Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Patient Characteristics
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
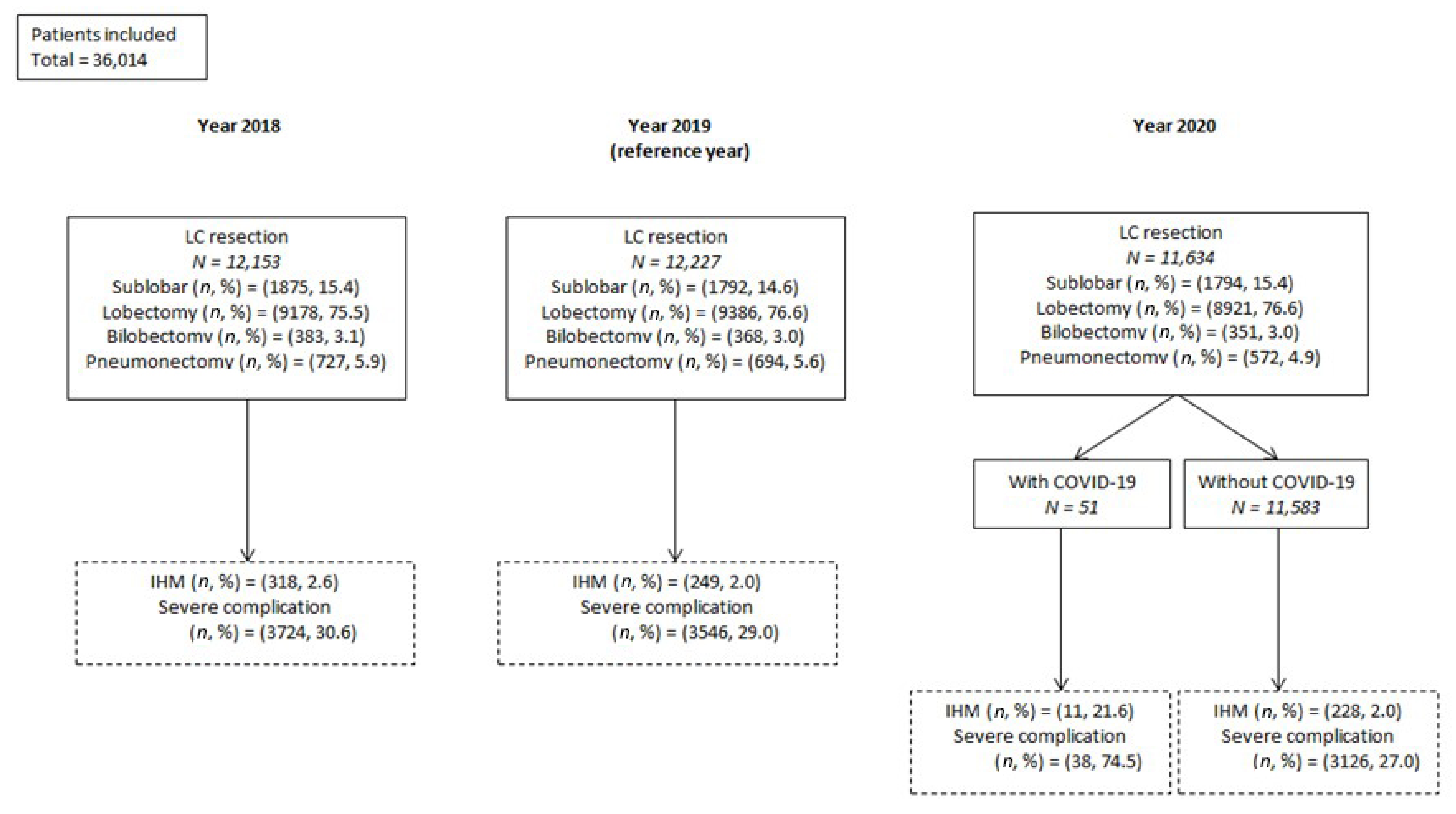
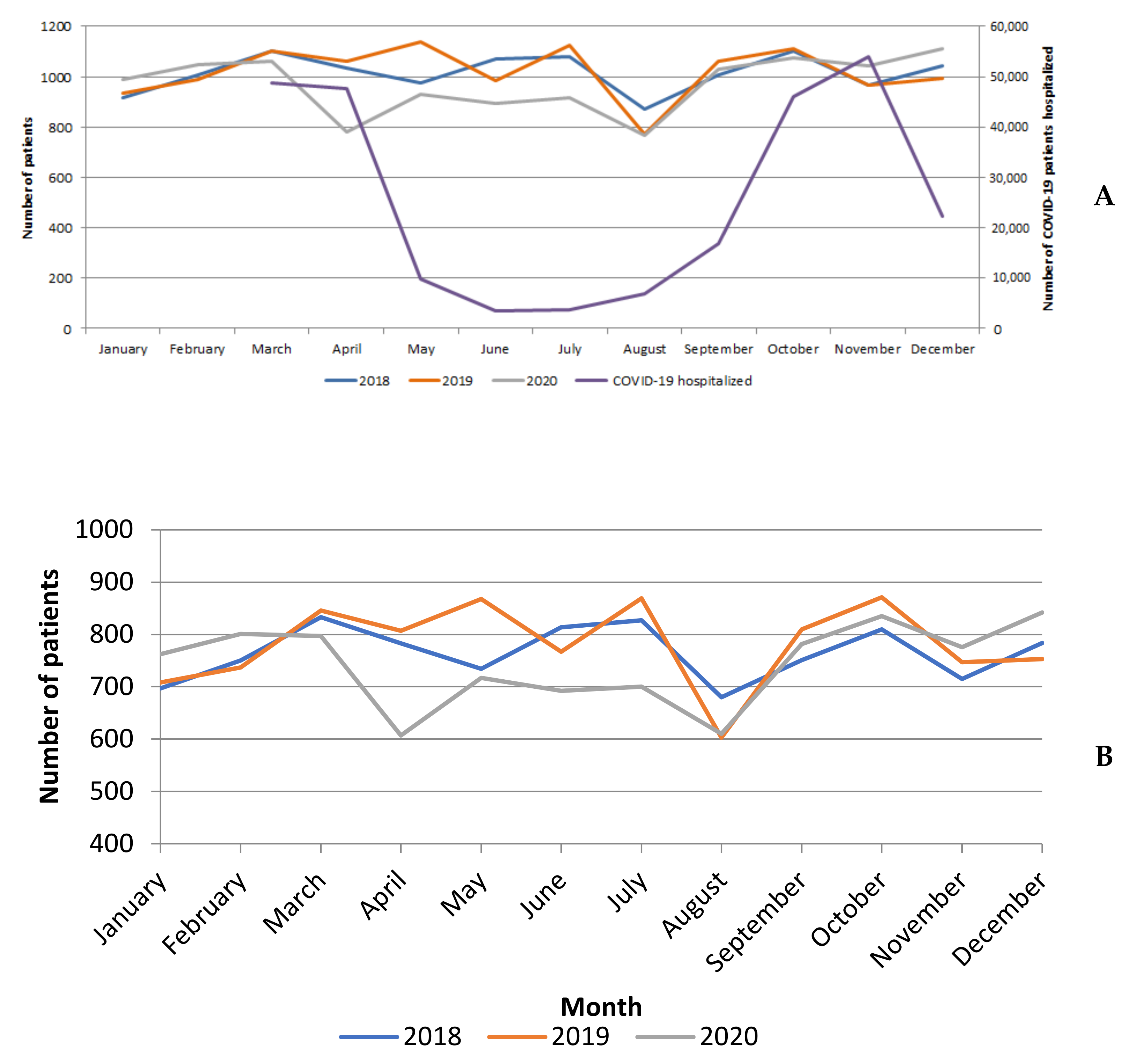
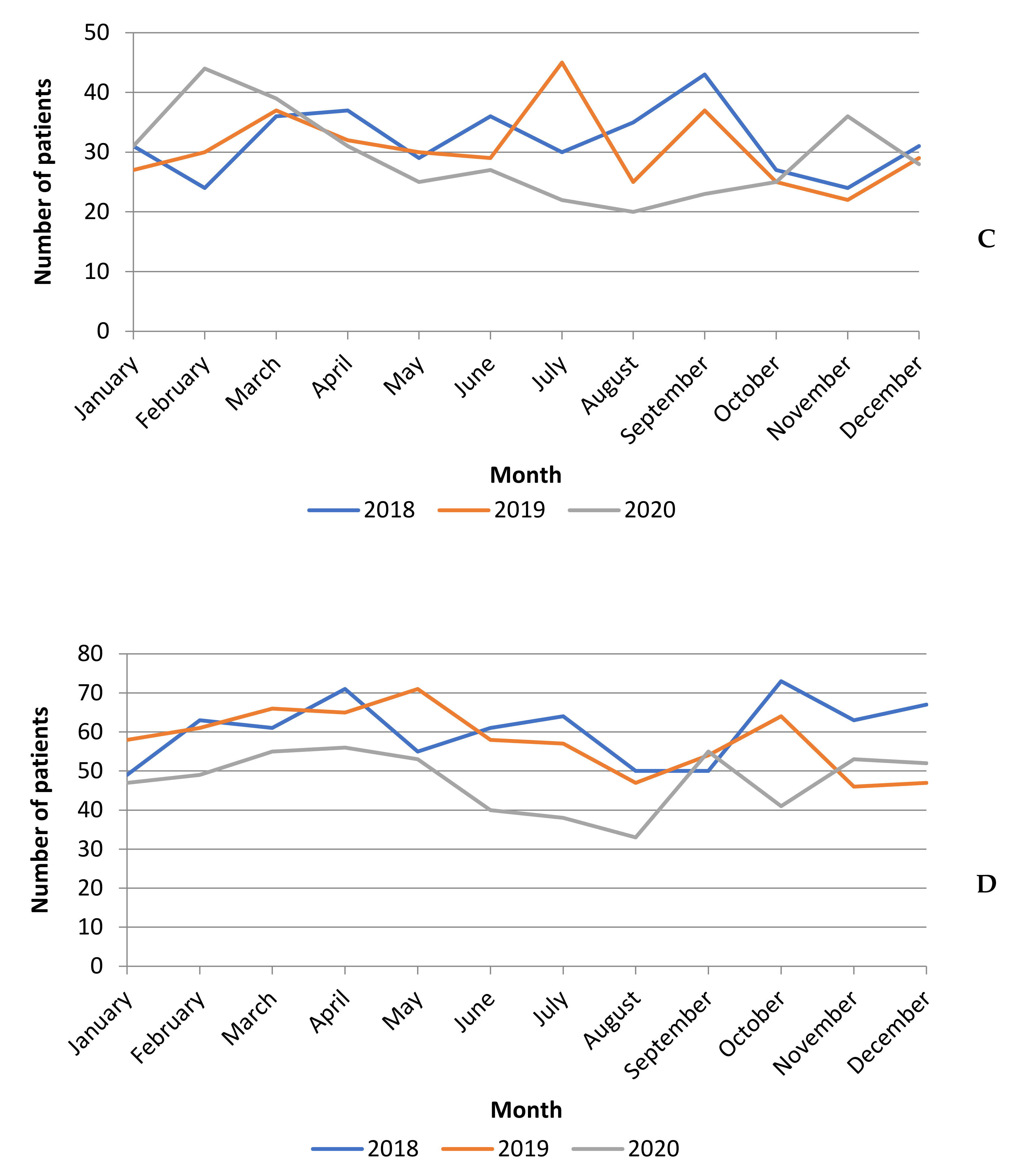
