1. Introduction
Soft tissue sarcomas (STS) of the limbs include a large variety of histologies, sizes, and grades, with clear and significant variances in their clinical course and prognosis [
1]. Despite these intrinsic varieties, STS are very rare tumors, which represent only 1% of all malignancies considering all locations in the adult population. These variabilities, together with a lack of knowledge of these rare cancers among general practitioners, often lead to a delayed presentation at referral centers [
2]. A delayed diagnosis can cause the observation of large lumps at baseline, thus increasing difficulties in surgical treatment. Therefore, an early diagnosis and a multidisciplinary approach are mandatory to choose the best strategy for each patient.
Limb salvage surgery with radiation therapy (RT) is the standard of care for most patients, while the role of chemotherapy (ChT) is more debated [
3]. There is still disagreement on which are the most appropriate surgical strategies when the tumor involves the principal vessels of the limb.
A decision must be made between an extended resection with the risk of causing a deficiency for the patient or limiting the excision with the risk of incomplete tumor removal [
4].
Magnetic Resonance Imaging (MRI) is the imaging tool of choice to evaluate STS of the extremities, with fundamental information in all the phases of the disease, in particular for surgical planning [
5]. An accurate MRI study at baseline is imperative for decision-making. Several MRI features can change drastically the surgical approach, above all the identification of the so-called “tail sign”, which can be observed in some “infiltrative” histotypes [
6]. In these cases, a wider resection is suggested [
7]. In addition, MRI is the imaging of choice to assess the relationship between the tumor mass and vascular bundles.
To date, many clinical studies tried to identify prognostic factors for local recurrence (LR) and overall survival (OS). Numerous factors have been suggested as possible risk factors for developing LR or distant metastasis, including histological STS-subtypes, surgical margins, tumor size, grade, administration of neo(adjuvant) RT or ChT [
8,
9,
10,
11,
12,
13]. However, an established prognostic profile for individual patients is still lacking, even though the risk factors for the occurrence of LR and distant metastasis have been studied in many series [
14].
Nonetheless, the correlation between the closeness between major vessels and the tumor and outcomes (LR and survival) has never been investigated in STS. Recently, Fujiwara et al. [
15] observed that the proximity to major blood vessels is a poor prognostic factor for local control and survival in patients affected by osteosarcoma.
The aim of this study was to explore the influence of the proximity of the tumor to the main vascular structures in a large population with deep STS of the thigh and popliteal fossa.
2. Material and Methods
Cases
All patients with a diagnosis of STS treated at a single Institution who underwent surgery from January 2000 to April 2017 were retrospectively analyzed.
Inclusion criteria included primary, deeply seated STS, location in the thigh and popliteal fossa, age ≥18 years and pre-operative MRI available for retrospective evaluation. Depths were evaluated according to preoperative MRI. Patients were excluded if treated with an amputation to analyze a more homogeneous series. Thus, a total of 529 patients meeting these criteria were selected from the whole series.
The study was approved by the local Ethics Committee.
All cases were histologically revised and classified according to the 2013 World Health Organization classification of STS [
16]. A 3-step system (FNCLCC) was used to assess STS grade [
16].
Some STS subtypes (myxofibrosarcoma and undifferentiated pleomorphic sarcoma) may present a specific focal-infiltrative pattern and were therefore classified as “infiltrative subtypes” [
7].
The larger diameter was measured on the surgical specimen to assess tumor size. All patients underwent surgery in order to obtain limb-sparing, function-sparing surgery with negative surgical margins according to the R classification [
17].
The use of RT and ChT was decided at the discretion of a multidisciplinary team (orthopedic surgeon, radiotherapist, and oncologist). Radiation therapy and ChT were administrated according to STS guidelines [
18].
