Developing a Nationwide Infrastructure for Therapeutic Drug Monitoring of Targeted Oral Anticancer Drugs: The ON-TARGET Study Protocol
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Primary Objective
2.3. Secondary Objectives
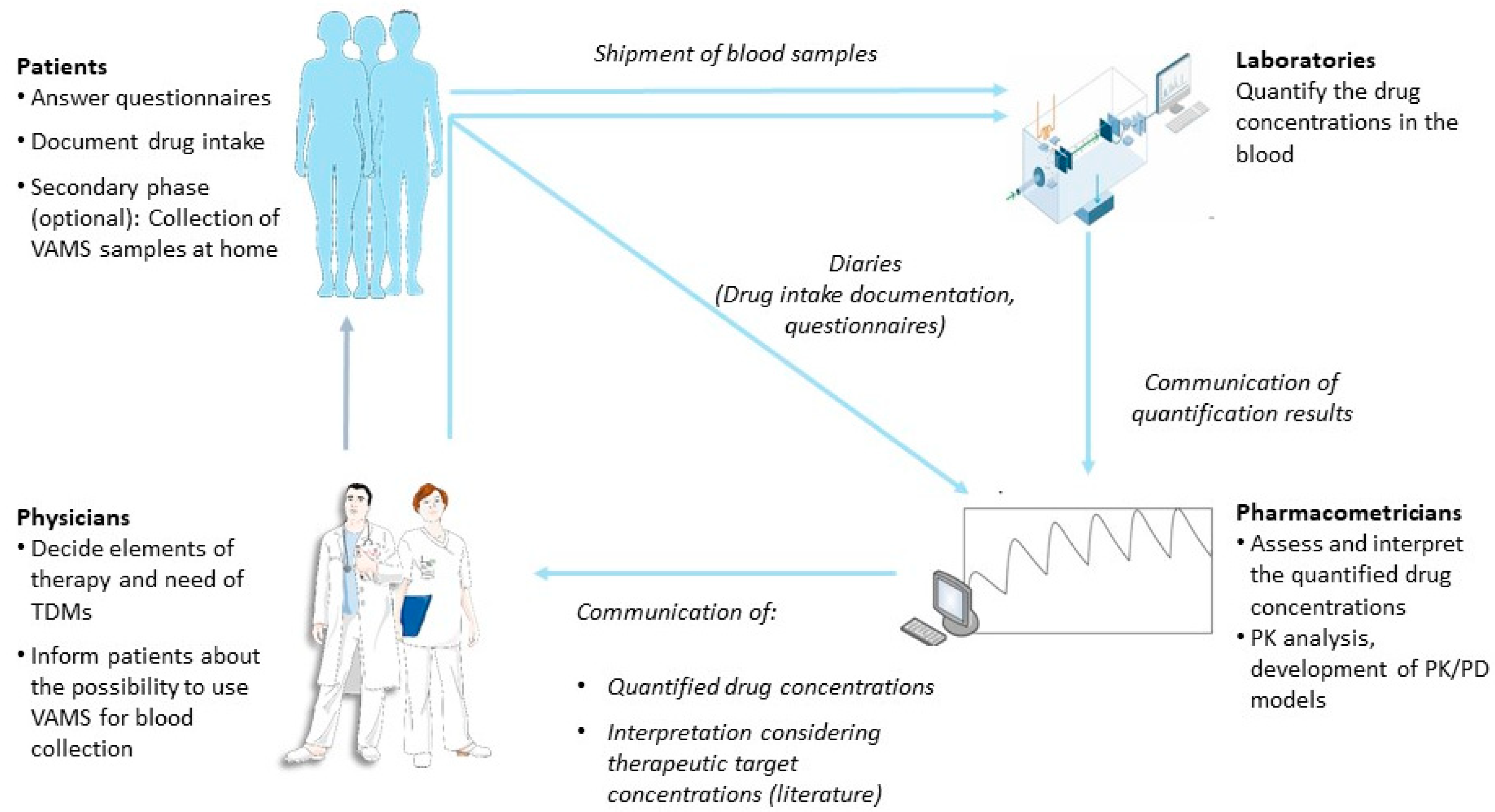
2.4. Study Design
2.4.1. Overview
2.4.2. First Study Phase
2.4.3. Second Study Phase
2.4.4. Toxicity Assessment
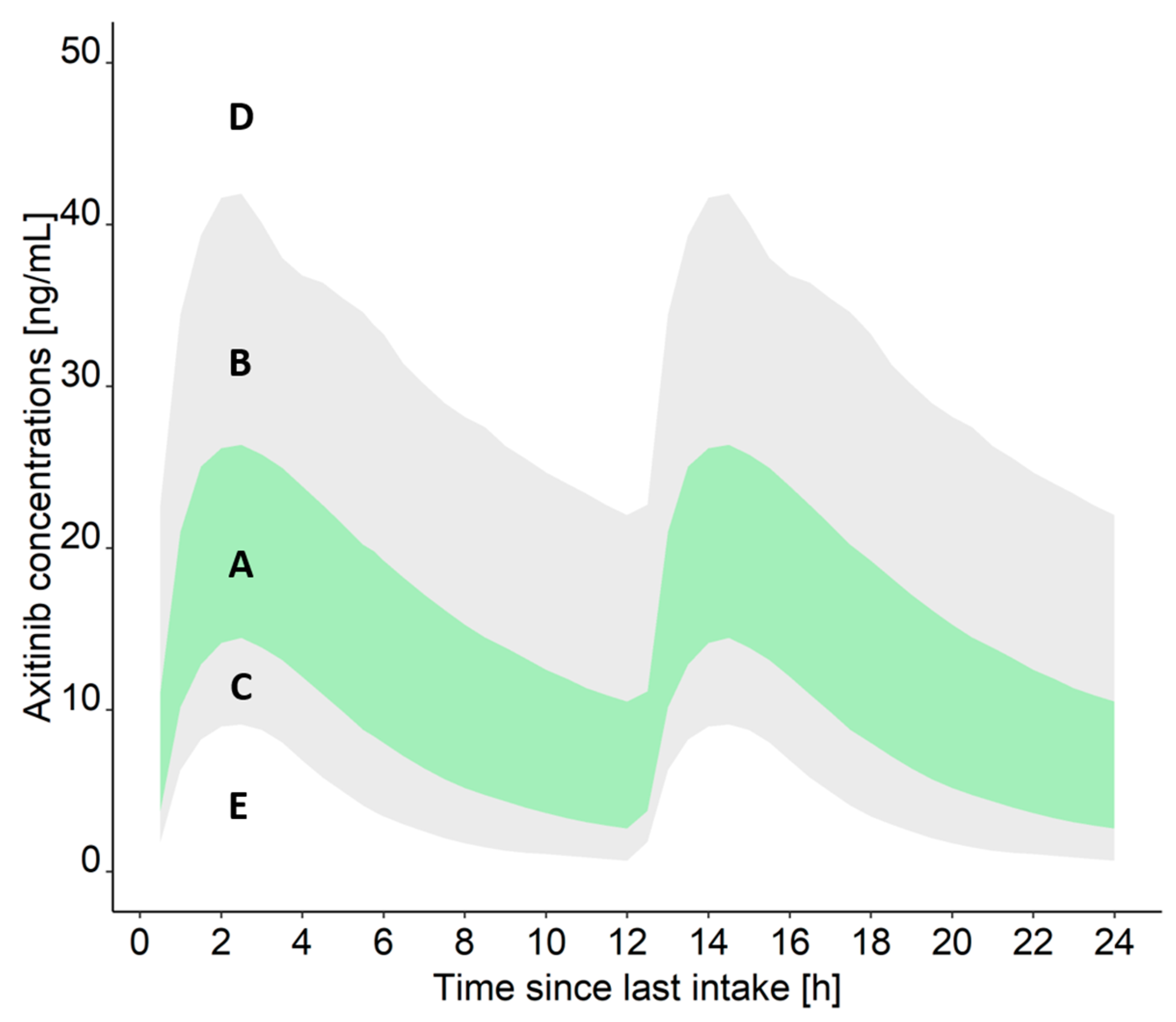
2.4.5. TDM Result Report
- Measured drug concentration is within the IQR (A): The measured concentration is in the xth (25th–75th) percentile of the concentrations that can be expected for axitinib/cabozantinib, considering the dosing regimen and relevant covariates of the patient. Thus, the risk of adverse drug reactions is low at the current dosing regimen.
- Measured drug concentration is within the 90% PI but higher than the IQR (B): The measured concentration is in the xth (76th–95th) percentile of the concentrations that can be expected for axitinib/cabozantinib, considering the dosing regimen and relevant covariates of the patient. Due to the elevated concentrations, the patient should be carefully monitored to detect severe adverse drug reactions early on.
