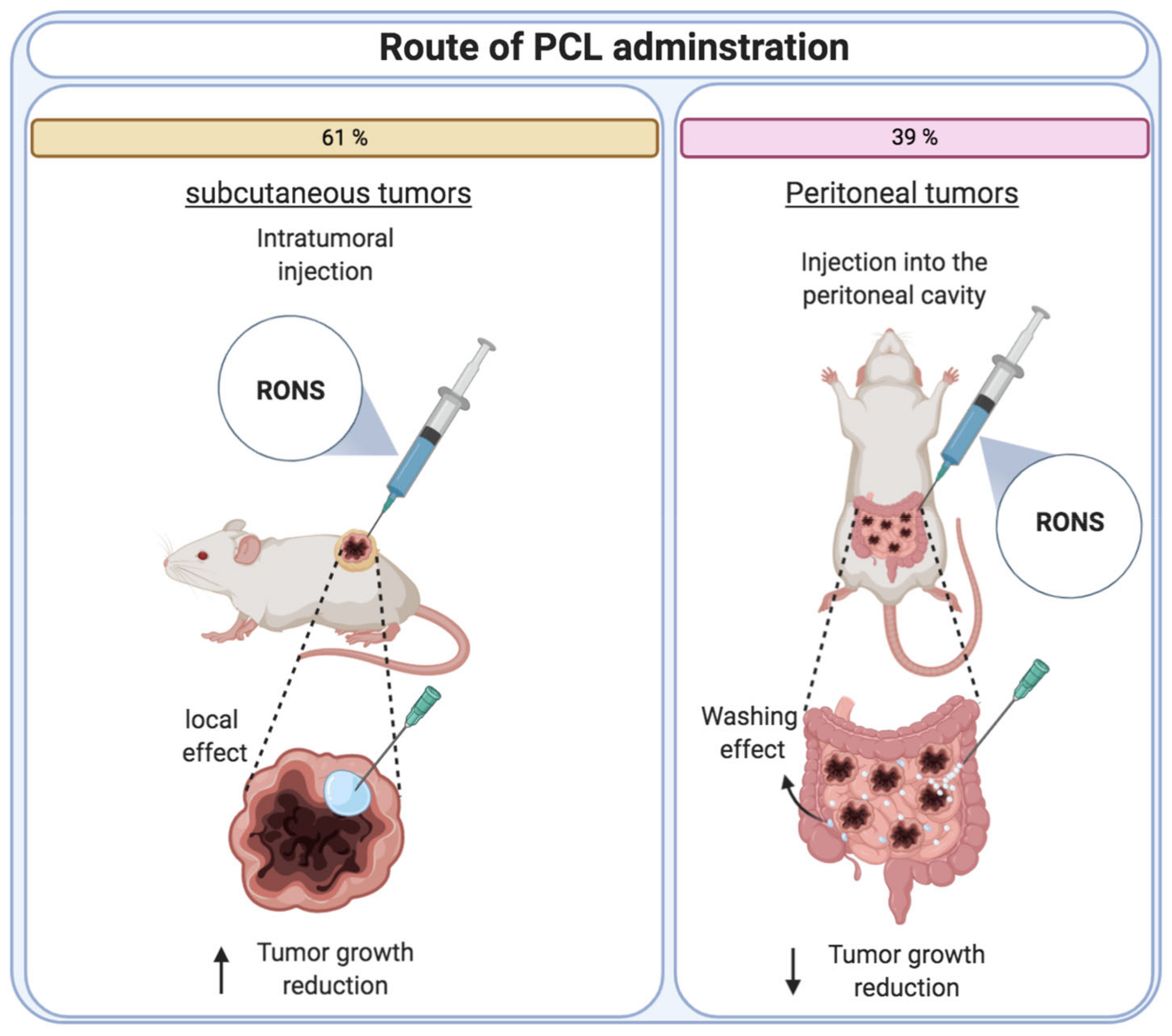
4.4. PCL Dosage Regimen
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zvereva, G.; Gorbanev, Y.; Graves, D.B.; Ceriani, E.; Petrovic, Z.L.; Tsai, P.A.; Verlet, J.R.R.; Jablonowski, H.; Reid, J.P.; Graham, W.G.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 53002. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D Appl. Phys. 2009, 42, 53001. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45. [Google Scholar] [CrossRef]
- Bauer, G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019, 26, 101291. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. Signal amplification by tumor cells: Clue to the understanding of the antitumor effects of cold atmospheric plasma and plasma-activated medium. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 2, 87–98. [Google Scholar] [CrossRef]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic Effect of H2O2 and NO2 in Cell Death Induced by Cold Atmospheric He Plasma. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Mohades, S.; Laroussi, M.; Sears, J.; Barekzi, N.; Razavi, H. Evaluation of the effects of a plasma activated medium on cancer cells. Phys. Plasmas 2015, 22, 122001. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Harley, J.C.; Suchowerska, N.; McKenzie, D.R. Cancer treatment with gas plasma and with gas plasma–activated liquid: Positives, potentials and problems of clinical translation. Biophys. Rev. 2020, 12, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. The Application of the Cold Atmospheric Plasma-Activated Solutions in Cancer Treatment. Anticancer Agents Med. Chem. 2017, 18, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Sanz, M.; Tornín, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.-P.; Layrolle, P.; Canal, C. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef]
- Adhikari, M.; Adhikari, B.; Kaushik, N.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Melanoma growth analysis in blood serum and tissue using xenograft model with response to cold atmospheric plasma activated medium. Appl. Sci. 2019, 9, 4227. [Google Scholar] [CrossRef]
- Adhikari, M.; Kaushik, N.; Ghimire, B.; Adhikari, B.; Baboota, S.; Al-Khedhairy, A.A.; Wahab, R.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion synergistically inhibits human melanoma tumorigenesis via targeting HGF/c-MET downstream pathway. Cell Commun. Signal. 2019, 17, 52. [Google Scholar] [CrossRef]
- Liu, J.R.; Wu, Y.M.; Xu, G.M.; Gao, L.G.; Ma, Y.; Shi, X.M.; Zhang, G.J. Low-temperature plasma induced melanoma apoptosis by triggering a p53/PIGs/caspase-dependent pathway in vivo and in vitro. J. Phys. D Appl. Phys. 2019, 52, 315204. [Google Scholar] [CrossRef]
- Lee, C.B.; Seo, I.H.; Chae, M.W.; Park, J.W.; Choi, E.H.; Uhm, H.S.; Baik, K.Y. Anticancer Activity of Liquid Treated with Microwave Plasma-Generated Gas through Macrophage Activation. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. Invivopen: A novel plasma source for in vivo cancer treatment. J. Cancer 2020, 11, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, 81576. