The Leukemic Phase of ALK-Negative Anaplastic Large Cell Lymphoma Is Associated with CD7 Positivity, Complex Karyotype, TP53 Deletion, and a Poor Prognosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and methods
2.1. Case Selection
2.2. Immunophenotypic Analysis
2.3. Conventional Cytogenetic Analysis
2.4. Fluorescence in Situ Hybridization
2.5. Statistical Analysis
3. Results
3.1. Clinical Findings
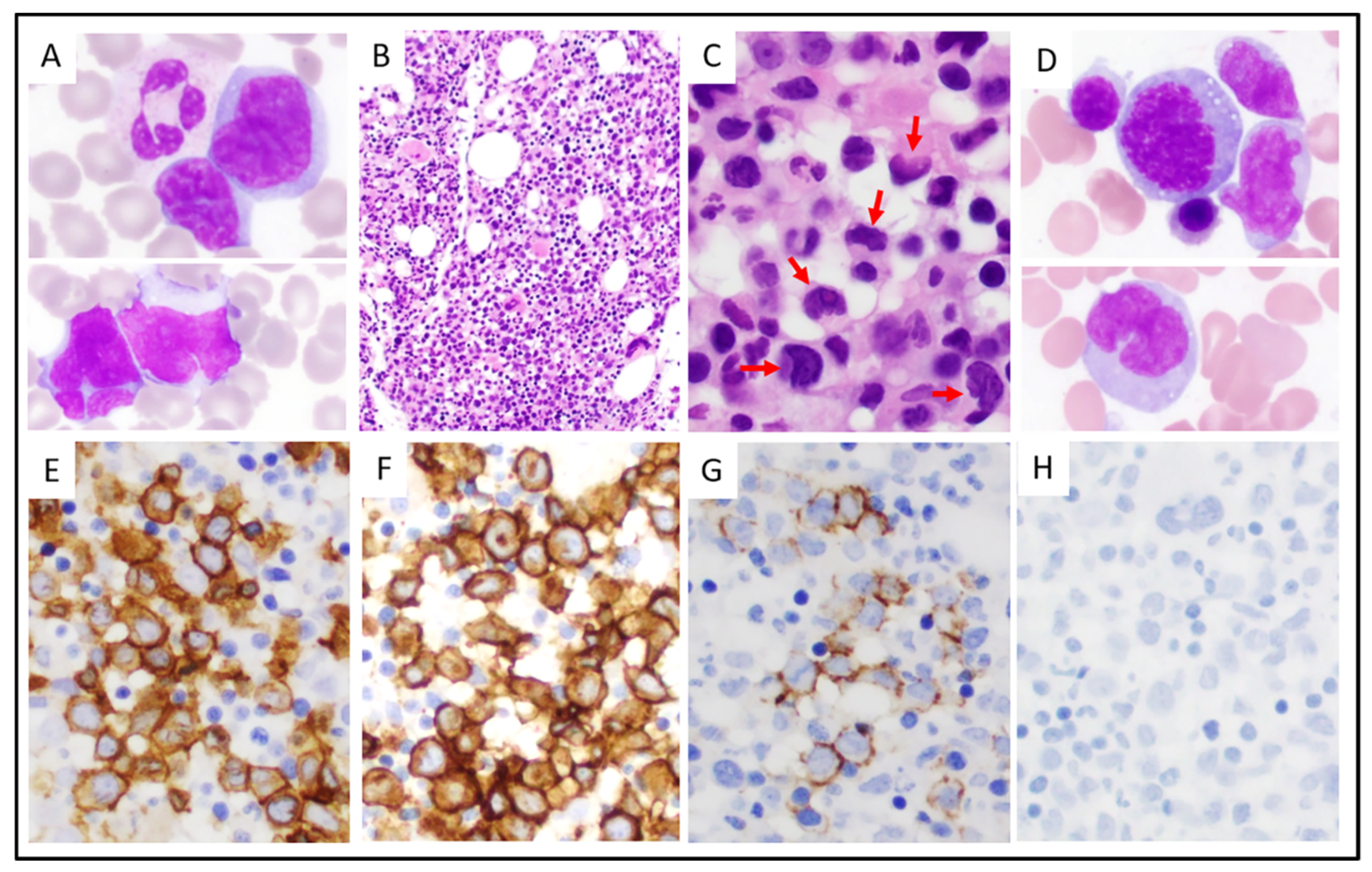
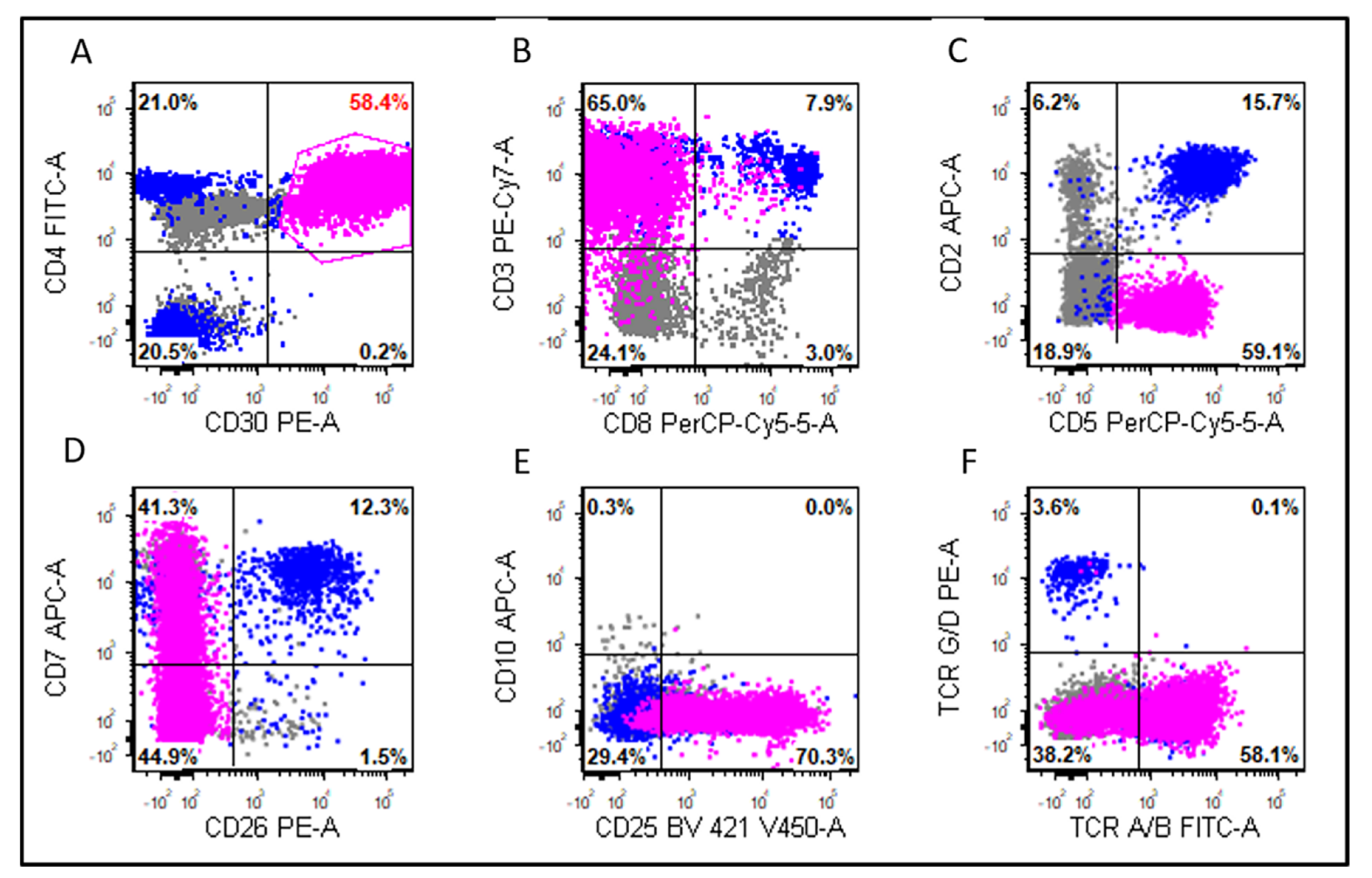
3.2. Morphologic and Immunophenotypic Findings
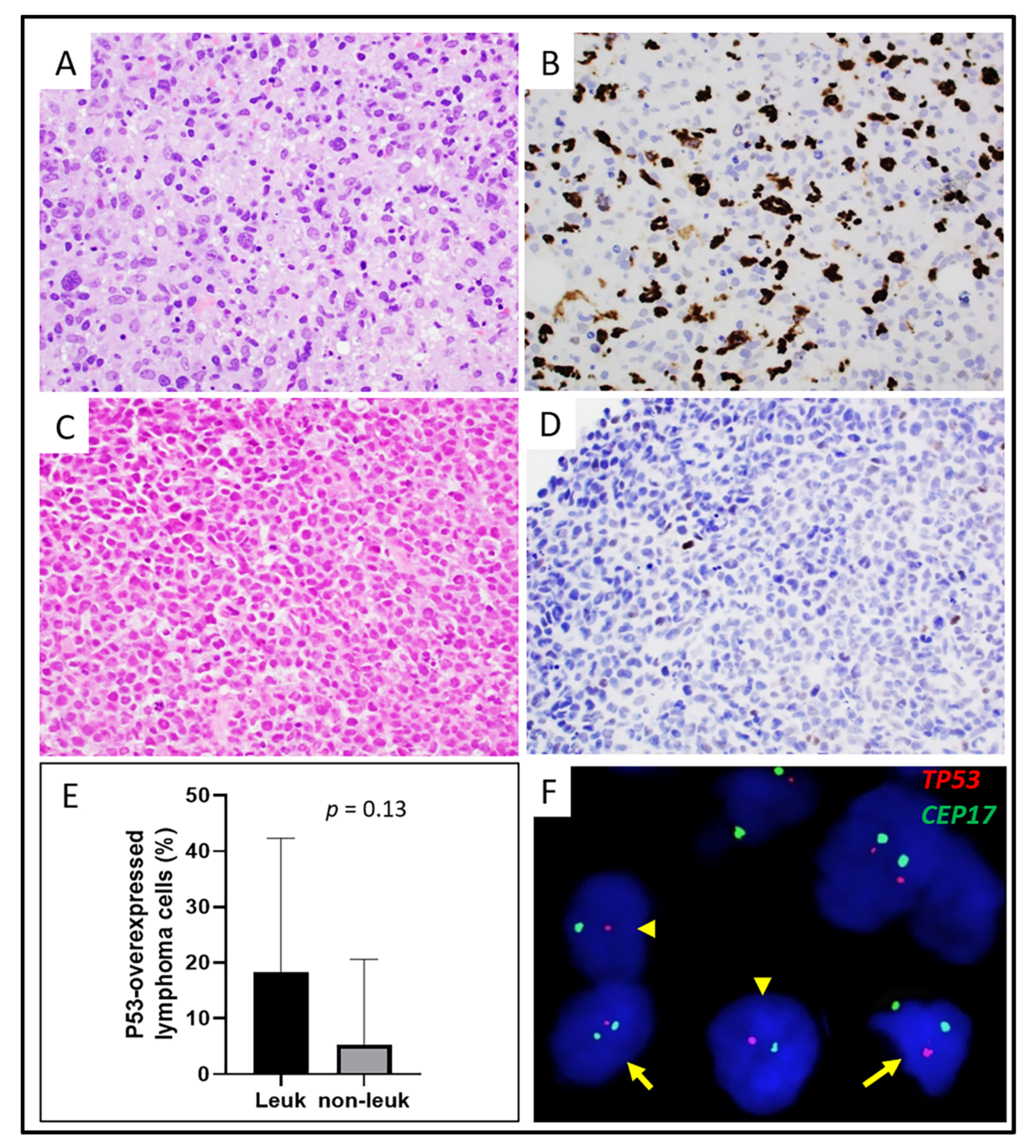
3.3. Cytogenetic and Molecular Findings
3.4. Treatment and Response
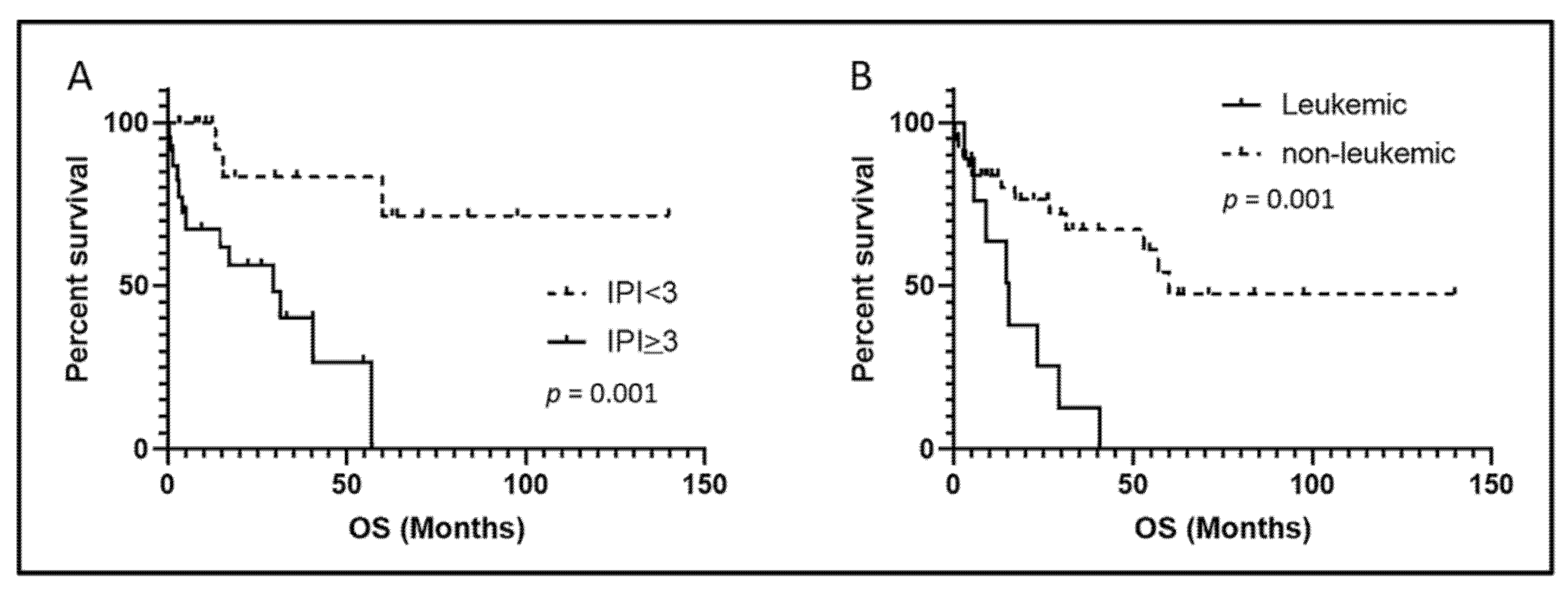
3.5. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, L.J.; Elenitoba-Johnson, K.S. Anaplastic Large Cell Lymphoma. Am. J. Clin. Pathol. 2007, 127, 707–722. [Google Scholar] [CrossRef] [Green Version]
- Vose, J.; Armitage, J.; Weisenburger, D.; International, T.C.L.P. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef]
- Falini, B.; Pileri, S.; Zinzani, P.L.; Carbone, A.; Zagonel, V.; Wolf-Peeters, C.; Verhoef, G.; Menestrina, F.; Todeschini, G.; Paulli, M.; et al. ALK+ lymphoma: Clinico-pathological findings and outcome. Blood 1999, 93, 2697–2706. [Google Scholar] [PubMed]
- Sibon, D.; Fournier, M.; Briere, J.; Lamant, L.; Haioun, C.; Coiffier, B.; Bologna, S.; Morel, P.; Gabarre, J.; Hermine, O.; et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3939–3946. [Google Scholar] [CrossRef]
- Ten Berge, R.L.; de Bruin, P.C.; Oudejans, J.J.; Ossenkoppele, G.J.; van der Valk, P.; Meijer, C.J. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology 2003, 43, 462–469. [Google Scholar] [CrossRef]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellin, F.; Landstrom, J.; Jerkeman, M.; Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014, 124, 1570–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ok, C.Y.; Wang, S.A.; Amin, H.M. Leukemic phase of ALK(+) anaplastic large-cell lymphoma, small-cell variant: Clinicopathologic pitfalls of a rare entity. Clin. Lymphoma Myeloma Leuk. 2014, 14, e123–e126. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Paillard, C.; Ducassou, S.; Perel, Y.; Plantaz, D.; Strullu, M.; Eischen, A.; Lutz, P.; Lamant, L.; Le Deley, M.C.; et al. Paediatric anaplastic large cell lymphoma with leukaemic presentation in children: A report of nine French cases. Br. J. Haematol. 2014, 165, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.T.; Condron, M.R.; Nguyen, N.D.; De, J.; Medeiros, L.J.; Padula, A. Anaplastic large cell lymphoma in leukemic phase: Extraordinarily high white blood cell count. Pathol. Int. 2009, 59, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Tsao, L.; Murty, V.V.; Nandula, S.V.; Donovan, V.; Oesterheld, J.; Bhagat, G.; Alobeid, B. Pediatric ALK+ anaplastic large cell lymphoma with t(3;8)(q26.2;q24) translocation and c-myc rearrangement terminating in a leukemic phase. Am. J. Hematol. 2007, 82, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Bayle, C.; Charpentier, A.; Duchayne, E.; Manel, A.M.; Pages, M.P.; Robert, A.; Lamant, L.; Dastugue, N.; Bertrand, Y.; Dijoud, F.; et al. Leukaemic presentation of small cell variant anaplastic large cell lymphoma: Report of four cases. Br. J. Haematol. 1999, 104, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.S.; Smith, L.B.; Winegarden, J.D., 3rd; Krauss, J.C.; Tworek, J.A.; Schnitzer, B. Highly aggressive ALK-positive anaplastic large cell lymphoma with a leukemic phase and multi-organ involvement: A report of three cases and a review of the literature. Ann. Hematol. 2007, 86, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, G. Leukemic phase of ALK-negative anaplastic large cell lymphoma in a patient who is on androgenic steroids: A case report. Ann. Med. Surg. 2020, 49, 1–4. [Google Scholar] [CrossRef]
- Karki, N.R.; Badin, K.; Savage, N.; Bryan, L. Leukaemic relapse of anaplastic large cell lymphoma, ALK negative. BMJ Case Rep. 2021, 14, e239213. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Hori, Y.; Kosako, H.; Oiwa, T.; Warigaya, K.; Mushino, T.; Murata, S.; Fujimoto, M.; Nishikawa, A.; Murata, S.I.; et al. Brentuximab vedotin for refractory anaplastic lymphoma kinase-negative anaplastic large cell lymphoma in leukemic phase with RUNX3 overexpression. Hematol. Rep. 2020, 12, 8368. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Wang, E.; Chen, W.; Huang, Q. ALK-negative anaplastic large cell lymphoma with extensive peripheral blood and bone marrow involvements manifested as “leukemic phase”. Leuk. Res. 2010, 34, 475–482. [Google Scholar] [CrossRef]
- Huang, Q.; Gaal, K.K.; Nademanee, A. Acute leukemic manifestation of recurrent anaplastic large-cell lymphoma 20 years after autologous bone marrow transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, e34–e36. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.S.; Liu, B.W.; Lam, F.S.; Wong, K.F. ALK-negative anaplastic large cell lymphoma in leukemic phase with near-pentaploidy. Leuk. Lymphoma 2010, 51, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Dalal, B.I.; Chhanabhai, M.; Horsman, D.E.; LeHuquet, J.; Coupland, R. Anaplastic large-cell lymphoma presenting as acute leukemia. Am. J. Hematol. 2005, 79, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, S.; Medeiros, L.J.; Lin, P.; Wang, S.A.; Tang, G.; Yin, C.C.; You, M.J.; Khoury, J.D.; Iyer, S.P.; et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Mod. Pathol. 2020, 33, 324–333. [Google Scholar] [CrossRef]
- Shen, J.; Medeiros, L.J.; Li, S.; Wang, S.A.; Lin, P.; Khanlari, M.; Iyer, S.P.; Yin, C.C.; Tang, G.; Jorgensen, J.L.; et al. CD8 expression in anaplastic large cell lymphoma correlates with noncommon morphologic variants and T-cell antigen expression suggesting biological differences with CD8-negative anaplastic large cell lymphoma. Hum. Pathol. 2020, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.C.; Peng, J.; Li, Y.; Kanagal-Shamanna, R.; Muzzafar, T.; DiNardo, C.; Khoury, J.D.; Li, S.; Medeiros, L.J.; Wang, S.A.; et al. Clinical significance of newly emerged isolated del(20q) in patients following cytotoxic therapies. Mod. Pathol. 2015, 28, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyapichev, K.A.; Tang, G.; Li, S.; You, M.J.; Cheng, T.J.; Miranda, R.N.; Iyer, S.; Yin, C.C.; Konoplev, S.; Bueso-Ramos, C.; et al. MYC expression is associated with older age, common morphology, increased MYC copy number, and poorer prognosis in patients with ALK+ anaplastic large cell lymphoma. Hum. Pathol. 