All patients had an MRI performed within a maximum of 21 days prior to surgery. All MRI were performed with a high field (1.5 Tesla or higher) and with the standard sequences, which included T1w, T2w, STIR, or T2 fat sat. Magnetic resonance imagines were retrospectively reviewed on a PACS (Carestream Vue PACS v. 11.4.1.1102) by two expert oncologic radiologists (PS and FP). Imaging analyses were performed in consensus. The closeness to major vessels was defined as the minimum distance between the tumor and the main vascular bundle. This distance was measured on the axial slices of preoperative T1-weighted MRI [
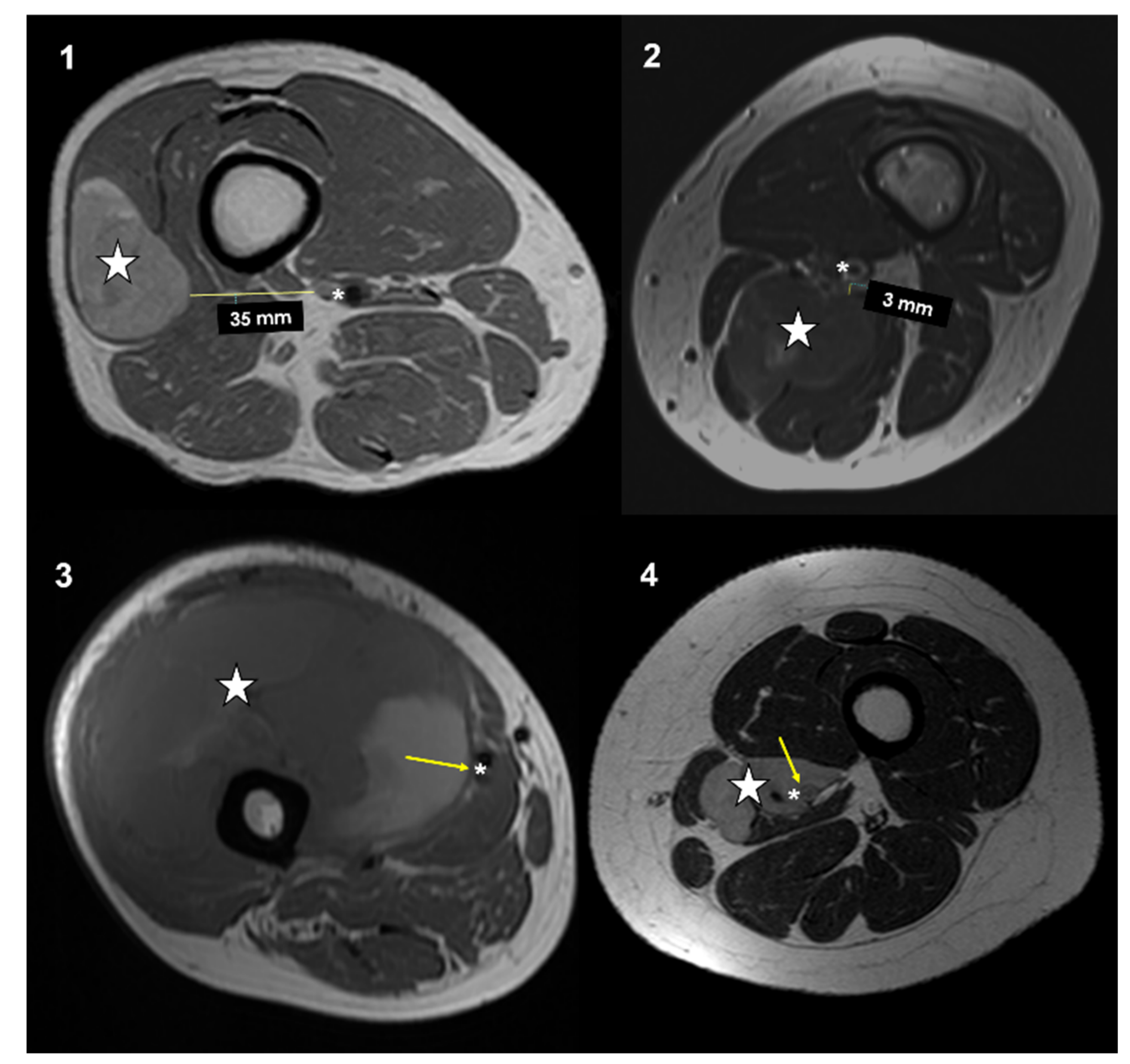
19]. Distance between the major vascular bundle and the tumor was classified into four types according to Fujiwara et al. [
15]: type 1, >5 mm; type 2, ≤5 mm and >0 mm; type 3, attached to the tumor; type 4, surrounded by the tumor (
Figure 1).
