Prognostic Impact of Sarcopenia in Patients with Metastatic Hormone-Sensitive Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
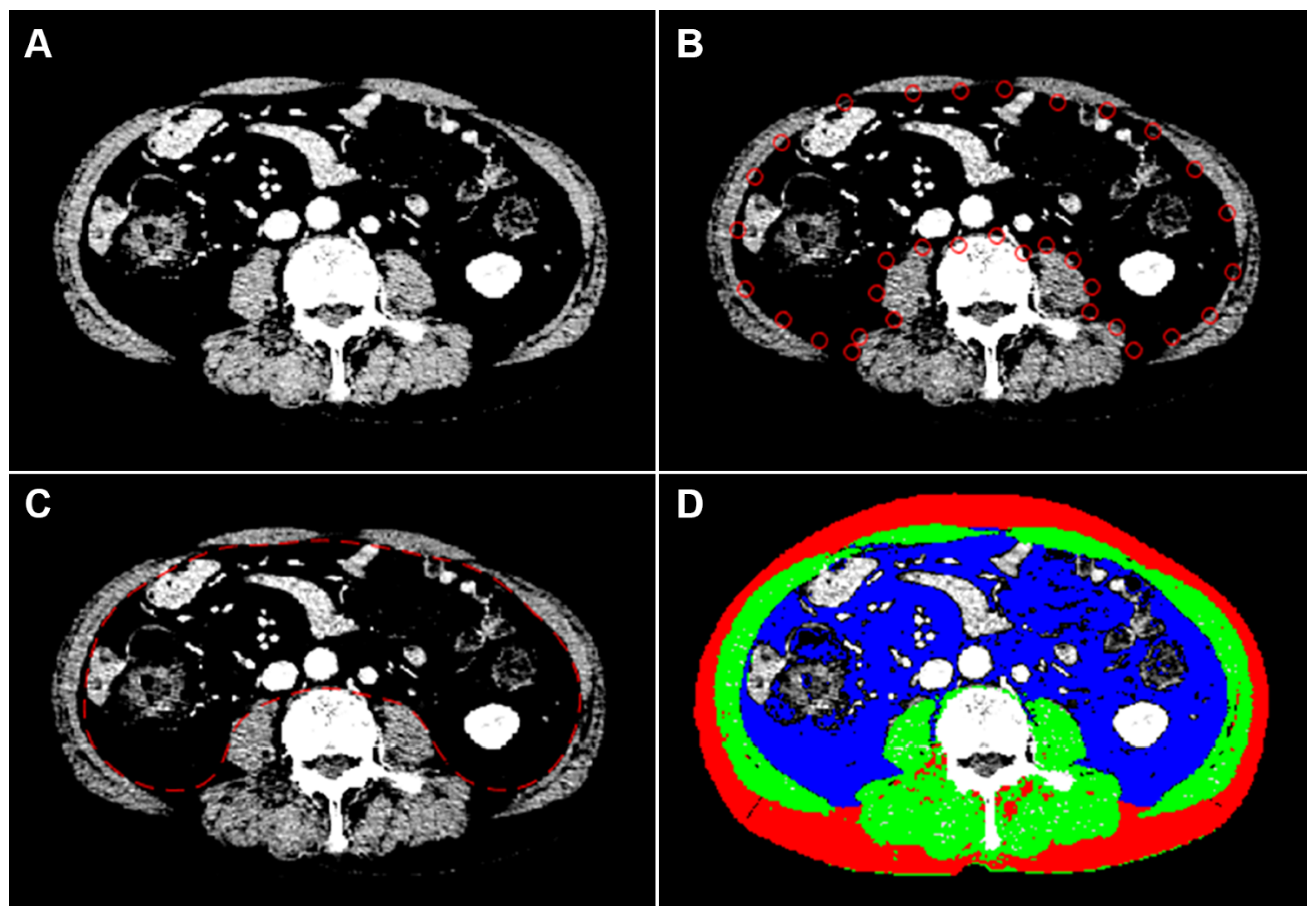
2.2. Image Analysis
2.3. Clinical Data Collection
2.4. RNA Sequencing Pre-Process and Gene Set Enrichment Analysis
2.5. Endpoints
2.6. Statistical Analysis
3. Results
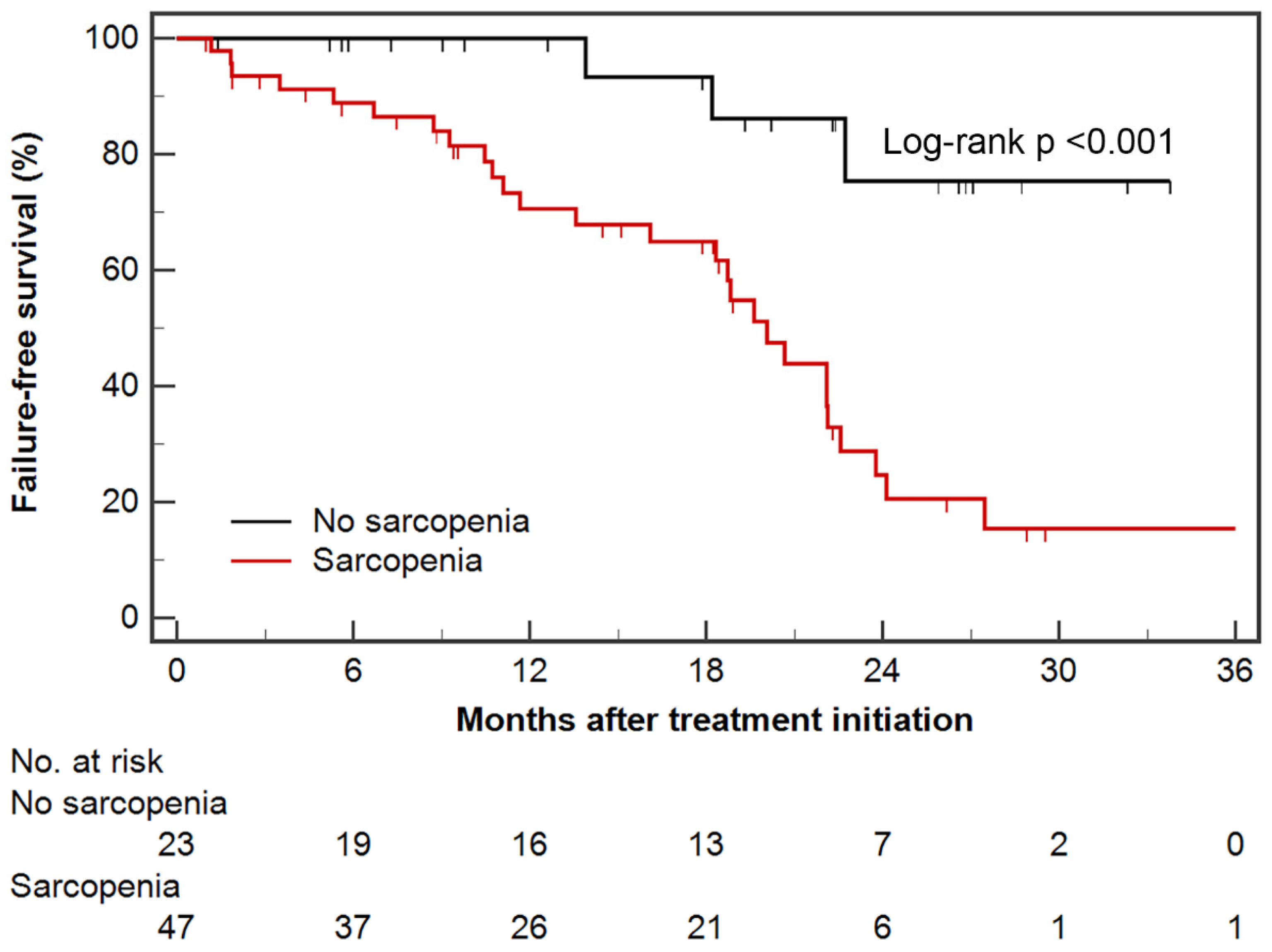
3.1. Failure-Free Survival
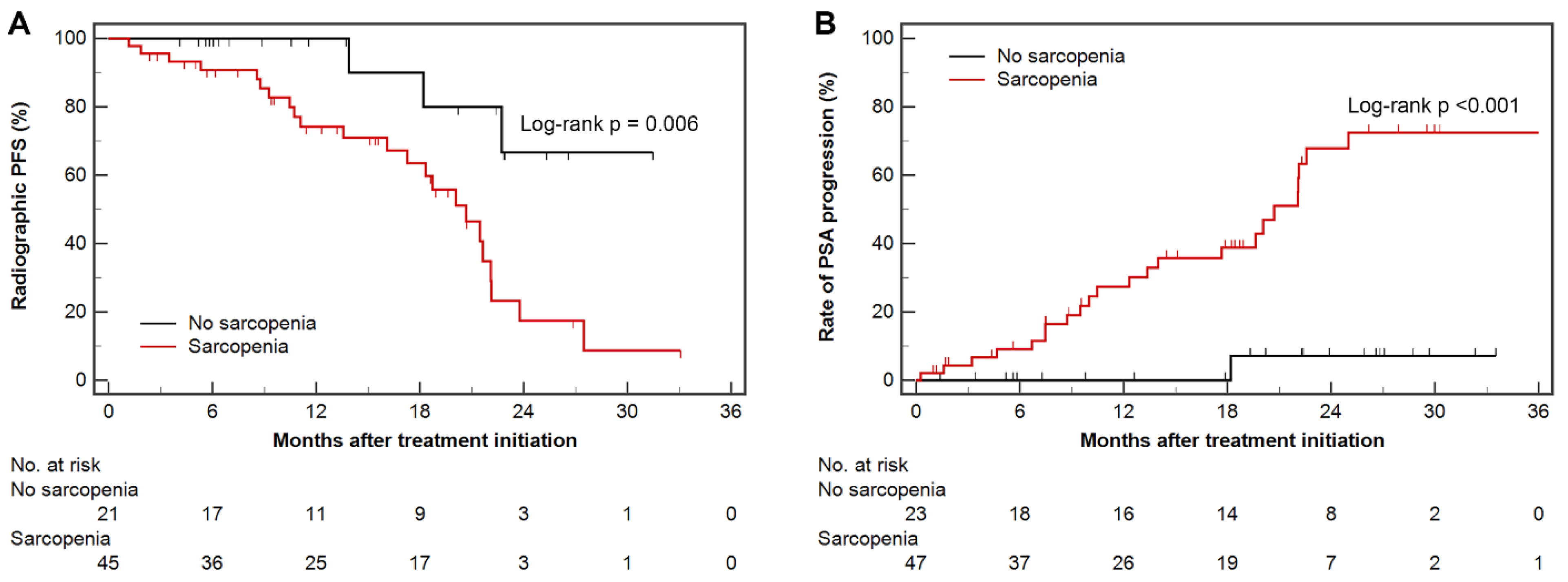
3.2. Radiographic Progression-Free Survival and Time to PSA Progression
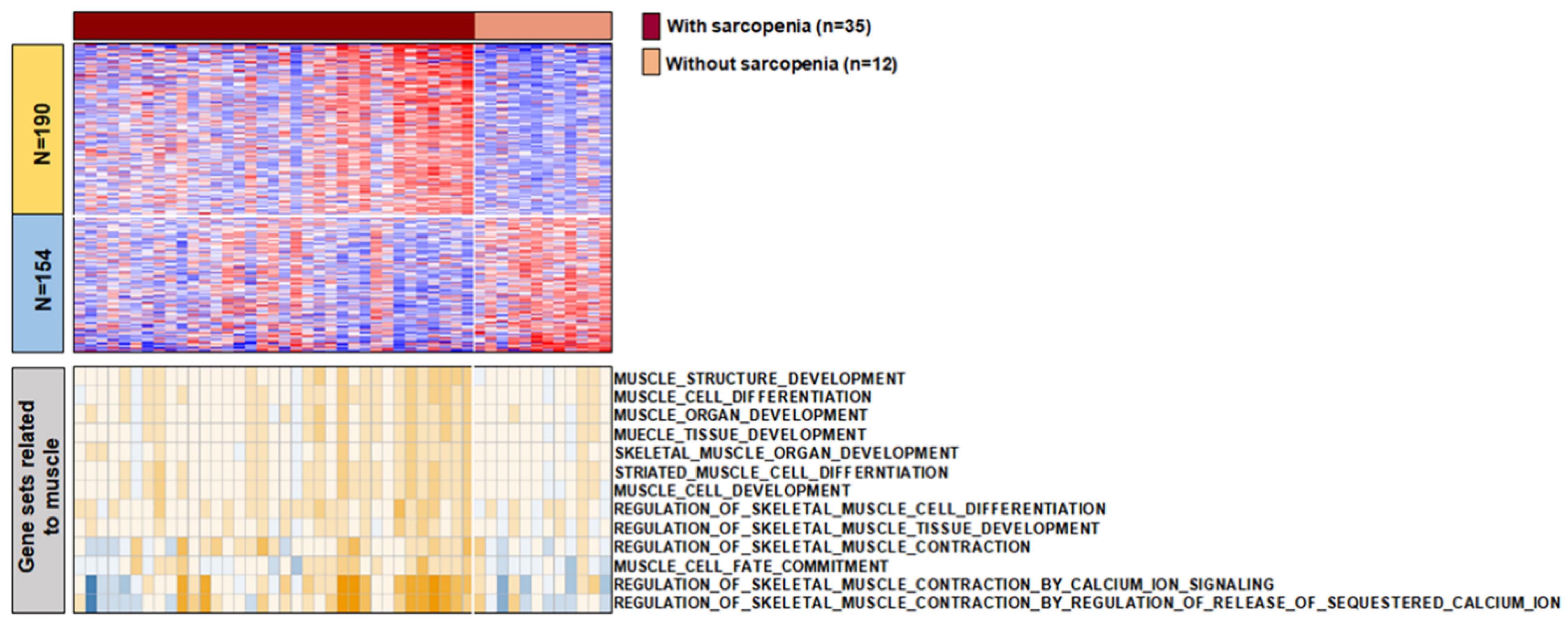
3.3. The Characteristics of Transcript between Samples with and without Sarcopenia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juarez Soto, A.; Merseburger, A.S.; Ozguroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Collins, J.; Noble, S.; Chester, J.; Coles, B.; Byrne, A. The assessment and impact of sarcopenia in lung cancer: A systematic literature review. BMJ Open 2014, 4, e003697. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Lee, J.; Jeong, W.K.; Kim, S.T.; Kim, J.H.; Hong, J.Y.; Kang, W.K.; Kim, K.M.; Sohn, I.; Choi, D. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer 2021, 24, 457–466. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, W.K.; Baik, S.K.; Cha, S.H.; Kim, M.Y. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J. Cachexia Sarcopenia Muscle 2018, 9, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, V.C.; Martin, P.; Lewandowski, P.A. Cancer cachexia: Impact, mechanisms and emerging treatments. J. Cachexia Sarcopenia Muscle 2013, 4, 95–109. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Kuo, F.; Petruzella, S.; Akin, O.; Paris, M.; Russo, P.; Chan, T.A.-t.; Mourtzakis, M.; Hakimi, A.A.; Furberg, H. Mechanisms underlying the association between sarcopenia and poor oncologic outcomes in clear cell renal cell carcinoma. J. Clin. Oncol. 2019, 37, 662. [Google Scholar] [CrossRef]