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.J.; Kang, S.U.; Kim, Y.E.; Park, J.K.; Shin, Y.S.; Kim, Y.S.; Lee, K.; Kim, C.H. Non-thermal plasma induces AKT degradation through turn-on the MUL1 E3 ligase in head and neck cancer. Oncotarget 2015, 6, 33382–33396. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Tori, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fuji, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; Von Bernstorff, W.; Von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-Activated Lactated Ringer’s Solution on Pancreatic Cancer Cells In Vitro and In Vivo. Ann. Surg. Oncol. 2018, 25, 299–307. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Freund, E.; Hackbarth, C.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clin. Plasma Med. 2018, 11, 10–17. [Google Scholar] [CrossRef]
- Nakamura, K.; Peng, Y.; Utsumi, F.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; Kajiyama, H. Novel Intraperitoneal Treatment With Non-Thermal Plasma-Activated Medium Inhibits Metastatic Potential of Ovarian Cancer Cells. Sci. Rep. 2017, 7, 6085. [Google Scholar] [CrossRef]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T.; et al. Intraperitoneal Administration of Plasma-Activated Medium: Proposal of a Novel Treatment Option for Peritoneal Metastasis From Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, J.I.; Tanaka, H.; Ishikawa, K.; Sakakita, H.; Ikehara, Y.; Hori, M. Plasma-activated medium (PAM) kills human cancer-initiating cells. Pathol. Int. 2018, 68, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Denayer, T.; Stöhrn, T.; Van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: Progress, challenges and future directions. Dis. Model. Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef]
- Frese, K.K.; Tuveson, D.A. Maximizing mouse cancer models. Nat. Rev. Cancer 2007, 7, 654–658. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived Xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Becher, O.J.; Holland, E.C. Genetically Engineered Models Have Advantages over Xenografts for Preclinical Studies. Cancer Res 2006, 66, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Da Hora, C.C.; Schweiger, M.W.; Wurdinger, T.; Tannous, B.A. Patient-Derived Glioma Models: From Patients to Dish to Animals. Cells 2019, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Nguyen, H.M.; Corey, E. Generation of prostate cancer patient-derived xenografts to investigate mechanisms of novel treatments and treatment resistance. Methods Mol. Biol. 2018, 1786, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Li, Y.; Nelson, S.D.; Dry, S.M.; Singh, A.S.; Elliott, I.A.; et al. Temozolomide regresses a doxorubicin-resistant undifferentiated spindle-cell sarcoma patient-derived orthotopic xenograft (PDOX): Precision-oncology nude-mouse model matching the patient with effective therapy. J. Cell. Biochem. 2018, 119, 6598–6603. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Zhang, G.; Kim, H.S.; Stinson, R.M.; Bechara, R.; Zhang, C.; Chen, Z.; Saba, N.F.; Pakkala, S.; Pillai, R.; et al. Patient-derived xenografts faithfully replicated clinical outcome in a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer. J. Transl. Med. 2016, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, D.A.; Vulimiri, S.V.; French, J.E.; Sonawane, B. The use of genetically modified mice in cancer risk assessment: Challenges and limitations. Crit. Rev. Toxicol. 2013, 43, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Walrath, J.C.; Hawes, J.J.; Van Dyke, T.; Reilly, K.M. Genetically Engineered Mouse Models in Cancer Research. Adv. Cancer Res. 2010, 106, 113–164. [Google Scholar] [CrossRef]