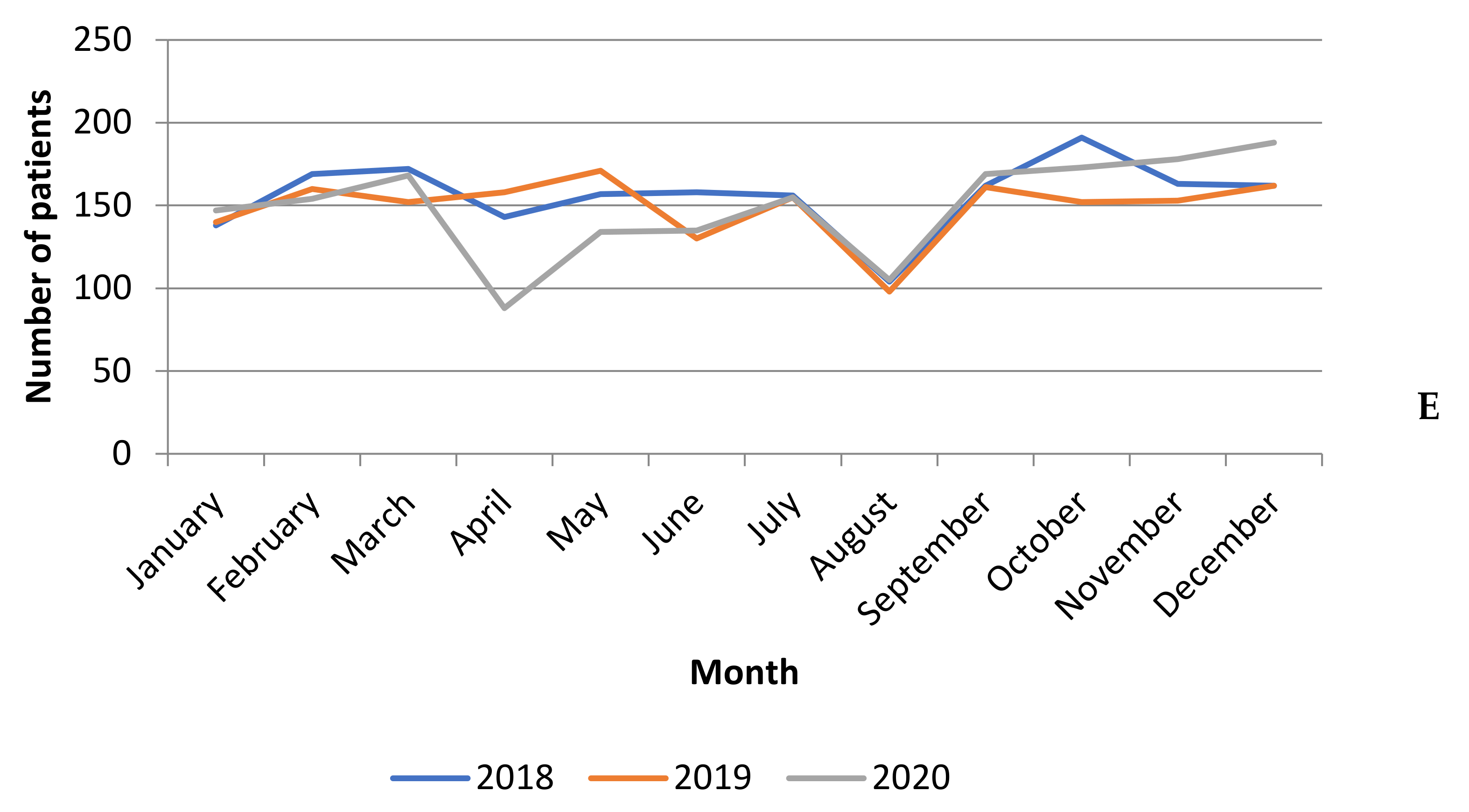
3.1. Surgical Activity Volume
3.2. Patient Characteristics
3.2.1. Whole Sample of Patients
3.2.2. LC Resections in Patients with SARS-CoV-2 Infection
3.3. Thirty-Day in-Hospital Mortality and Postoperative Complications
3.3.1. Whole Sample of Patients
3.3.2. LC Resections in Patients without SARS-CoV-2 Infection
3.3.3. LC Resections in Patients with SARS-CoV-2 Infection
3.4. Factors Associated with 30-Day in-Hospital Mortality and Severe Complications in Patients with SARS-CoV-2 Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- COVID-19 Dashboard. French Public Health Agency (Santé Publique France). [Internet]. Available online: https://dashboard.covid19.data.gouv.fr/vue-d-ensemble?location=FRA (accessed on 30 September 2021).