- Albiges, L.; Hakimi, A.A.; Xie, W.; McKay, R.R.; Simantov, R.; Lin, X.; Lee, J.L.; Rini, B.I.; Srinivas, S.; Bjarnason, G.A.; et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J. Clin. Oncol. 2016, 34, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.; Park, S.Y.; Shin, T.J.; You, D.; Jeong, I.G.; Hong, J.H.; Kim, C.S.; Ahn, H. Association of Muscle Mass with Survival after Radical Prostatectomy in Patients with Prostate Cancer. J. Urol. 2019, 202, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.M.; Llaniguez, J.T.; Telemi, E.; Chuang, M.; Abouelleil, M.; Wilkinson, B.; Chandra, A.; Boyce-Fappiano, D.; Elibe, E.; Schultz, L.; et al. Sarcopenia Predicts Overall Survival in Patients with Lung, Breast, Prostate, or Myeloma Spine Metastases Undergoing Stereotactic Body Radiation Therapy (SBRT), Independent of Histology. Neurosurgery 2020, 86, 705–716. [Google Scholar] [CrossRef]
- Ikeda, T.; Ishihara, H.; Iizuka, J.; Hashimoto, Y.; Yoshida, K.; Kakuta, Y.; Takagi, T.; Okumi, M.; Ishida, H.; Kondo, T.; et al. Prognostic impact of sarcopenia in patients with metastatic hormone-sensitive prostate cancer. Jpn. J. Clin. Oncol. 2020, 50, 933–939. [Google Scholar] [CrossRef]
- Ohtaka, A.; Aoki, H.; Nagata, M.; Kanayama, M.; Shimizu, F.; Ide, H.; Tsujimura, A.; Horie, S. Sarcopenia is a poor prognostic factor of castration-resistant prostate cancer treated with docetaxel therapy. Prostate Int. 2019, 7, 9–14. [Google Scholar] [CrossRef]