- Lampreht Tratar, U.; Horvat, S.; Cemazar, M. Transgenic Mouse Models in Cancer Research. Front. Oncol. 2018, 8, 268. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.; Shi, W.; Ma, A.; Vere White, R.W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- Walsh, N.C.; Kenney, L.L.; Jangalwe, S.; Aryee, K.-E.; Greiner, D.L.; Brehm, M.A.; Shultz, L.D. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 187–215. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Kondo, T.; Sekine, M.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Cell survival of glioblastoma grown in medium containing hydrogen peroxide and/or nitrite, or in plasma-activated medium. Arch. Biochem. Biophys. 2016, 605, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Tornin Cavielles, J.; Labay, C.P.; Tampieri, F.; Ginebra Molins, M.P.; Canal Barnils, C. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures. Nat. Protoc. 2021. accepted. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Tornín, J.; Villasante, A.; Solé-Martí, X.; Ginebra, M.-P.; Canal, C. Osteosarcoma Tissue-Engineered Model Challenges Oxidative Stress Therapy Revealing Promoted Cancer Stem Cell Properties. Free Radic. Biol. Med. 2021, 164, 107–118. [Google Scholar] [CrossRef]
- Griseti, E.; Merbahi, N.; Golzio, M. Anti-Cancer Potential of Two Plasma-Activated Liquids: Implication of Long-Lived Reactive Oxygen and Nitrogen Species. Cancers 2020, 12, 721. [Google Scholar] [CrossRef]
- Yan, D.; Nourmohammadi, N.; Bian, K.; Murad, F.; Sherman, J.H.; Keidar, M. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci. Rep. 2016, 6, 26016. [Google Scholar] [CrossRef]
- Justilien, V.; Fields, A.P. Utility and Applications of Orthotopic Models of Human Non-Small Cell Lung Cancer (NSCLC) for the Evaluation of Novel and Emerging Cancer Therapeutics. Curr. Protoc. Pharmacol. 2013, 62, 14.27.1–14.27.17. [Google Scholar] [CrossRef]
- Jung, J. Human Tumor Xenograft Models for Preclinical Assessment of Anticancer Drug Development. Toxicol. Res 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Ravoori, M.K.; Margalit, O.; Singh, S.; Kim, S.H.; Wei, W.; Menter, D.G.; DuBois, R.N.; Kundra, V. Magnetic Resonance Imaging and Bioluminescence Imaging for Evaluating Tumor Burden in Orthotopic Colon Cancer. Sci. Rep. 2019, 9, 6100. [Google Scholar] [CrossRef]
- Bouvet, M.; Hoffman, R.M. Tumor imaging technologies in mouse models. Methods Mol. Biol. 2015, 1267, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Krupnick, A.S.; Tidwell, V.K.; Engelbach, J.A.; Alli, V.V.; Nehorai, A.; You, M.; Vikis, H.G.; Gelman, A.E.; Kreisel, D.; Garbow, J.R. Quantitative monitoring of mouse lung tumors by magnetic resonance imaging. Nat. Protoc. 2012, 7, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kon, N.; Jiang, L.; Tan, M.; Ludwig, T.; Zhao, Y.; Baer, R.; Gu, W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, B.; Cüce, G.; Ayan, İ.Ç.; Gültekin, B.; Canbaz, H.T.; Dursun, H.G.; Şahin, Z.; Keskin, İ.; Kalkan, S.S. Evaluation of Apoptosis Pathway of Geraniol on Ishikawa Cells. Nutr. Cancer 2020, 1–6. [Google Scholar] [CrossRef]