- Measured drug concentration is within the 90% PI but lower than the IQR (C): The measured concentration is in the xth (5th–24th) percentile of the concentrations that can be expected for axitinib/cabozantinib, considering the dosing regimen and relevant covariates of the patient. Thus, the risk of adverse drug reactions is low at the current dosing regimen.
- Measured drug concentration is higher than the 90% PI (D): The measured concentration is in the xth (>95th) percentile of the concentrations that can be expected for axitinib/cabozantinib, considering the dosing regimen and relevant covariates of the patient. Thus, a risk of adverse drug reactions is increased at the current dosing regimen and the patient should be carefully monitored.
- Measured drug concentration is lower than the 90% PI (E): The measured concentration is in the xth (<5th) percentile of the concentrations that can be expected for axitinib/cabozantinib, considering the dosing regimen and relevant covariates of the patient. Thus, according to the result, the patient would not be adequately treated with axitinib/cabozantinib and further investigation for the cause is recommended. Possible causes include a delayed absorption of the drug, non-adherence, drug–drug interactions, or inaccurate documentation of the sample collection time.
2.5. Statistics
2.6. Ethics Considerations, Administration, Data Collection, and Data Protection
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klümpen, H.J.; Samer, C.F.; Mathijssen, R.H.J.; Schellens, J.H.M.; Gurney, H. Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat. Rev. 2011, 37, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheijen, R.B.; Yu, H.; Schellens, J.H.M.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin. Pharmacol. Ther. 2017, 102, 765–776. [Google Scholar] [CrossRef]
- Mueller-Schoell, A.; Groenland, S.L.; Scherf-Clavel, O.; van Dyk, M.; Huisinga, W.; Michelet, R.; Jaehde, U.; Steeghs, N.; Huitema, A.D.R.; Kloft, C. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur. J. Clin. Pharmacol. 2021, 77, 441–464. [Google Scholar] [CrossRef]
- Groenland, S.L.; Mathijssen, R.H.J.; Beijnen, J.H.; Huitema, A.D.R.; Steeghs, N. Individualized dosing of oral targeted therapies in oncology is crucial in the era of precision medicine. Eur. J. Clin. Pharmacol. 2019, 75, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; Van Eerden, R.A.G.; Verheijen, R.B.; Koolen, S.L.W.; Moes, D.J.A.R.; Desar, I.M.E.; Reyners, A.K.L.; Gelderblom, H.J.; Van Erp, N.P.; Mathijssen, R.H.J.; et al. Therapeutic drug monitoring of oral anticancer drugs: The dutch pharmacology oncology group-therapeutic drug monitoring protocol for a prospective study. Ther. Drug Monit. 2019, 41, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, L.A.; Widmer, N.; Zaman, K.; Cardoso, E.; Buclin, T.; Csajka, C. Therapeutic drug monitoring of targeted anticancer therapy. Biomark. Med. 2015, 9, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Shipkova, M.; Hesselink, D.A.; Holt, D.W.; Billaud, E.M.; Van Gelder, T.; Kunicki, P.K.; Brunet, M.; Budde, K.; Barten, M.J.; De Simone, P.; et al. Therapeutic drug monitoring of everolimus: A consensus report. Ther. Drug Monit. 2016, 38, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Lankheet, N.A.G.; Kloth, J.S.L.; Gadellaa-Van Hooijdonk, C.G.M.; Cirkel, G.A.; Mathijssen, R.H.J.; Lolkema, M.P.J.K.; Schellens, J.H.M.; Voest, E.E.; Sleijfer, S.; De Jonge, M.J.A.; et al. Pharmacokinetically guided sunitinib dosing: A feasibility study in patients with advanced solid tumours. Br. J. Cancer 2014, 110, 2441–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotta, V.; Widmer, N.; Decosterd, L.A.; Chalandon, Y.; Heim, D.; Gregor, M.; Benz, R.; Leoncini-Franscini, L.; Baerlocher, G.M.; Duchosal, M.A.; et al. Clinical usefulness of therapeutic concentration monitoring for imatinib dosage individualization: Results from a randomized controlled trial. Cancer Chemother. Pharmacol. 2014, 74, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Lankheet, N.A.G.; Desar, I.M.E.; Mulder, S.F.; Burger, D.M.; Kweekel, D.M.; van Herpen, C.M.L.; van der Graaf, W.T.A.; van Erp, N.P. Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br. J. Clin. Pharmacol. 2017, 83, 2195–2204. [Google Scholar] [CrossRef]