- Stangl-Kremser, J.; Suarez-Ibarrola, R.; Andrea, D.; Korn, S.M.; Pones, M.; Kramer, G.; Marhold, M.; Krainer, M.; Enikeev, D.V.; Glybochko, P.V.; et al. Assessment of body composition in the advanced stage of castration-resistant prostate cancer: Special focus on sarcopenia. Prostate Cancer Prostatic Dis. 2020, 23, 309–315. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. (1985) 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients with Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-Induced Skeletal Muscle Atrophy and Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Sydes, M.R.; Mason, M.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.; Ritchie, A.W.S.; Russell, M.; Gilson, C.; Jones, R.; de Bono, J.; et al. Adding abiraterone acetate plus prednisolone (AAP) or docetaxel for patients (pts) with high-risk prostate cancer (PCa) starting long-term androgen deprivation therapy (ADT): Directly randomised data from STAMPEDE (NCT00268476). Ann. Oncol. 2017, 28, v619. [Google Scholar] [CrossRef]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.B.; Goldman, B.; Tangen, C.M.; Hussain, M.; Petrylak, D.P.; Page, S.; Klein, E.A.; Crawford, E.D.; Southwest Oncology, G. Association of body mass index with response and survival in men with metastatic prostate cancer: Southwest Oncology Group trials 8894 and 9916. J. Urol. 2007, 178, 1946–1951, discussion 1951. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Perna, S.; Guido, D.; Grassi, M.; Rondanelli, M. Association between muscle mass and adipo-metabolic profile: A cross-sectional study in older subjects. Clin. Interv. Aging 2015, 10, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneppers, A.E.M.; Langen, R.C.J.; Gosker, H.R.; Verdijk, L.B.; Cebron