- Hu, R.; Wang, M.; Niu, W.; Wang, Y.; Liu, Y.; Liu, L.; Wang, M.; Zhong, J.; You, H.; Wu, X.; et al. SKA3 promotes cell proliferation and migration in cervical cancer by activating the PI3K/Akt signaling pathway. Cancer Cell Int. 2018, 18, 183. [Google Scholar] [CrossRef]
- Delmas, D.; Xiao, J.; Vejux, A.; Aires, V. Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules 2020, 25, 2009. [Google Scholar] [CrossRef]
- Ramasamy, K.; Agarwal, R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef]
| Tumor type | Reference | Plasma Device/Gas | Treatment Conditions of Liquids for In Vivo Cancer Therapy | Quantification of RONS in PCL | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas Flow (slm) | Liquid Type | Liquid Volume (mL) | Distance (mm) | Treatment Time (min) | In Vivo | In Vitro * | |||||
| [RONS] | [RONS] (µM) | ||||||||||
| Subcutaneous tumors | Melanoma | [16] | Plasma jet/Helium | 4 | DMEM | 1 | 15 | 6 | - | - | - |
| [17] | micro DBD | 1.5 | RPMI | - | 2 | 10 | - | - | - | ||
| [18] | micro DBD | 1.5 | RPMI | - | 2 | 10 | - | - | - | ||
| [19] | Plasma jet/Helium | 3 | NaCl 8.5% | 1 | 30 | 1, 2, 3, 4 and 5 | - | H2O2: ~ 35 | Longer treatment time and different liquid type were used for the in vivo condition | ||
| [20] | Microwave plasma generator | - | Water:DMEM (1:10) | 1000 | - | 50 | - | - | - | ||
| Breast | [21] | Plasma jet/Helium | 1 | DMEM | 2 | 13 | 15 | - | H2O2: ~ 200 | Longer treatment time was used for the in vivo condition | |
| [22] | Plasma jet/Helium | 0.2 | PBS | 2 | 13 | 10 | - | - | - | ||
| Ovarian | [23] | Plasma jet/Argon | 2 | RPMI | 4 | 15 | 10 | - | - | Smaller volume of liquid and longer treatment time were used for the in vivo condition | |
| [24] | Plasma jet/Argon | 2 | Ringer’s lactate | 5.5 | 3 | 10 | - | H2O2: ~ 8 | Smaller volume of liquid and longer treatment time were used for the in vivo condition | ||
| Neck | [25] | Plasma jet/Helium + Oxygen | 2 | Serum-free culture media. Not specified | 15 | 10–20 | 15 | O3: 1.154 ppm H2O2: 1.833 ppm O2: 4.767 ppm NO3−: 0.167 ppm | same as in vivo | - | |
| Pancreas | [26] | Plasma jet/Argon | 2 | RPMI | 4 | 15 | 10 | - | - | Smaller volume of liquid and longer treatment time were used for the in vivo condition | |
| Peritoneal tumors | Pancreas | [27] | kINPen Med/Argon | 3 | DMEM | 4 | 5 | 10 | - | - | - |
| [28] | Plasma jet/Argon | 2 | Ringer’s lactate | 8 | 3 | 10 | - | - | Bigger volume of liquid, longer treatment time, and shorter distance of the treatment were used for the in vivo condition | ||
| [29] | kINPen Med/Argon | 5 | DMEM | 5 | - | 10 | - | - | |||
| Ovarian | [30] | Plasma jet/Argon | 2 | RPMI | 5.5 | 3 | 10 | - | H2O2 at different dilution ratios: 0.68 to 1692.16 | Not specified which dilution ratio was used for the in vivo | |
| Gastric | [31] | Plasma jet/Argon | 2 | RPMI | 6 | 3 | 5 | - | - | - | |
| [32] | Plasma jet/Argon | 2 | DMEM | 8 | 3 | 5 | H2O2: 227 µM | same as in vivo | - | ||