Patients’ characteristics are presented by frequencies and percentages for categorical variables, median, and range for continuous variables.
The Kaplan–Meier method was used to estimate OS and LR-free survival. The Kaplan–Meier method was used as a univariate unadjusted analysis of prognostic factors to estimate LR-free survival and OS. The estimated local recurrence-free survival interval was defined as the time between surgery and first LR. Being a retrospective study, patients were censored at the last available follow-up, in case of death, or in case of amputation not related to local recurrence. Overall survival interval was defined as the time between surgery and death, during the time of follow-up. Patients who died of other causes were censored. Differences in survival rates were assessed by the log-rank test. Multivariable analysis of LR and OS was based on cause-specific hazards and therefore carried out by Cox regression models. The following factors were included in multivariate analysis: histotype, FNLCC grade, proximity to major vessels, radiotherapy, chemotherapy, tumour size, age, and margins. p values < 0.05 were considered significant. All analysis was completed using the Statistical Package for Social Science (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA).
3. Results
Median age at the time of surgical procedures was 58 years (range, 18 to 92). (
Table 1) Most of the patients (288, 54.4%) were male and 241 (45.6%) were female.
The majority of STS were grade 3 (421, 79.6%), 9.6% were grade 2, and 10.8% grade 1. Size of the tumour was larger than 10 cm in 47.4% of the cases, 5–10 cm in 36.5%, and smaller than 5 cm in 16.1%. Non-infiltrative histotypes accounted for 66.5% of the whole series, whereas in 33.5% were infiltrative STS.
Type 1 involvement was found in 285 (53.9%) of the cases on pre-operative MRI, whereas 61 (11.5%) were type 2. The tumour was attached to major vessels (type 3) in 108 (20.4%) of the cases and surrounded by the tumor 75 (14.2%). Type 3–4 vessels involvement were mostly observed among non-infiltrative STS (p = 0.030) and large tumors (p < 0.001). Fifty-eight patients (11.0%) were metastatic at the time of diagnosis (43 to the lungs, in 7 cases to lymph nodes, 2 cases of bone metastasis, and 5 to the liver). A vascular by-pass was required in 21 (4.0%) of the cases, mostly in type 3–4 vessels involvement (p < 0.001). All the cases had only artery reconstructed.
Type 3–4 vessels involvement was mostly found in STS larger than 10 cm (p < 0.001) and in non-infiltrative histotypes (p = 0.030). However, no correlation was found with FNLCC grade (p = 0.258).
R0 margins were obtained in 494 cases (93.3%), R1 margins in 35 (6.7%). No correlation was found between the quality of surgical margins and type of vessels involvement (p = 0.288). No amelioration in the quality of surgical margin was observed in patients who underwent artery sacrifice and reconstruction with a by-pass (p = 0.727).
Chemotherapy was used in 253 (47.8%) STS, mostly in non-infiltrative histotypes (p = 0.021), in patients with metastasis at presentation (p < 0.001), in grade 3 tumors (p < 0.001), and in those presenting with a type 3–4 vessels involvement (p = 0.005). Patients younger than 65 years at the time of diagnosis received ChT more frequently (p < 0.001).
In 369 (69.8%) cases, RT was given in addition to surgery as part of the primary treatment, mainly in grade 3 STS (p < 0.001), large tumors (p < 0.001), and in those with a pre-operative type 3–4 vessels involvement (p = 0.003). Radiotherapy was administered in the pre-operative setting in 94 cases, with no amelioration of surgical margins adequacy (p = 0.645).
At the last follow-up (median 31 months, range 2 to 269), 363 patients (68.6%) were alive; 107 (20.2%) died of the disease; 10 patients (1.9%) died of other causes. Forty-nine patients (9.3%) were alive with progressive metastatic disease.
3.1. Local Recurrence
In 86 cases (16.3%), a LR occurred after a median time of 17 months (range, 2 to 96).
Local recurrence-rate was 19.1% (C.I. 95% 15.0%–23.2%) at five years and 41.4% (C.I. 95% 23.8–39.0%) at ten years.