Lipovec, N.; Leermakers, P.A.; Kelders, M.; de Theije, C.C.; Omersa, D.; Lainscak, M.; et al. Increased Myogenic and Protein Turnover Signaling in Skeletal Muscle of Chronic Obstructive Pulmonary Disease Patients with Sarcopenia. J. Am. Med. Dir. Assoc. 2017, 18, 637.e1–637.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzeszczynska, J.; Meyer, A.; McGregor, R.; Schilb, A.; Degen, S.; Tadini, V.; Johns, N.; Langen, R.; Schols, A.; Glass, D.J.; et al. Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. J. Cachexia Sarcopenia Muscle 2018, 9, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Tlsty, T.D.; Gascard, P. Stromal directives can control cancer. Science 2019, 365, 122–123. [Google Scholar] [CrossRef]
- Pattison, J.S.; Folk, L.C.; Madsen, R.W.; Childs, T.E.; Booth, F.W. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol. Genom. 2003, 15, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.H.; Serie, D.J.; Parasramka, M.; Cheville, J.C.; Bot, B.M.; Tan, W.; Wang, L.; Joseph, R.W.; Hilton, T.; Leibovich, B.C.; et al. Differential gene expression profiling of matched primary renal cell carcinoma and metastases reveals upregulation of extracellular matrix genes. Ann. Oncol. 2017, 28, 604–610. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia with Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, D.; Buttner, S.; Kim, Y.; Gani, F.; Xu, L.; Margonis, G.A.; Amini, N.; Kamel, I.R.; Pawlik, T.M. Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br. J. Surg. 2016, 103, e83–e92. [Google Scholar] [CrossRef] [PubMed]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Botta, F.; Mariani, A.; Bagnardi, V.; De Carlis, L. The Impact of Sarcopenia on Postoperative Course following Pancreatoduodenectomy: Single-Center Experience of 110 Consecutive Cases. Dig. Surg. 2020, 37, 312–320. [Google Scholar] [CrossRef]