2021, 108, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Gur, H.D.; Loghavi, S.; Konoplev, S.N.; Konopleva, M.; Daver, N.; Tashakori, M.; Kadia, T.; Routbort, M.; Salem, A.; et al. P53 protein overexpression in de novo acute myeloid leukemia patients with normal diploid karyotype correlates with FLT3 internal tandem duplication and worse relapse-free survival. Am. J. Hematol. 2018, 93, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onciu, M.; Behm, F.G.; Raimondi, S.C.; Moore, S.; Harwood, E.L.; Pui, C.H.; Sandlund, J.T. ALK-positive anaplastic large cell lymphoma with leukemic peripheral blood involvement is a clinicopathologic entity with an unfavorable prognosis. Report of three cases and review of the literature. Am. J. Clin. Pathol. 2003, 120, 617–625. [Google Scholar] [CrossRef]
- Khanlari, M.; Li, S.; Miranda, R.N.; Iyer, S.; Konoplev, S.; Lin, P.; Yin, C.C.; Tang, G.; Qiu, L.; Vega, F.; et al. Small cell/lymphohistiocytic morphology is associated with peripheral blood involvement, CD8 positivity and retained T-cell antigens, but not outcome in adults with ALK+ anaplastic large cell lymphoma. Mod. Pathol. 2021, 1–17. [Google Scholar] [CrossRef]
- Muzzafar, T.; Wei, E.X.; Lin, P.; Medeiros, L.J.; Jorgensen, J.L. Flow cytometric immunophenotyping of anaplastic large cell lymphoma. Arch. Pathol. Lab. Med. 2009, 133, 49–56. [Google Scholar] [CrossRef]
- Haynes, B.F.; Martin, M.E.; Kay, H.H.; Kurtzberg, J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J. Exp. Med. 1988, 168, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Png, Y.T.; Vinanica, N.; Kamiya, T.; Shimasaki, N.; Coustan-Smith, E.; Campana, D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 2017, 1, 2348–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozenova, K.A.; Jevremovic, D.; Reichard, K.K.; Nguyen, P.; Otteson, G.E.; Timm, M.M.; Horna, P.; Olteanu, H.; Shi, M. CD2 and CD7 are sensitive flow cytometry screening markers for T-lineage acute leukemia(s): A study of 465 acute leukemia cases. Hum. Pathol. 