A higher LR rate was found in infiltrative STS subtype (29.6% vs. 14.5% at five years,
p = 0.005). (
Figure 2) FNLCC grade 2 and 3 STS had also an increased risk of LR (15.5% and 21.8% at five years, respectively) compared to grade 1 (1.8% at 5 years) (
p = 0.035). Proximity to major vessels type 1 and 2 had a LR rate lower (12.0% and 12.4% at five years, respectively) than type 3 and 4 (26.3% and 40.6% at five years, respectively) (
p < 0.001). No significant differences were observed neither for size of the tumor (
p = 0.156) nor for RT administration (
p = 0.181) with LR rate. Patients with adequate surgical margins had a lower LR-rate, even though this did not reach statistical significance (
p = 0.069). No amelioration of local control of the disease was observed among patients treated with a vascular by-pass (
p = 0.223). However, among type 4 vascular involvement cases, a vascular by-pass allowed for an amelioration in local control of the disease (LR rate 21.2% at five years, lower than in type 3 vascular involvement without vascular sacrifice).
On multivariate analysis infiltrative histotypes, high FNLCC grade and type 3–4 of proximity to major vessels were confirmed to be independent prognostic factors for LR (
Table 2). Radiotherapy was also found to be an independent prognostic factor for LR.
3.2. Sarcoma Specific Survival
Estimated overall survival, measured with a Kaplan–Meier analysis was 73.1% (C.I. 95% 68.2–78.0%) at five years and 63.2% (C.I. 95% 56.4–70.0%) at ten years.
A significant worse OS was observed in STS with distant metastasis at presentation (20.4% vs. 79.5% at five years follow up,
p < 0.001). Patients treated with ChT had a better OS (66.6% vs. 80.3% at five years,
p = 0.001) (
Figure 3).
Grade 2 and 3 STS had an inferior prognosis (OS 78.6% and 69.1% at five years, respectively) if compared to grade 1 (100% OS at five years, p < 0.001). Regarding histology, the worst OS was found in infiltrative over non-infiltrative subtypes (65.6% vs. 76.7 at five years, p = 0.005). Patients with a major vessels involvement type 1 and 2 had a better OS (77.5%, 81.7%, respectively, at five years) compared to type 3 and 4 (69.5%, 55.9, respectively, at five years) (p = 0.008).
On multivariate analysis, proximity to major vessels was not confirmed to be an independent prognostic factor for OS (
Table 3).
4. Discussion
One of the most important factors to be considered by surgeons when treating patients affected by STS is the distance between the tumor and the major vessels. However, to the best of our knowledge, no previous study focused specifically on the prognostic implication of the proximity of the tumor to major vessels in STS.
In the present series, proximity to major vessels was observed to be an independent prognostic factor for local recurrence in primary STS of the thigh and popliteal fossa, thus providing a possible prognostic estimation based on preoperative MRI assessment.
Moreover, we confirm that two of the most important predictors of outcome are histology/histologic subtype and grade [
1]. Specific infiltrative STS subtypes such as myxofibrosarcoma and undifferentiated pleomorphic sarcoma have an increased risk of LR regardless of the adequacy of surgical margins [
7,
20,
21,
22]. A non-significant difference in the LR rate was observed concerning the adequacy of surgical margins. However, it must be considered that the interpretation of the surgical margins can be subjective and might vary depending on who assessed the specimen.
Radiation therapy seemed to have a strong impact to reduce the risk of a recurrence. Moreover, patients who received RT were unfavorably selected, the choice being a clinical one performed on an individual basis. For example, RTE was mostly administered in large and type 3–4 tumors.
Overall survival was related to the histological tumor grade. The main difference in outcome was identified between grade 2 and grade 3 tumors, whereas less important differences were observed between grade 1 and 2 STS. All the patients affected by grade 1 sarcoma were alive at the final follow-up. Chemotherapy was not found to ameliorate OS on multivariate analysis. However, patients who underwent ChT were unfavorably selected, being selected for ChT those at a higher risk. In particular, patients older than 65 years received ChT in fewer cases than younger patients. Proximity to major vessels was not found to influence OS on multivariate analysis. Biological aggressiveness of the tumor rather than vessel proximity is the major factor behind patient survival. The age of the patient was also found to be an independent prognostic factor for survival. Acem et al. previously observed that this can only partially be explained by differences in tumor and treatment characteristics, suggesting that STS may have a more aggressive tumor behavior in elderly patients when compared with their younger counterparts, which may coincide with a weaker tumor-specific immune response in elderly patients [
11].