- Wagner, D.; Marsoner, K.; Tomberger, A.; Haybaeck, J.; Haas, J.; Werkgartner, G.; Cerwenka, H.; Bacher, H.; Mischinger, H.J.; Kornprat, P. Low skeletal muscle mass outperforms the Charlson Comorbidity Index in risk prediction in patients undergoing pancreatic resections. Eur. J. Surg. Oncol. 2018, 44, 658–663. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 70) | No Sarcopenia (n = 23) | Sarcopenia (n = 47) | p |
|---|---|---|---|---|
| Age (years) * | 66.5 (60.0, 73.0) | 63.0 (58.0, 70.0) | 68.0 (63.3, 73.0) | 0.070 |
| BMI (km/m2) * | 24.3 (21.7, 25.8) | 25.6 (25.1, 28.6) | 22.4 (21.2, 25.0) | <0.001 |
| Obesity, n † | 29 (41.4%) | 17 (73.9%) | 12 (25.5%) | <0.001 |
| SMI (cm2/m2) * | 49.6 (44.9, 53.1) | 58.1 (53.8, 62.4) | 46.5 (43.7, 48.6) | <0.001 |
| SFI (cm2/m2) * | 38.3 (29.4, 47.9) | 48.1 (38.3, 65.3) | 34.5 (27.8, 45.5) | <0.001 |
| VFI (cm2/m2) * | 47.2 (30.3, 62.8) | 48.5 (37.6, 70.6) | 45.3 (25.0, 62.5) | 0.165 |
| VSR * | 1.09 (0.76, 1.58) | 0.93 (0.82, 1.52) | 1.16 (0.78, 1.59) | 0.516 |
| PSA (ng/mL) * | 299.4 (89.6, 801.3) | 237.3 (94.4, 540.2) | 338.0 (101.7, 1248.7) | 0.241 |
| Treatment agent, n † | 0.353 | |||
| Docetaxel | 42 (60.0%) | 12 (52.2%) | 30 (63.8%) | |
| Abiraterone acetate | 28 (40.0%) | 11 (47.8%) | 17 (36.2%) | |
| ECOG PS > 0, n † | 34 (48.6%) | 10 (43.5%) | 24 (51.1%) | 0.554 |
| Gleason score ≥ 8, n ‡ | 63 (94.0%) | 19 (90.5%) | 44 (95.7%) | 0.584 |
| Stage (cT4), n † | 37 (55.2%) | 16 (69.6%) | 21 (47.7%) | 0.090 |