- Heath, E.I.; Chiorean, E.G.; Sweeney, C.J.; Hodge, J.P.; Lager, J.J.; Forman, K.; Malburg, L.; Arumugham, T.; Dar, M.M.; Suttle, A.B.; et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin. Pharmacol. Ther. 2010, 88, 818–823. [Google Scholar] [CrossRef]
- Lubberman, F.J.E.; Gelderblom, H.; Hamberg, P.; Vervenne, W.L.; Mulder, S.F.; Jansman, F.G.A.; Colbers, A.; van der Graaf, W.T.A.; Burger, D.M.; Luelmo, S.; et al. The Effect of Using Pazopanib With Food vs. Fasted on Pharmacokinetics, Patient Safety, and Preference (DIET Study). Clin. Pharmacol. Ther. 2019, 106, 1076–1082. [Google Scholar] [CrossRef]
- Groenland, S.L.; van Eerden, R.A.G.; Verheijen, R.B.; de Vries, N.; Thijssen, B.; Rosing, H.; Beijnen, J.H.; Koolen, S.L.W.; Mathijssen, R.H.J.; Huitema, A.D.R.; et al. Cost-neutral optimization of pazopanib exposure by splitting intake moments: A prospective pharmacokinetic study in cancer patients. Clin. Pharmacokinet. 2020, 59, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.B.; Bins, S.; Mathijssen, R.H.J.; Lolkema, M.P.; van Doorn, L.; Schellens, J.H.M.; Beijnen, J.H.; Langenberg, M.H.G.; Huitema, A.D.R.; Steeghs, N. Individualized pazopanib dosing: A prospective feasibility study in cancer patients. Clin. Cancer Res. 2016, 22, 5738–5746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rini, B.I.; Melichar, B.; Fishman, M.N.; Oya, M.; Pithavala, Y.K.; Chen, Y.; Bair, A.H.; Grünwald, V. Axitinib dose titration: Analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann. Oncol. 2015, 26, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Balleine, R.L.; Lee, C.; Gao, B.; Balakrishnar, B.; Menzies, A.M.; Yeap, S.H.; Ali, S.S.; Gebski, V.; Provan, P.; et al. Dose escalation of tamoxifen in patients with low endoxifen level: Evidence for therapeutic drug monitoring—The TADE study. Clin. Cancer Res. 2016, 22, 3164–3171. [Google Scholar] [CrossRef] [Green Version]
- Kok, M.G.M.; Fillet, M. Volumetric absorptive microsampling: Current advances and applications. J. Pharm. Biomed. Anal. 2018, 147, 288–296. [Google Scholar] [CrossRef]
- Harahap, Y.; Diptasaadya, R.; Purwanto, D.J. Volumetric absorptive microsampling as a sampling alternative in clinical trials and therapeutic drug monitoring during the COVID-19 pandemic: A review. Drug Des. Devel. Ther. 2020, 14, 5757–5771. [Google Scholar] [CrossRef] [PubMed]
- Kip, A.E.; Kiers, K.C.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H.; Dorlo, T.P.C. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J. Pharm. Biomed. Anal. 2017, 135, 160–166. [Google Scholar] [CrossRef]
- Velghe, S.; Stove, C.P. Volumetric absorptive microsampling as an alternative tool for therapeutic drug monitoring of first-generation anti-epileptic drugs. Anal. Bioanal. Chem. 2018, 410, 2331–2341. [Google Scholar] [CrossRef] [Green Version]
- Gustavsen, M.T.; Midtvedt, K.; Vethe, N.T.; Robertsen, I.; Bergan, S.; Åsberg, A. Tacrolimus Area Under the Concentration Versus Time Curve Monitoring, Using Home-Based Volumetric Absorptive Capillary Microsampling. Ther. Drug Monit. 2020, 42, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Berends, S.E.; D’Haens, G.R.A.M.; Schaap, T.; de Vries, A.; Rispens, T.; Bloem, K.; Mathôt, R.A.A. Dried blood samples can support monitoring of infliximab concentrations in patients with inflammatory bowel disease: A clinical validation. Br. J. Clin. Pharmacol. 2019, 85, 1544–1551. [Google Scholar] [CrossRef]
- Stern, M.; Giebels, M.; Fey, T.; Lübking, M.; Alferink, J.; Hempel, G. Validation and clinical application of a volumetric absorptive microsampling method for 14 psychiatric drugs. Bioanalysis 2020, 12, 1129–1147. [Google Scholar] [CrossRef]
- National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov (accessed on 31 May 2021).