Most of the deeply seated STS of the thigh and popliteal fossa included in the present series had a distance to major vessels >5 mm. Nonetheless, approximately one third of the cases were attached or surrounded vascular bundle (type 3 and 4, respectively), particularly evident among large tumors and non-infiltrative histotypes. In many cases, the main vessels are just pushed by the STS, but not actually penetrated. Thus, surgery with additional complex resection of the vessels may represent an overtreatment in these situations, growing the risk for an unnecessarily increased risk of surgical morbidity. Nonetheless, when a major vascular bundle is found to surround the sarcoma on preoperative MRI, a vascular by-pass reduced local recurrence risk, with no amelioration on survival rate. A combined ortho-vascular approach for STS patients is a challenge and can increase the incidence of postoperative complications, but can help in ameliorating surgical outcomes. [
4,
23,
24]
In our opinion, these results may offer valuable prognostic information for treating oncologists and could be helpful to advise patients on the management of their STS. The strategy of treatment should be tailored for each case, considering the possible survival benefits, and expected morbidity.
The retrospective design of this study is one of the main limitations, with possible selection biases regarding diagnosis, treatment, and follow-up of patients included.
Moreover, margins classification on a retrospective series could have been affected by their previous assessment. In addition, the artery and vein were considered together as “major vessels”.
However, this series includes data from a selected cohort of patients, which include only adults affected by primary sarcomas of the thigh and popliteal fossa. A limited follow-up in some of the patients could also slightly affect the results. Moreover, the quality of the MRI was not always consistent between patients. We believe that a prospective study with matching between the magnetic resonance images, the pathological reports and excised gross specimen, as well as a precise lesion-by-lesion correlation would be necessary. Recent advantages in regards of quantitative imaging may offer more precise and reliable information, in all the phases of the disease. Several quantitative tools applicable to MRI such as diffusion (DWI) sequences, dynamic contrast-enhanced, and radiomics analyses are increasing in use to obtain a solid and more reliable evaluation [
25,
26]. In the near future, the radiomic features extrapolated from MRI studies will permit to build artificial intelligence algorithms, in order to help in diagnosis, to predict patients’ prognosis and for tumor grade prediction, and to evaluate responses to treatments.
In the literature, there is a common lack of awareness about the importance of the tumor-vascular relationship as a prognostic factor for local recurrence. The lack of association between tumor-vascular encasement and overall sarcoma-specific survival, confirmed the results similarly obtained by Crombé et al [
26]. in a smaller sample of patients [
27]. On the contrary, to our knowledge, the association observed in the present series between tumor-vascular proximity and the increased risk of LR has never been reported before in patients affected by STS.
Our results encourage us to consider in the conventional MRI analysis tumor proximity to major vessels. Tumor-vascular closeness must be evaluated, quantified, and considered among the other already known MRI features related to patients’ prognosis, which include peritumoral enhancement, signs of necrosis, signal intensity inhomogeneity, ill-defined borders, and signs of infiltrations [
27,
28,
29,
30].
In the case of patients with contraindications to MRI exams, the same evaluation in regards to tumor-vascular proximity can be reached with contrast-enhanced computed tomography.
5. Conclusions
In conclusion, our experience seems to confirm that the closeness between STS and major blood vessels can predict a poorer local control, but not survival.
We observed an increased risk of local recurrence as the tumor developed adjacent to the major vessels, despite the use of radiation therapy. Even if RTE can independently reduce the risk of LR, in the case of pre-operative MRI findings of the tumor attached to the major vascular bundle, a vascular resection and by-pass reconstruction offered a better local control.
Stratification of risks is mandatory and can contribute to an amelioration of the treatment decisions, thus improving clinical results in patients affected by STS.