2021, 114, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tan, Y.; Wang, G.; Deng, B.; Ling, Z.; Song, W.; Seery, S.; Zhang, Y.; Peng, S.; Xu, J.; et al. Donor-Derived CD7 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia: First-in-Human, Phase I Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3340–3351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.J.; Seo, E.J.; Suh, C. A case of ALK negative anaplastic large cell lymphoma with leukaemic manifestation, transformed from CD4 positive T-cell large granular lymphocytic leukaemia. Pathology 2015, 47, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Rassidakis, G.Z.; Thomaides, A.; Wang, S.; Jiang, Y.; Fourtouna, A.; Lai, R.; Medeiros, L.J. p53 gene mutations are uncommon but p53 is commonly expressed in anaplastic large-cell lymphoma. Leukemia 2005, 19, 1663–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobello, C.; Tichy, B.; Bystry, V.; Radova, L.; Filip, D.; Mraz, M.; Montes-Mojarro, I.A.; Prokoph, N.; Larose, H.; Liang, H.C.; et al. STAT3 and TP53 mutations associate with poor prognosis in anaplastic large cell lymphoma. Leukemia 2021, 35, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ding, N.; Mi, L.; Shi, Y.; Liu, W.; Song, Y.; Shu, S.; Zhu, J. Correlation of mutational landscape and survival outcome of peripheral T-cell lymphomas. Exp. Hematol. Oncol. 2021, 10, 9. [Google Scholar] [CrossRef]
- Cui, Y.X.; Kerby, A.; McDuff, F.K.; Ye, H.; Turner, S.D. NPM-ALK inhibits the p53 tumor suppressor pathway in an MDM2 and JNK-dependent manner. Blood 2009, 113, 5217–5227. [Google Scholar] [CrossRef] [Green Version]
- Drakos, E.; Atsaves, V.; Schlette, E.; Li, J.; Papanastasi, I.; Rassidakis, G.Z.; Medeiros, L.J. The therapeutic potential of p53 reactivation by nutlin-3a in ALK+ anaplastic large cell lymphoma with wild-type or mutated p53. Leukemia 2009, 23, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Uchihara, Y.; Tago, K.; Tamura, H.; Funakoshi-Tago, M. EBP2, a novel NPM-ALK-interacting protein in the nucleolus, contributes to the proliferation of ALCL cells by regulating tumor suppressor p53. Mol. Oncol. 