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Jia, X.; Heller, G.; Barz, A.; Sit, L.; Fruscione, M.; Appawu, M.; Iasonos, A.; Atkinson, T.; Goldfarb, S.; et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J. Natl. Cancer Inst. 2009, 101, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Galizia, D.; Milani, A.; Geuna, E.; Martinello, R.; Cagnazzo, C.; Foresto, M.; Longo, V.; Berchialla, P.; Solinas, G.; Calori, A.; et al. Self-evaluation of duration of adjuvant chemotherapy side effects in breast cancer patients: A prospective study. Cancer Med. 2018, 7, 4339–4344. [Google Scholar] [CrossRef] [PubMed]
- Veitch, Z.W.; Shepshelovich, D.; Gallagher, C.; Wang, L.; Abdul Razak, A.R.; Spreafico, A.; Bedard, P.L.; Siu, L.L.; Minasian, L.; Hansen, A.R. Underreporting of Symptomatic Adverse Events in Phase I Clinical Trials. JNCI J. Natl. Cancer Inst. 2021, 113, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Zentrum für Krebsregisterdaten im Robert Koch-Institut: Datenbankabfrage mit Schätzung der Inzidenz, Prävalenz und des Überlebens ovn Krebs in Deutschland auf Basis der Epidemiologischen Landeskrebsregisterdaten. Available online: www.krebsdaten.de/abfrage (accessed on 7 December 2021).
- Food and Drug Administration. Center for Drug Evaluation and Research. Axitinib Clinical Pharmacology and Biopharmaceutics Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202324Orig1s000ClinPharmR.pdf (accessed on 1 January 2012).
- Lacy, S.; Nielsen, J.; Yang, B.; Miles, D.; Nguyen, L.; Hutmacher, M. Population exposure–response analysis of cabozantinib efficacy and safety endpoints in patients with renal cell carcinoma. Cancer Chemother. Pharmacol. 2018, 81, 1061–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation; EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**; European Medicines Agency: Amsterdam, The Netherlands, 2012; Volume 44, pp. 1–23.
- Kanefendt, F.; Lindauer, A.; Mross, K.; Fuhr, U.; Jaehde, U. Determination of soluble vascular endothelial growth factor receptor 3 (sVEGFR-3) in plasma as pharmacodynamic biomarker. J. Pharm. Biomed. Anal. 2012, 70, 485–491. [Google Scholar] [CrossRef]
- Diekstra, M.H.; Fritsch, A.; Kanefendt, F.; Swen, J.J.; Djar, M.; Sörgel, F.; Kinzig, M.; Stelzer, C.; Schindele, D.; Gauler, T.; et al. Population modeling integrating pharmacokinetics, pharmacodynamics, pharmacogenetics, and clinical outcome in patients with sunitinib-treated cancer. CPT Pharmacometrics Syst. Pharmacol. 2017, 6, 604–613. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Health PRO-CTCAE Item Library: Version 1.0. 24 March 2016. Available online: https://healthcaredelivery.cancer.gov/pro-ctcae/pro-ctcae_german.pdf (accessed on 27 September 2019).
- Kirsch, M.; Mitchell, S.A.; Dobbels, F.; Stussi, G.; Basch, E.; Halter, J.P.; De Geest, S. Linguistic and content validation of a German-language PRO-CTCAE-based patient-reported outcomes instrument to evaluate the late effect symptom experience after allogeneic hematopoietic stem cell transplantation. Eur. J. Oncol. Nurs. 2015, 19, 66–74. [Google Scholar] [CrossRef]
- Electronic Medicines Compendium. Inlyta 1 mg Film-Coated Tablets: Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/4325/smpc (accessed on 1 June 2021).
- Electronic Medicines Compendium. Cabometyx 20 mg Film-Coated Tablets: Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/4331/smpc (accessed on 1 June 2021).