| Regional LN metastasis, n ‡ | 56 (80.0%) | 22 (95.7%) | 34 (72.3%) | 0.026 |
| Bone metastasis, n ‡ | 58 (82.9%) | 14 (60.9%) | 44 (93.6%) | 0.001 |
| Visceral metastasis, n † | 20 (28.6%) | 7 (30.4%) | 13 (27.7%) | 0.811 |
| High-volume, n † | 56 (80.0%) | 14 (60.9%) | 42 (89.4%) | 0.006 |
| High-risk, n ‡ | 59 (84.3%) | 14 (60.9%) | 45 (95.7%) | <0.001 |
| Variables (Reference) | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 65 years (<65 years) | 1.78 (0.82–3.87) | 0.145 | 1.33 (0.55–3.24) | 0.529 |
| Obesity (non-obesity) | 0.42 (0.19–0.93) | 0.033 | 2.51 (0.60–10.49) | 0.207 |
| Sarcopenia (no sarcopenia) | 6.18 (1.87–20.44) | 0.003 | 6.69 (1.57–28.49) | 0.010 |
| SFI ≥ median (<median) | 0.34 (0.16–0.75) | 0.007 | 0.30 (0.08–1.07) | 0.063 |
| VFI ≥ median (<median) | 1.04 (0.51–2.15) | 0.912 | ||
| VSR ≥ median (<median) | 1.42 (0.69–2.93) | 0.342 | ||
| PSA ≥ median (<median) | 1.17 (0.56–2.41) | 0.677 | 0.91 (0.36–2.30) | 0.843 |
| Abiraterone acetate (docetaxel) | 0.88 (0.37–2.08) | 0.774 | ||
| ECOG PS ≥ 1 (0) | 2.39 (1.12–5.14) | 0.025 | 2.10 (0.93–4.72) | 0.073 |
| Gleason score ≥ 8 (<8) | N/A | 0.955 | ||
| Stage cT4 (≤cT3) | 0.82 (0.38–1.77) | 0.618 | ||
| Regional LN metastasis (no) | 1.47 (0.56–3.85) | 0.431 | ||
| Bone metastasis (no) | 1.27 (0.48–3.31) | 0.632 | ||
| Visceral metastasis (no) | 1.13 (0.51–2.46) | 0.768 | ||
| High-volume (low-volume) | 2.43 (0.84–7.04) | 0.102 | 1.41 (0.24–8.33) | 0.704 |
| High-risk (low-risk) | 2.95 (0.89–9.78) | 0.078 | 0.911 (0.36–2.30) | 0.843 |
| Variables (Reference) | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 65 years (<65 years) | 2.33 (0.98–5.55) | 0.055 | 1.63 (0.63–4.21) | 0.314 |