2021, 15, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Boi, M.; Rinaldi, A.; Kwee, I.; Bonetti, P.; Todaro, M.; Tabbo, F.; Piva, R.; Rancoita, P.M.; Matolcsy, A.; Timar, B.; et al. PRDM1/BLIMP1 is commonly inactivated in anaplastic large T-cell lymphoma. Blood 2013, 122, 2683–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shustov, A.; Cabrera, M.E.; Civallero, M.; Bellei, M.; Ko, Y.H.; Manni, M.; Skrypets, T.; Horwitz, S.M.; De Souza, C.A.; Radford, J.A.; et al. ALK-negative anaplastic large cell lymphoma: Features and outcomes of 235 patients from the International T-Cell Project. Blood Adv. 2021, 5, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.B.; Hamilton-Dutoit, S.J.; Bendix, K.; Ketterling, R.P.; Bedroske, P.P.; Luoma, I.M.; Sattler, C.A.; Boddicker, R.L.; Bennani, N.N.; Norgaard, P.; et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: A Danish cohort study. Blood 2017, 130, 554–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Case ID | Age | Gender | WBC (×109/L) a | Hb (g/dL) a | Platelet (×109/L) a | LDH (U/L) a | LN a | BM a | Leukemic Onset (Months) b | Circulating Lymphoma Cell (%) | Overall Survival (Months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | F | n/a | n/a | n/a | n/a | N | n/a | +4 | 7 | 5.1 | Alive |
| 2 | 55 | M | 3.4 | 10.7 | 155 | 627 | P | P | 0 | 6 | 3.1 | Dead |
| 3 | 67 | M | 6.4 | 13.4 | 312 | n/a | N | N | +19.4 | n/a | 23.4 | Dead |
| 4 | 74 | M | 106.5 | 11.5 | 34 | 7262 | P | P | 0 | 75 | 14.6 | Dead |
| 5 | 62 | M | n/a | n/a | n/a | n/a | P | N | +5.8 | 2 | 29.5 | Dead |
| 6 | 58 | F | n/a | n/a | n/a | n/a | P | N | 0 | n/a | 9.1 | Dead |
| 7 | 21 | M | n/a | n/a | n/a | n/a | N | N | +15.2 | 4 | 15.5 | Dead |
| 8 | 39 | M | 9.31 | 10.4 | 73 | n/a | N | P | 0 | n/a | 5.6 | Dead |
| 9 | 70 | M | 15.6 | 15.4 | 85 | 5057 | P | P | 0 | 37 | 40.6 | Dead |
| Clinical Features | Leukemic (n = 9) | Non-Leukemic (n = 39) | p Value |
|---|---|---|---|
| Male: Female | 3.5:1 (7/2) | 3.3:1 (30/9) | 1 |
| Median age, years (range) | 61 (21–74) | 60 (36–79) | 0.8 |
| B symptoms | 83% (5/6) | 62% (23/37) | 0.4 |
| Nodal presentation | 56% (5/9) | 64% (25/39) | 0.71 |