Author Contributions
Conceptualization, A.S. and P.S.; data curation, A.M., F.P. and V.D.; formal analysis, M.F.; methodology, L.C.; supervision, M.M., M.D.P. and D.M.D.; writing—original draft, A.S. and E.C.; writing—review and editing, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Istituto Ortopedico Rizzoli (protocol code SAPAMO, date of approval 9 September 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Patient consent was waived in case of death of the patient.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014, 260, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soomers, V.; Husson, O.; Young, R.; Desar, I.; Van der Graaf, W. The sarcoma diagnostic interval: A systematic review on length, contributing factors and patient outcomes. ESMO Open 2020, 5, e000592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, M.; Alldinger, I.; Böckler, D.; Ulrich, A.; Hyhlik-Dürr, A. Vascular reconstruction after retroperitoneal and lower extremity sarcoma resection. Eur. J. Surg. Oncol. 2017, 43, 407–415. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanaka, Y.; Errani, C. Imaging of Soft Tissue Tumors. Curr. Med. Imaging 2021, 17, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Spinnato, P.; Clinca, R. MRI Tail Sign in Soft-Tissue Sarcoma. Radiology 2021, 299, 276. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Caldari, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Pirini, M.G.; Spinnato, P.; Cappelli, A.; Donati, D.M.; De Paolis, M. Margin Assessment in Soft Tissue Sarcomas: Review of the Literature. Cancers 2021, 13, 1687. [Google Scholar] [CrossRef]
- Smolle, M.A.; Sande, M.V.; Callegaro, D.; Wunder, J.; Hayes, A.; Leitner, L.; Bergovec, M.; Tunn, P.U.; van Praag, V.; Fiocco, M.; et al. Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models. Cancers 2019, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Rueten-Budde, A.J.; van Praag, V.M.; van de Sande, M.A.J.; Fiocco, M.; Group, P.S. External validation and adaptation of a dynamic prediction model for patients with high-grade extremity soft tissue sarcoma. J. Surg. Oncol. 2021, 123, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Soomers, V.L.M.N.; Husson, O.; Desar, I.M.E.; van de Sande, M.A.J.; de Haan, J.J.; Verhoef, C.; Vriens, I.J.H.; van Houdt, W.J.; van de Poll-Franse, L.; van der Graaf, W.T.A. Patient and diagnostic intervals of survivors of sarcoma: Results from the SURVSARC study. Cancer 2020, 126, 5283–5292. [Google Scholar] [CrossRef]
- Acem, I.; Verhoef, C.; Rueten-Budde, A.J.; Grünhagen, D.J.; van Houdt, W.J.; van de Sande, M.A.J.; group, P.s. Age-related differences of oncological outcomes in primary extremity soft tissue sarcoma: A multistate model including 6260 patients. Eur. J. Cancer 2020, 141, 128–136. [Google Scholar] [CrossRef]
- Kalisvaart, G.M.; Bloem, J.L.; Bovée, J.V.M.G.; van de Sande, M.A.J.; Gelderblom, H.; van der Hage, J.A.; Hartgrink, H.H.; Krol, A.D.G.; de Geus-Oei, L.F.; Grootjans, W. Personalising sarcoma care using quantitative multimodality imaging for response assessment. Clin. Radiol. 2021, 76, 313.e1–313.e13. [Google Scholar] [CrossRef]
- Fujiwara, T.; Evans, S.; Stevenson, J.; Tsuda, Y.; Gregory, J.; Grimer, R.J.; Abudu, S. Regional variation in the survival of patients with a soft-tissue sarcoma of the extremity and trunk wall under a centralized care system: What has been the impact of national policies in the UK? Bone Jt. J. 2021, 103-B, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, J.J.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.; Pollock, R.; Aston, W.; Dijkstra, P.D.; Ferguson, P.C.; Griffin, A.M.; Wunder, J.S.; et al. Individualised risk assessment for local recurrence and distant metastases in a retrospective transatlantic cohort of 687 patients with high-grade soft tissue sarcomas of the extremities: A multistate model. BMJ Open 2017, 7, e012930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, T.; Medellin, M.R.; Sambri, A.; Tsuda, Y.; Balko, J.; Sumathi, V.; Gregory, J.; Jeys, L.; Abudu, A. Preoperative surgical risk stratification in osteosarcoma based on the proximity to the major vessels. Bone Jt. J. 2019, 101-B, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. The pathologist and the residual tumor (R) classification. Pathol. Res. Pract. 1994, 190, 115–123. [Google Scholar] [CrossRef]