- Rini, B.I.; Garrett, M.; Poland, B.; Dutcher, J.P.; Rixe, O.; Wilding, G.; Stadler, W.M.; Pithavala, Y.K.; Kim, S.; Tarazi, J.; et al. Axitinib in metastatic renal cell carcinoma: Results of a pharmacokinetic and pharmacodynamic analysis. J. Clin. Pharmacol. 2013, 53, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.; Chapel, S.; Tran, B.D.; Lacy, S. Updated population pharmacokinetic model of cabozantinib integrating various cancer types including hepatocellular carcinoma. J. Clin. Pharmacol. 2019, 59, 1551–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ferrer, M.; Wojnicz, A.; Mejía, G.; Koller, D.; Zubiaur, P.; Abad-Santos, F. Utility of Therapeutic Drug Monitoring of Imatinib, Nilotinib, and Dasatinib in Chronic Myeloid Leukemia: A Systematic Review and Meta-analysis. Clin. Ther. 2019, 41, 2558–2570.e7. [Google Scholar] [CrossRef]
- Miura, M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol. Pharm. Bull. 2015, 38, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Vithanachchi, D.T.; Maujean, A.; Downes, M.J.; Scuffham, P. A comprehensive review of economic evaluations of therapeutic drug monitoring interventions for cancer treatments. Br. J. Clin. Pharmacol. 2021, 87, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; McMillin, G.A.; Bernard, P.S.; Tantravahi, S.; Walker, B.S.; Schmidt, R.L. Cost effectiveness of therapeutic drug monitoring for imatinib administration in chronic myeloid leukemia. PLoS ONE 2019, 14, e0226552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Velde, D.; van der Graaf, J.L.; Boussaidi, M.; Huisman, R.; Hesselink, D.A.; Russcher, H.; Kooij-Egas, A.C.; van Maarseveen, E.; de Winter, B.C.M. Development and Validation of Hematocrit Level Measurement in Dried Blood Spots Using Near-Infrared Spectroscopy. Ther. Drug Monit. 2021, 43, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Friedl, B.; Kurlbaum, M.; Kroiss, M.; Fassnacht, M.; Scherf-Clavel, O. A method for the minimally invasive drug monitoring of mitotane by means of volumetric absorptive microsampling for a home-based therapeutic drug monitoring. Anal. Bioanal. Chem. 2019, 411, 3951–3962. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurain, A.A.; Williams, D.B.; Mackenzie, L.; Roberts, M.S.; Wiese, M.D. Simultaneous LC-MS/MS quantification of oxycodone, tramadol and fentanyl and their metabolites (noroxycodone, oxymorphone, O-desmethyltramadol, N-desmethyltramadol, and norfentanyl) in human plasma and whole blood collected via venepuncture and volumetr. J. Pharm. Biomed. Anal. 2021, 203, 114171. [Google Scholar] [CrossRef] [PubMed]
- Canisius, T.P.I.J.M.; Soons, J.W.P.H.; Verschuure, P.; Wammes-Van Der Heijden, E.A.; Rouhl, R.P.W.; Majoie, H.J.M. Therapeutic drug monitoring of anti-epileptic drugs—A clinical verification of volumetric absorptive micro sampling. Clin. Chem. Lab. Med. 2020, 58, 828–835. [Google Scholar] [CrossRef]
- D’Urso, A.; Rudge, J.; Patsalos, P.N.; De Grazia, U. Volumetric Absorptive Microsampling: A New Sampling Tool for Therapeutic Drug Monitoring of Antiepileptic Drugs. Ther. Drug Monit. 2019, 41, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.W.; Cowan, D.A.; Walker, C.J.; Wojek, N.; Brailsford, A.D. Determination of anabolic steroids in dried blood using microsampling and gas chromatography-tandem mass spectrometry: Application to a testosterone gel administration study. J. Chromatogr. A 2020, 1628, 461445. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bigwarfe, T.; Myzithras, M.; Waltz, E.; Ahlberg, J. Application of Mitra® microsampling for pharmacokinetic bioanalysis of monoclonal antibodies in rats. Bioanalysis 2019, 11, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Myzithras, M.; Bolella, E.; Leonard, A.; Ahlberg, J. Whole blood stability evaluation of monoclonal antibody therapeutics using volumetric absorptive microsampling. Bioanalysis 2021, 13, 621–629. [Google Scholar] [CrossRef]