| Extra-nodal involvement | 44% (4/9) | 69% (27/39) | 0.25 |
| Bone marrow involvement | 50% (4/8) | 14% (5/37) | 0.04 |
| Stage III or IV | 100% (8/8) | 72% (28/39) | 0.17 |
| Leukocytosis | 40% (2/5) | 36% (13/36) | 1 |
| Absolute lymphocytosis | 50% (2/4) | 0% (0/35) | 0.008 |
| Anemia | 80% (4/5) | 72% (26/36) | 1 |
| Thrombocytopenia | 60% (3/5) | 11% (4/36) | 0.03 |
| Elevated LDH | 100% (3/3) | 52% (15/29) | 0.24 |
| High ECOG-PS (≥2) | 0% (0/5) | 6% (2/34) | 1.0 |
| High IPI (≥3) | 83% (5/6) | 51% (18/35) | 0.21 |
| Initial treatment | 0.13 | ||
| CHOP or modified CHOP | 78% (7/9) | 57% (21/37) | 0.45 |
| Others | 22% (2/9) | 43% (16/37) | |
| Initial CR | 33% (3/9) | 64% (23/36) | 0.14 |
| SCT+ | 38% (3/8) | 28% (9/32) | 0.68 |
| Pathologic Features | Leukemic (n = 9) | Non-Leukemic (n = 39) | p Value |
|---|---|---|---|
| Immunophenotype | |||
| CD2+ | 75% (6/8) | 74% (20/27) | 1 |
| CD3+ | 78% (7/9) | 54% (20/37) | 0.27 |
| CD4+ | 75% (6/8) | 75% (24/32) | 1 |
| CD5+ | 13% (1/8) | 47% (14/30) | 0.11 |
| CD7+ | 71% (5/7) | 19% (5/26) | 0.02 |
| CD8+ | 25% (2/8) | 14% (4/28) | 0.6 |
| CD25+ | 50% (3/6) | 75% (3/4) | 1 |
| CD43+ | 100% (4/4) | 79% (11/14) | 1 |
| CD45+ | 100% (2/2) | 77% (20/26) | 1 |
| CD56+ | 0% (0/6) | 6% (1/16) | 1 |
| TCR A/B+ | 83% (5/6) | 60% (3/5) | 0.55 |
| TCR G/D+ | 0% (0/4) | 0% (0/4) | 1 |
| Granzyme B+ | 33% (1/3) | 60% (9/15) | 0.56 |
| TIA1+ | 50% (2/4) | 55% (6/11) | 1 |
| EMA+ | 60% (3/5) | 26% (6/23) | 0.29 |
| P53 overexpression | 50% (3/6) | 17% (3/18) | 0.14 |
| Karyotype | |||
| Complex+ | 80% (4/5) | 14% (1/7) | 0.03 |
| FISH | |||
| DUSP22-R+ | 20% (1/5) | 35% (7/20) | 1.0 |
| Case ID | Karyotype | DUSP22-R (FISH) | TP53 | ||
|---|---|---|---|---|---|
| Deletion (FISH) | Mutation (NGS) | Over-Expression a (IHC) | |||
| 1 | 46,XX[20] b | n/a | P | n/a | P |
| 2 | n/a | N | P | n/a | N |
| 3 | 49~53, XY,−2, del(2)(p13), +3, add(3)(p26), del(3)(p23p24), add(4)(p16), +5, +5, −15, add(17)(q25), −18, −21, +4~8mar[cp20]/ | N | P | N | P |
| 4 | 46, XY, der(3)add(3)(p22)t(3;11)(q29;q13), t(6;8)(p23;q13), del(13)(q12), add(19)(p13.3)[8]/46, XY[12] | P | P | n/a | N |