| Obesity (non-obesity) | 0.54 (0.23–1.26) | 0.155 | ||
| Sarcopenia (no sarcopenia) | 4.73 (1.40–15.96) | 0.012 | 3.77 (0.95–14.99) | 0.060 |
| SFI ≥ median (<median) | 0.65 (0.30–1.45) | 0.294 | ||
| VFI ≥ median (<median) | 0.97 (0.44–2.15) | 0.944 | ||
| VSR ≥ median (<median) | 0.90 (0.42–1.96) | 0.798 | ||
| PSA ≥ median (<median) | 1.11 (0.51–2.42) | 0.790 | 0.86 (0.33–2.24) | 0.759 |
| Abiraterone acetate (docetaxel) | 0.70 (0.28–1.79) | 0.463 | ||
| ECOG PS ≥ 1 (0) | 2.61 (1.12–6.07) | 0.026 | 2.27 (0.96–5.39) | 0.063 |
| Gleason score ≥ 8 (<8) | N/A | 0.963 | ||
| Stage cT4 (≤cT3) | 0.96 (0.41–2.22) | 0.918 | ||
| Regional LN metastasis (no) | 0.91 (0.34–2.44) | 0.853 | ||
| Bone metastasis (no) | 1.18 (0.44–3.14) | 0.748 | ||
| Visceral metastasis (no) | 0.97 (0.40–2.33) | 0.946 | ||
| High-volume (low-volume) | 2.15 (0.72–6.41) | 0.169 | 1.17 (0.21–6.53) | 0.855 |
| High-risk (low-risk) | 2.46 (0.73–8.30) | 0.147 | 0.89 (0.33–2.24) | 0.759 |
| Variables (Reference) | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 65 years (<65 years) | 1.95 (0.80–4.75) | 0.143 | 1.41 (0.53–3.74) | 0.494 |
| Obesity (non-obesity) | 0.34 (0.13–0.87) | 0.025 | 2.53 (0.47–13.47) | 0.278 |
| Sarcopenia (no sarcopenia) | 16.07 (2.16–119.46) | 0.007 | 12.91 (1.08–153.85) | 0.043 |
| SFI ≥ median (<median) | 0.31 (0.13–0.75) | 0.009 | 0.29 (0.06–1.34) | 0.114 |
| VFI ≥ median (<median) | 1.15 (0.51–2.58) | 0.734 | ||
| VSR ≥ median (<median) | 1.75 (0.76–4.01) | 0.185 | ||
| PSA ≥ median (<median) | 1.84 (0.78–4.30) | 0.162 | 1.12 (0.40–3.10) | 0.832 |
| Abiraterone acetate (docetaxel) | 1.18 (0.48–2.87) | 0.720 | ||
| ECOG PS ≥ 1 (0) | 3.65 (1.44–9.22) | 0.006 | 2.73 (1.04–7.14) | 0.041 |