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. ESMO 2014, 25 (Suppl. 3), iii102–iii112. [Google Scholar] [CrossRef]
- Van Trommel, M.F.; Kroon, H.M.; Bloem, J.L.; Hogendoorn, P.C.; Taminiau, A.H. MR imaging based strategies in limb salvage surgery for osteosarcoma of the distal femur. Skeletal. Radiol. 1997, 26, 636–641. [Google Scholar] [CrossRef]
- Harati, K.; Goertz, O.; Pieper, A.; Daigeler, A.; Joneidi-Jafari, H.; Niggemann, H.; Stricker, I.; Lehnhardt, M. Soft Tissue Sarcomas of the Extremities: Surgical Margins Can Be Close as Long as the Resected Tumor Has No Ink on It. Oncologist 2017, 22, 1400–1410. [Google Scholar] [CrossRef] [Green Version]
- Sambri, A.; Bianchi, G.; Righi, A.; Ferrari, C.; Donati, D. Surgical margins do not affect prognosis in high grade myxofibrosarcoma. Eur. J. Surg. Oncol. 2016, 42, 1042–1048. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Miceli, R.; Grosso, F.; Fiore, M.; Puma, E.; Pennacchioli, E.; Barisella, M.; Sangalli, C.; Mariani, L.; Casali, P.G.; et al. Myxofibrosarcoma: Prognostic factors and survival in a series of patients treated at a single institution. Ann. Surg. Oncol. 2011, 18, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Piazza, M.; Pagliarini, E.; Trovarelli, G.; Spertino, A.; Ruggieri, P. The Orthopedic-Vascular Multidisciplinary Approach Improves Patient Safety in Surgery for Musculoskeletal Tumors: A Large-Volume Center Experience. J. Pers. Med. 2021, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Cananzi, F.C.M.; Ruspi, L.; Fiore, M.; Sicoli, F.; Quagliuolo, V.; Gronchi, A. Major vascular resection in retroperitoneal sarcoma surgery. Surgery 2021, 170, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, Y.; Xu, N.; Tu, Y.; Yin, Z.; Zhang, Y.; Liu, Y.; Duan, Z.; Liu, W.; Wang, S. An Update in Imaging Evaluation of Histopathological Grade of Soft Tissue Sarcomas Using Structural and Quantitative Imaging and Radiomics. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef]
- Spinnato, P.; Kind, M.; Le Loarer, F.; Bianchi, G.; Colangeli, M.; Sambri, A.; Ponti, F.; van Langevelde, K.; Crombé, A. Soft Tissue Sarcomas: The Role of Quantitative MRI in Treatment Response Evaluation. Acad. Radiol. 2021. [Google Scholar] [CrossRef]
- Crombe, A.; Marcellin, P.J.; Buy, X.; Stoeckle, E.; Brouste, V.; Italiano, A.; Le Loarer, F.; Kind, M. Soft-Tissue Sarcomas: Assessment of MRI Features Correlating with Histologic Grade and Patient Outcome. Radiology 2019, 291, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Spinnato, P. The Importance of Accurate Tumor Measurements and Staging in Oncologic Imaging: Impact on Patients’ Health. Acad. Radiol. 2021, 28, 767–768. [Google Scholar] [CrossRef]
- Spinnato, P.; Clinca, R.; Vara, G.; Cesari, M.; Ponti, F.; Facchini, G.; Longhi, A.; Donati, D.M.; Bianchi, G.; Sambri, A. MRI Features as Prognostic Factors in Myxofibrosarcoma: Proposal of MRI Grading System. Acad. Radiol. 2020, 28, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Spinnato, P.; Bazzocchi, A.; Tuzzato, G.M.; Donati, D.; Bianchi, G. Does pre-operative MRI predict the risk of local recurrence in primary myxofibrosarcoma of the extremities? Asia Pac. J. Clin. Oncol. 2019, 15, e181–e186. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).