- Farid, Y.; Schettino, M.; Kapila, A.K.; Hamdi, M.; Cuylits, N.; Wauthy, P.; Ortiz, S. Decrease in surgical activity in the COVID-19 pandemic: An economic crisis. Br. J. Surg. 2020, 107, e300. [Google Scholar] [CrossRef]
- Goldberg, M.; Jougla, E.; Fassa, M.; Padieu, R.; Quantin, C. The French public health information system. J. Int. Assoc. Stat. 2012, 28, 31–41. [Google Scholar]
- Pagès, P.-B.; Cottenet, J.; Mariet, A.-S.; Bernard, A.; Quantin, C. In-hospital mortality following lung cancer resection: Nationwide administrative database. Eur. Respir J. 2016, 47, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Pagès, P.-B.; Mariet, A.-S.; Madelaine, L.; Cottenet, J.; Hanna, H.A.; Quantin, C.; Bernard, A. Impact of video-assisted thoracic surgery approach on postoperative mortality after lobectomy in octogenarians. J. Thorac. Cardiovasc. Surg. 2019, 157, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- French High Council for Public Health (Haut Conseil de la sante publique, HCSP). COVID-19 Guidelines for Cancer Patients. Accessed August 18, 2020 [Internet]. Available online: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier¼hcspx20200314_aprrlpelpecdcclprdfs.pdf (accessed on 30 September 2021).
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Dingemans, A.-M.C.; Soo, R.A.; Jazieh, A.R.; Rice, S.J.; Kim, Y.T.; Teo, L.L.; Warren, G.W.; Xiao, S.-Y.; Smit, E.F.; Aerts, J.G.; et al. Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2020, 15, 1119–1136. [Google Scholar] [CrossRef]
- Seitlinger, J.; Banga Nkomo, D.; Streit, A.; Stasiak, F.; Renaud, S. Letter to the Editor Concerning the Article From Cai focusing on Coronavirus Disease 2019 Impact on Lung Cancer Resection, Published in June 2020. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, e172–e173. [Google Scholar]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Hu, J.; Zhang, H.; Xie, C. The Management of Patients With Lung Cancer During the Outbreak of Coronavirus Disease 2019. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, e106–e107. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Weinberg, D.S.; Edelman, M.J.; Horwitz, E.M.; Uzzo, R.G.; Fisher, R.I. A War on Two Fronts: Cancer Care in the Time of COVID-19. Ann. Intern. Med. 2020, 172, 756–758. [Google Scholar] [CrossRef] [Green Version]
- Soe, W.M.; Balakrishnan, A.; Adhiyaman, V. Nosocomial COVID-19 on a green ward. Clin. Med. Lond. Engl. 2020, 20, e282. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Baker, M.; Vaidya, V.; Tucker, R.; Resnick, A.; Morris, C.A.; Klompas, M.; CDC Prevention Epicenters Program. Incidence of Nosocomial COVID-19 in Patients Hospitalized at a Large US Academic Medical Center. JAMA Netw. Open 2020, 3, e2020498. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; Collins, J.; Barlow-Pay, F.; Rickard, F.; Bruce, E.; Verduri, A.; Quinn, T.; Mitchell, E.; Price, A.; Vilches-Moraga, A.; et al. Nosocomial COVID-19 infection: Examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J. Hosp. Infect. 2020, 106, 376–384. [Google Scholar] [CrossRef]
- Risk Factors for Severity and Mortality in Adult COVID-19 Inpatients in Wuhan. Available online: https://pubmed.ncbi.nlm.nih.gov/32294485/ (accessed on 30 September 2021).