| Gleason score ≥ 8 (<8) | N/A | 0.958 | ||
| Stage cT4 (≤cT3) | 0.59 (0.24–1.43) | 0.246 | ||
| Regional LN metastasis (no) | 2.03 (0.61–6.82) | 0.250 | ||
| Bone metastasis (no) | 6.01 (0.81–44.67) | 0.079 | 0.43 (0.04–5.04) | 0.500 |
| Visceral metastasis (no) | 1.39 (0.60–3.26) | 0.446 | ||
| High-volume (low-volume) | 8.62 (1.16–64.07) | 0.035 | 1.37 (0.16–11.96) | 0.773 |
| High-risk (low-risk) | N/A | 0.950 | N/A | 0.954 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Jee, B.A.; Kim, J.-H.; Bae, H.; Chung, J.H.; Song, W.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; et al. Prognostic Impact of Sarcopenia in Patients with Metastatic Hormone-Sensitive Prostate Cancer. Cancers 2021, 13, 6345. https://doi.org/10.3390/cancers13246345
Lee JH, Jee BA, Kim J-H, Bae H, Chung JH, Song W, Sung HH, Jeon HG, Jeong BC, Seo SI, et al. Prognostic Impact of Sarcopenia in Patients with Metastatic Hormone-Sensitive Prostate Cancer. Cancers. 2021; 13(24):6345. https://doi.org/10.3390/cancers13246345
Chicago/Turabian StyleLee, Ji Hyun, Byul A Jee, Jae-Hun Kim, Hoyoung Bae, Jae Hoon Chung, Wan Song, Hyun Hwan Sung, Hwang Gyun Jeon, Byong Chang Jeong, Seong Il Seo, and et al. 2021. "Prognostic Impact of Sarcopenia in Patients with Metastatic Hormone-Sensitive Prostate Cancer" Cancers 13, no. 24: 6345. https://doi.org/10.3390/cancers13246345
APA StyleLee, J. H., Jee, B. A., Kim, J.-H., Bae, H., Chung, J. H., Song, W., Sung, H. H., Jeon, H. G., Jeong, B. C., Seo, S. I., Jeon, S. S., Lee, H. M., Park, S. H., & Kang, M. (2021). Prognostic Impact of Sarcopenia in Patients with Metastatic Hormone-Sensitive Prostate Cancer. Cancers, 13(24), 6345. https://doi.org/10.3390/cancers13246345