- Verougstraete, N.; Stove, C.P. Volumetric absorptive microsampling as a suitable tool to monitor tyrosine kinase inhibitors. J. Pharm. Biomed. Anal. 2022, 207, 114418. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Yoshida, Y.; Yoshida, T.; Kondo, T.; Inotsume, N.; Toda, T. Simultaneous Quantification of BCR-ABL and Bruton Tyrosine Kinase Inhibitors in Dried Plasma Spots and Its Application to Clinical Sample Analysis. Ther. Drug Monit. 2021, 43, 386–393. [Google Scholar]
- De Kesel, P.M.M.; Lambert, W.E.; Stove, C.P. Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal. Chim. Acta 2015, 881, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, R.B.; Thijssen, B.; Atrafi, F.; Schellens, J.H.M.; Rosing, H.; de Vries, N.; Beijnen, J.H.; Mathijssen, R.H.J.; Steeghs, N.; Huitema, A.D.R. Validation and clinical application of an LC-MS/MS method for the quantification of everolimus using volumetric absorptive microsampling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1104, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.; Mandrioli, R.; Mercolini, L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 2019, 1046, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Capiau, S.; Veenhof, H.; Koster, R.A.; Bergqvist, Y.; Boettcher, M.; Halmingh, O.; Keevil, B.G.; Koch, B.C.P.; Linden, R.; Pistos, C.; et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: Development and Validation of Dried Blood Spot-Based Methods for Therapeutic Drug Monitoring. Ther. Drug Monit. 2019, 41, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Enderle, Y.; Foerster, K.; Burhenne, J. Clinical feasibility of dried blood spots: Analytics, validation, and applications. J. Pharm. Biomed. Anal. 2016, 130, 231–243. [Google Scholar] [CrossRef]
- Groenland, S.L.; van Nuland, M.; Verheijen, R.B.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R.; Steeghs, N. Therapeutic drug monitoring of oral anti-hormonal drugs in oncology. Clin. Pharmacokinet. 2019, 58, 299–308. [Google Scholar] [CrossRef]
- Groenland, S.L.; van Nuland, M.; Bergman, A.M.; de Feijter, J.M.; Dezentje, V.O.; Rosing, H.; Beijnen, J.H.; Huitema, A.D.R.; Steeghs, N. Concomitant intake of abiraterone acetate and food to increase pharmacokinetic exposure: Real life data from a therapeutic drug monitoring programme. Eur. J. Cancer 2020, 130, 32–38. [Google Scholar] [CrossRef]
- Hohmann, N.; Bozorgmehr, F.; Christopoulos, P.; Mikus, G.; Blank, A.; Burhenne, J.; Thomas, M.; Haefeli, W.E. Pharmacoenhancement of Low Crizotinib Plasma Concentrations in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer using the CYP3A Inhibitor Cobicistat. Clin. Transl. Sci. 2021, 14, 487–491. [Google Scholar] [CrossRef]
- Al-Barrak, J.; Cheung, W.Y. Adherence to imatinib therapy in gastrointestinal stromal tumors and chronic myeloid leukemia. Support. Care Cancer 2013, 21, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Kekäle, M.; Talvensaari, K.; Koskenvesa, P.; Porkka, K.; Airaksinen, M. Chronic myeloid leukemia patients’ adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Prefer. Adherence 2014, 8, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Chen, C.Y.; Lin, S.J.; Chang, C.S. Medication adherence to oral anticancer drugs: Systematic review. Expert Rev. Anticancer Ther. 2016, 16, 423–432. [Google Scholar] [CrossRef]
- Cardoso, E.; Csajka, C.; Schneider, M.P.; Widmer, N. Effect of adherence on pharmacokinetic/pharmacodynamic relationships of oral targeted anticancer drugs. Clin. Pharmacokinet. 2018, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.; Amoyal, N.; Nisotel, L.; Fishbein, J.N.; MacDonald, J.; Stagl, J.; Lennes, I.; Temel, J.S.; Safren, S.A.; Pirl, W.F. A systematic review of adherence to oral antineoplastic therapies. Oncologist 2016, 21, 354–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, K.P.; Chen, L.C.; Cheung, K.L.; Chang, C.S.; Yang, Y.H. Interruption and non-adherence to long-term adjuvant hormone therapy is associated with adverse survival outcome of breast cancer women—An Asian population-based study. PLoS ONE 2014, 9, e87027. [Google Scholar] [CrossRef]