- Jordan, R.E.; Adab, P.; Cheng, K.K. Covid-19: Risk factors for severe disease and death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef] [Green Version]
- Morgant, M.-C.; Pagès, P.-B.; Orsini, B.; Falcoz, P.-E.; Thomas, P.; Le Pimpec-Barthes, F.; Dahan, M.; Bernard, A. Time trends in surgery for lung cancer in France from 2005 to 2012: A nationwide study. Eur. Respir J. 2015, 46, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Broderick, S.R.; Grau-Sepulveda, M.; Kosinski, A.S.; Kurlansky, P.A.; Shahian, D.M.; Jacobs, J.P.; Becker, S.; DeCamp, M.M.; Seder, C.W.; Grogan, E.L.; et al. The Society of Thoracic Surgeons Composite Score Rating for Pulmonary Resection for Lung Cancer. Ann. Thorac. Surg. 2020, 109, 848–855. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Russano, M.; Citarella, F.; Vincenzi, B.; Tonini, G.; Santini, D. Coronavirus Disease 2019 or Lung Cancer: What Should We Treat? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, e105–e106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, C.; Huang, Y. Treatment and Outcome of a Patient With Lung Cancer Infected With Severe Acute Respiratory Syndrome Coronavirus-2. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, e63–e64. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Cottenet, J.; Bonniaud, P.; Piroth, L.; Arveux, P.; Tubert-Bitter, P.; Quantin, C. Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level. Cancers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Peng, S.; Huang, L.; Zhao, B.; Zhou, S.; Braithwaite, I.; Zhang, N.; Fu, X. Clinical course of coronavirus disease 2019 in 11 patients after thoracic surgery and challenges in diagnosis. J. Thorac. Cardiovasc. Surg. 2020, 160, 585–592.e2. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Gatteschi, L.; Salvicchi, A.; Bongiolatti, S.; Lavorini, F.; Voltolini, L. Clinical courses and outcomes of five patients with primary lung cancer surgically treated while affected by Severe acute respiratory syndrome coronavirus 2. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Hao, Z.; Gao, Y.; Ping, W.; Wang, Q.; Peng, S.; Zhao, B.; Sun, W.; Zhu, M.; Li, K.; et al. Coronavirus Disease 2019 in the Perioperative Period of Lung Resection: A Brief Report From a Single Thoracic Surgery Department in Wuhan, People’s Republic of China. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1065–1072. [Google Scholar] [CrossRef]
- Hoyos Mejía, L.; Romero Román, A.; Gil Barturen, M.; Córdoba Pelaez, M.D.M.; Campo-Cañaveral de la Cruz, J.L.; Naranjo, J.M.; Crolwey Carrasco, S.; Tanaka, S.; Sánchez Calle, A.; Varela de Ugarte, A.; et al. Thoracic surgery during the coronavirus disease 2019 (COVID-19) pandemic in Madrid, Spain: Single-centre report. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Zervos, M.; Kent, A.; Chachoua, A.; Bizekis, C.; Pass, H.; Cerfolio, R.J. Safety of patients and providers in lung cancer surgery during the COVID-19 pandemic. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 1222–1227. [Google Scholar] [CrossRef]
- Maurizi, G.; Rendina, E.A. A High-Volume Thoracic Surgery Division Into the Storm of the COVID-19 Pandemic. Ann. Thorac. Surg. 2020, 110, 353–354. [Google Scholar] [CrossRef]
- Yang, C.-F.J.; Wang, H.; Kumar, A.; Wang, X.; Hartwig, M.G.; D’Amico, T.A.; Berry, M.F. Impact of Timing of Lobectomy on Survival for Clinical Stage IA Lung Squamous Cell Carcinoma. Chest 2017, 152, 1239–1250. [Google Scholar] [CrossRef]
- Wu, C.F.; Chao, Y.-K. Saving time is saving lives: A delayed lobectomy predicts poorer overall survival in patients with clinical stage IA squamous cell carcinoma of the lung. J. Thorac. Dis. 2018, 10 (Suppl. 26), S3147–S3148. [Google Scholar] [CrossRef] [PubMed]
- Serna-Gallegos, D.; Mercado, F.; Imai, T.; Berz, D.; Soukiasian, H.; American Association for Thoracic Surgery. Effects of Time from Completed Clinical Staging to Surgery: Does it Make a Difference in Stage 1 Non-Small Cell Lung Cancer? [abstract 67]. In San Diego, CA; 2018. Available online: https://aats.org/aatsimis/AATS/Meetings/Active_Meetings/98th_Annual_Meeting/Preliminary_Program/Abstracts/67.aspx (accessed on 30 September 2021).