| 5 | Insufficient for analysis | n/a | n/a | n/a | n/a |
| 6 | 46, XX[45]/47.XX, −3, add(11)(q23-24), +mar1-2[5] | n/a | n/a | n/a | n/a |
| 7 | n/a | N | P | n/a | N |
| 8 | n/a | n/a | n/a | n/a | n/a |
| 9 | 65~68, add(X)(p11.2),−Y, add(1)(p13), del(1)(p13)×2, del(1)(q11.2)×2, der(2;5)(p10;q10), der(3)t(1;3)(p22;q29)×2, −5, del(5)(q13q33), del(6)(q21q25)×2, +7, add(7)(p13)×2, −8, −9, −10, −11, −12, −13, −15, add(16)(q24))×2, der(16)(add(16)(p13.1)add(16)(q12-13), der(17)del(17)(p11.2)del(17)(q11.2), del(17)(p13), i(17)(q10), −19, −20, add(21)(p11.2), +22, +8~9mar[cp12]/46, XY[8] | N | P | P c | P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Medeiros, L.J.; Tang, G.; Khanlari, M.; Li, S.; Konoplev, S.; Wang, S.A.; Yin, C.C.; Khoury, J.D.; Wang, W.; et al. The Leukemic Phase of ALK-Negative Anaplastic Large Cell Lymphoma Is Associated with CD7 Positivity, Complex Karyotype, TP53 Deletion, and a Poor Prognosis. Cancers 2021, 13, 6316. https://doi.org/10.3390/cancers13246316
Qiu L, Medeiros LJ, Tang G, Khanlari M, Li S, Konoplev S, Wang SA, Yin CC, Khoury JD, Wang W, et al. The Leukemic Phase of ALK-Negative Anaplastic Large Cell Lymphoma Is Associated with CD7 Positivity, Complex Karyotype, TP53 Deletion, and a Poor Prognosis. Cancers. 2021; 13(24):6316. https://doi.org/10.3390/cancers13246316
Chicago/Turabian StyleQiu, Lianqun, L. Jeffrey Medeiros, Guilin Tang, Mahsa Khanlari, Shaoying Li, Sergej Konoplev, Sa A. Wang, C. Cameron Yin, Joseph D. Khoury, Wei Wang, and et al. 2021. "The Leukemic Phase of ALK-Negative Anaplastic Large Cell Lymphoma Is Associated with CD7 Positivity, Complex Karyotype, TP53 Deletion, and a Poor Prognosis" Cancers 13, no. 24: 6316. https://doi.org/10.3390/cancers13246316
APA StyleQiu, L., Medeiros, L. J., Tang, G., Khanlari, M., Li, S., Konoplev, S., Wang, S. A., Yin, C. C., Khoury, J. D., Wang, W., Miranda, R. N., Iyer, S., You, M. J., & Xu, J. (2021). The Leukemic Phase of ALK-Negative Anaplastic Large Cell Lymphoma Is Associated with CD7 Positivity, Complex Karyotype, TP53 Deletion, and a Poor Prognosis. Cancers, 13(24), 6316. https://doi.org/10.3390/cancers13246316