| Colon | [33] | kINPen/Argon | 5 | NaCl 0.9% | 50 | - | 60 | - | H2O2: 100 O2−: (Abs. 0.08) NO2−: 2.5 NO3−: 8 | Different liquid type was used for the in vivo condition | |
| Endometrial | [32] | Plasma jet/Argon | 2 | DMEM | 8 | 3 | 5 | H2O2: 227 µM | same as in vivo | - | |
| Tumor type | Reference | Initial Tumor Size (mm Ø) | PCL | Dose Volume (μL) | Administration Route | Dosage Regimen | Treatment Duration (Days) | Therapeutic Effect | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Growth Reduction (%) | ||||||||||
| Subcutaneous tumors | Melanoma | [16] | 5–6 | DMEM | 400 | Subcutaneous | Daily | 25 | 76.7 * | |
| [17] | 4.65 | RPMI | 200 | Subcutaneous | Daily | 4 | ≈20 | |||
| [18] | 4.65 | RPMI | 200 | Subcutaneous | Daily | 3 | No reduction ** | |||
| [19] | 5 | NaCl 8.5% | 200 | subcutaneous (intratumoral) | Daily | 14 | 78.7 | |||
| [20] | Not quantified | Water:DMEM (1:10) | 1000 | Subcutaneous | Daily | 12 | ≈85 | |||
| Breast | [21] | 5 | DMEM | 200 | Subcutaneous | Daily | 30 | ≈80 | ≈10 a | |
| [22] | 5 | PBS | 200 | Subcutaneous | Daily | 30 | ≈60 | |||
| Ovarian | [23] | No tumor formation | RPMI | 200 | Subcutaneous | 3 times/week | 30 | ≈66.7 | ≈37.5 a | |
| [24] | No tumor formation | Ringer’s lactate | 200 | Subcutaneous | 3 times/week | 42 | ≈75 | |||
| Neck | [25] | 4.65 | Serum-free culture media. Not specified | 200 | Subcutaneous (intratumoral) | Daily | 6 | ≈50 | ||
| Pancreas | [26] | No tumor formation | RPMI | 200 | Subcutaneous | 3 times/week | 30 | ≈66.7 | ||
| Peritoneal tumors | Pancreas | [27] | No tumor formation | DMEM | 1000 | Intraperitoneal | Daily | 35 | ≈21.1 | |
| [28] | No tumor formation | Ringer’s lactate | 2500 | Intraperitoneal | Days 2–4 and Days 8–11 | 15 | Not quantified | |||
| [29] | Not quantified | DMEM | 1000 | Intraperitoneal | Daily | 21 | ≈27.8 | |||
| Ovarian | [30] | No tumor formation | RPMI | Not specified | Intraperitoneal | Daily | 3 | Not quantified | ||
| Gastric | [31] | No tumor formation | RPMI | Not specified | Intraperitoneal | Days 1–4 and Days 8–11 | 15 | Not quantified | ||
| [32] | No tumor formation | DMEM | 1000 | Intraperitoneal | Days 0, 1, 2, 6, 7, and 8 | 8 | Not quantified | |||
| Colon | [33] | Not quantified | NaCl 0.9% | 300 | Intraperitoneal | Days 2–6 | Not specified | ≈66.7 | ||
| Endometrial | [32] | No tumor formation | DMEM | 1000 | Intraperitoneal | Days 0, 1, 2, 6, 7, and 8 | 8 | Not quantified | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solé-Martí, X.; Espona-Noguera, A.; Ginebra, M.-P.; Canal, C. Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions. Cancers 2021, 13, 452. https://doi.org/10.3390/cancers13030452
Solé-Martí X, Espona-Noguera A, Ginebra M-P, Canal C. Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions. Cancers. 2021; 13(3):452. https://doi.org/10.3390/cancers13030452
Chicago/Turabian StyleSolé-Martí, Xavi, Albert Espona-Noguera, Maria-Pau Ginebra, and Cristina Canal. 2021. "Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions" Cancers 13, no. 3: 452. https://doi.org/10.3390/cancers13030452
APA StyleSolé-Martí, X., Espona-Noguera, A., Ginebra, M.-P., & Canal, C. (2021). Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions. Cancers, 13(3), 452. https://doi.org/10.3390/cancers13030452