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Navani, N.; Fisher, D.J.; Tierney, J.F.; Stephens, R.J.; Burdett, S.; NSCLC Meta-analysis Collaborative Group. The Accuracy of Clinical Staging of Stage I-IIIa Non-Small Cell Lung Cancer: An Analysis Based on Individual Participant Data. Chest 2019, 155, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bade, B.C.; Silvestri, G.A. Setting the Stage for Success in Lung Cancer: The Importance of Remembering Your (Guide)Lines. Chest 2019, 155, 456–457. [Google Scholar] [CrossRef] [Green Version]
- Goueslard, K.; Petit, J.-M.; Cottenet, J.; Chauvet-Gelinier, J.-C.; Jollant, F.; Quantin, C. Increased Risk of Rehospitalization for Acute Diabetes Complications and Suicide Attempts in Patients With Type 1 Diabetes and Comorbid Schizophrenia. Diabetes Care 2018, 41, 2316–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, A.; Falcoz, P.-E.; Thomas, P.A.; Rivera, C.; Brouchet, L.; Baste, J.M.; Puyraveau, M.; Quantin, C.; Pages, P.B.; Dahan, M. Comparison of Epithor clinical national database and medico-administrative database to identify the influence of case-mix on the estimation of hospital outliers. PLoS ONE 2019, 14, e0219672. [Google Scholar] [CrossRef] [Green Version]
- Bernard, A.; Cottenet, J.; Mariet, A.-S.; Quantin, C.; Pagès, P.-B. Is an activity volume threshold really realistic for lung cancer resection? J. Thorac. Dis. 2018, 10, 5685–5694. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallah, I.; Pauly, V.; Viprey, M.; Orleans, V.; Fond, G.; Auquier, P.; D’Journo, X.B.; Boyer, L.; Thomas, P.A. Unplanned readmission and survival after video-assisted thoracic surgery and open thoracotomy in patients with non-small-cell lung cancer: A 12-month nationwide cohort study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 59, 987–995. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Years | p-Value (Comparison between the 3 Years) | 2020 SARS-CoV-2 Patients | 2020 Non-SARS-CoV-2 Patients | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | Characteristics | p-Value (Comparison with 2019) | Characteristics | p-Value (Comparison with 2019) | p-Value (Comparison with 2020 SARS-CoV-2) | ||
| Hospitalized patients with lung cancer resection (n) | 12,153 | 12,227 | 11,634 | 51 | 11,583 | ||||
| Age Mean +/− std | 65 +/− 10 ‡ | 66 +/− 10 | 66 +/− 10 | 0.0002 | 68 +/− 8 | 0.0615 | 66 +/− 10 | 0.8598 | 0.0641 |
| Median (Q1–Q3) | 66 (60–72) | 67 (60–72) | 67 (60–73) | 70 (61–73) | 67 (60–73) | ||||
| Min-Max | 18–92 | 18–91 | 18–94 | 50–85 | 18–94 | ||||
| Men (%) | 63.4 * | 62 * | 60.6 | <0.0001 | 62.8 | 0.9150 | 60.6 | 0.0204 | 0.7494 |
| Pulmonary disease (%) | 33.1 * | 31.6 | 30.9 | 0.0012 | 72.6 | <0.0001 | 30.8 | 0.1494 | <0.0001 |
| Heart disease (%) | 15.7 | 15.5 | 16.3 | 0.1949 | 11.8 | 0.4638 | 16.3 | 0.0782 | 0.3798 |
| Peripheral vascular disease (%) | 9.7 | 9.2 | 9.1 | 0.1926 | 11.8 | 0.4668 | 9.1 | 0.8200 | 0.4693 |
| Neurological disease (%) | 4.2 | 4.1 | 4.3 | 0.6038 | 9.8 | 0.0560 | 4.3 | 0.3663 | 0.0678 |
| Liver disease (%) | 0.9 | 1 | 0.8 | 0.1705 | 2 | 0.3946 | 0.8 | 0.0643 | 0.3216 |
| Renal disease (%) | 2.9 | 2.8 | 3.1 | 0.2805 | 3.9 | 0.6523 | 3.1 | 0.1161 | 0.6725 |