- Pistilli, B.; Paci, A.; Ferreira, A.R.; Di Meglio, A.; Poinsignon, V.; Bardet, A.; Menvielle, G.; Dumas, A.; Pinto, S.; Dauchy, S.; et al. Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J. Clin. Oncol. 2020, 38, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, F.; Michelet, R.; Mueller-Schoell, A.; Maier, C.; Klopp-Schulze, L.; van Dyk, M.; Mikus, G.; Huisinga, W.; Kloft, C. Perspectives on model-informed precision dosing in the digital health era: Challenges, opportunities, and recommendations. Clin. Pharmacol. Ther. 2021, 109, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Schoell, A.; Klopp-Schulze, L.; Michelet, R.; Van Dyk, M.; Mürdter, T.E.; Schwab, M.; Joerger, M.; Huisinga, W.; Mikus, G.; Kloft, C. Simulation-based assessment of the impact of non-adherence on endoxifen target attainment in different tamoxifen dosing strategies. Pharmaceuticals 2021, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Klopp-Schulze, L.; Mueller-Schoell, A.; Neven, P.; Koolen, S.L.; Mathijssen, R.; Joerger, M.; Kloft, C. Integrated data analysis of six clinical studies points toward model-informed precision dosing of tamoxifen. Front. Pharmacol. 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
| Data Collection Parameter | Baseline Visit | Follow-Up Visits | Continuous |
|---|---|---|---|
| Vital parameters | X | X | |
| Information on current tumor therapy (treatment setting, current medication, concomitant treatment) | X | X | |
| Information on previous tumor therapies | X | ||
| Information on tumor diagnostics | X | X 1 | |
| Tumor classification according to TNM | X | X 1 | |
| Concomitant diseases | X | X 2 | |
| Current supportive medication | X | X 2 | |
| Current comedication | X | X 2 | |
| Toxicity according to CTCAE 5.0 | X | X 2 | |
| Symptomatic toxicity according to PRO-CTCAE | X 3 | ||
| Drug intake | X 4 | ||
| ECOG performance status | X | X | |
| Clinical chemistry parameters | X | X | |
| If applicable, information on the treatment termination (Date, reason(s)) | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mc Laughlin, A.M.; Schmulenson, E.; Teplytska, O.; Zimmermann, S.; Opitz, P.; Groenland, S.L.; Huitema, A.D.R.; Steeghs, N.; Müller, L.; Fuxius, S.; et al. Developing a Nationwide Infrastructure for Therapeutic Drug Monitoring of Targeted Oral Anticancer Drugs: The ON-TARGET Study Protocol. Cancers 2021, 13, 6281. https://doi.org/10.3390/cancers13246281
Mc Laughlin AM, Schmulenson E, Teplytska O, Zimmermann S, Opitz P, Groenland SL, Huitema ADR, Steeghs N, Müller L, Fuxius S, et al. Developing a Nationwide Infrastructure for Therapeutic Drug Monitoring of Targeted Oral Anticancer Drugs: The ON-TARGET Study Protocol. Cancers. 2021; 13(24):6281. https://doi.org/10.3390/cancers13246281
Chicago/Turabian StyleMc Laughlin, Anna M., Eduard Schmulenson, Olga Teplytska, Sebastian Zimmermann, Patrick Opitz, Stefanie L. Groenland, Alwin D. R. Huitema, Neeltje Steeghs, Lothar Müller, Stefan Fuxius, and et al. 2021. "Developing a Nationwide Infrastructure for Therapeutic Drug Monitoring of Targeted Oral Anticancer Drugs: The ON-TARGET Study Protocol" Cancers 13, no. 24: 6281. https://doi.org/10.3390/cancers13246281
APA StyleMc Laughlin, A. M., Schmulenson, E., Teplytska, O., Zimmermann, S., Opitz, P., Groenland, S. L., Huitema, A. D. R., Steeghs, N., Müller, L., Fuxius, S., Illerhaus, G., Joerger, M., Mayer, F., Fuhr, U., Holdenrieder, S., Hempel, G., Scherf-Clavel, O., Jaehde, U., Kloft, C., & for the ON-TARGET Study Consortium. (2021). Developing a Nationwide Infrastructure for Therapeutic Drug Monitoring of Targeted Oral Anticancer Drugs: The ON-TARGET Study Protocol. Cancers, 13(24), 6281. https://doi.org/10.3390/cancers13246281