| Endocrine disease (%) | 12.6 | 12.6 | 12.9 | 0.6701 | 21.6 | 0.0557 | 12.9 | 0.5711 | 0.0649 |
| Metabolic disease (%) | 14.1 | 13.7 | 13.8 | 0.6054 | 29.4 | 0.0012 | 13.7 | 0.9888 | 0.0012 |
| Infectious disease (%) | 0.6 | 0.7 | 0.6 | 0.7268 | 2 | 0.2959 | 0.6 | 0.4208 | 0.2654 |
| Hematological disease (%) | 5.2 | 5.3 | 5.2 | 0.9183 | 7.8 | 0.3506 | 5.2 | 0.6855 | 0.3411 |
| Other malignant lesions (%) | 43.4 * | 41.7 | 41.8 | 0.0116 | 45.1 | 0.6253 | 41.8 | 0.9497 | 0.6295 |
| Other therapies (%) | 11.2 * | 9.4 | 9.5 | <0.0001 | 9.8 | 0.8112 | 9.5 | 0.7757 | 0.8131 |
| Charlson index (%) | 0.0084 | 0.0238 | 0.0013 | 0.0199 | |||||
| 0 | 43.5 | 43.9 | 44.5 | 25.5 | 44.6 | ||||
| 1 | 24 | 24.9 | 22.8 | 35.3 | 22.8 | ||||
| 2 | 8 | 7.7 | 7.9 | 13.7 | 7.9 | ||||
| ≥3 | 24.6 | 23.5 | 24.8 | 25.5 | 24.8 | ||||
| Postoperative Complications | Year | p-Value (Comparison between the 3 Years) | 2020 SARS-CoV-2 Patients | 2020 non-SARS-CoV-2 Patients | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | Characteristics | p-Value (Comparison with 2019) | Characteristics | p-Value (Comparison with 2019) | p-Value (Comparison with 2020 SARS-CoV-2) | ||
| Hospitalized patients with lung cancer resection (n) | 12,153 | 12,227 | 11,634 | 51 | 11,583 | ||||
| Postoperative complications÷Pneumonia (%) | 15.7 * | 15.4 | 14.7 | 0.0805 | 60.8 | <0.0001 | 14.5 | 0.0592 | <0.0001 |
| Acute respiratory distress syndrome (%) | 7.7 * | 6 | 6.2 | <0.0001 | 29.4 | <0.0001 | 6.1 | 0.8522 | <0.0001 |
| Bleeding (%) | 8.7 | 9.6 | 9.5 | 0.0397 | 5.9 | 0.3671 | 9.5 | 0.7492 | 0.3803 |
| Respiratory failure %) | 13.8 * | 12.3 * | 11.3 | <0.0001 | 49 | <0.0001 | 11.1 | 0.0067 | <0.0001 |
| Heart failure (%) | 2.6 * | 2.5 | 2.2 | 0.0802 | 5.9 | 0.1414 | 2.2 | 0.0756 | 0.1026 |
| Acute renal failure (%) | 5.5 * | 5.1 | 4.7 | 0.0146 | 17.7 | 0.0010 | 4.6 | 0.0987 | 0.0005 |
| Phlebitis (%) | 1.6 | 1.6 | 1.6 | 0.9476 | 3.9 | 0.1926 | 1.6 | 0.9193 | 0.1897 |
| Pulmonary embolism (%) | 1.2 | 1.2 | 1.3 | 0.6432 | 3.9 | 0.1341 | 1.3 | 0.5542 | 0.1492 |
| Infectious complications (including sepsis) (%) | 12.7 * | 11.6 * | 10.3 | <0.0001 | 29.4 | <0.0001 | 10.2 | 0.0005 | <0.0001 |
| Severe complications a (%) | 30.6 * | 29 * | 27.2 | <0.0001 | 74.5 | <0.0001 | 27 | 0.0005 | <0.0001 |
| Clavien Dindo complications b (%) | 21.9 * | 20.0 * | 18.3 | <0.0001 | 64.7 | <0.0001 | 18.1 | 0.0002 | <0.0001 |
| Transfer to intensive care unit (%) | 22.8 | 22 ‡ | 23.3 | 0.0717 | 58.8 | <0.0001 | 23.1 | 0.0483 | <0.0001 |
| In-hospital mortality(%) | 2.6 * | 2 | 2.1 | 0.0024 | 21.6 | <0.0001 | 2 | 0.7079 | <0.0001 |
| Characteristics | In-hospital Mortality | Severe Complications * | ||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Year (reference = 2019, non-SARS-CoV-2) | ||||
| 2020 without SARS-CoV-2 a | 1.050 (0.862–1.281) | 1.097 (0.892–1.349) | 0.908 (0.853–0.967) | 0.904 (0.842–0.970) |
| 2020 with SARS-CoV-2 b | 13.431 (6.793–26.555) | 7.166 (3.302–15.552) | 7.279 (3.872–13.684) | 4.757 (2.309–9.799) |
| Type of lung cancer resection (ref = limited resection) | ||||
| Bilobectomy | 3.479 (2.266–5.341) | 2.356 (1.483–3.743) | 2.345 (1.944–2.830) | 1.923 (1.549–2.386) |
| Lobectomy | 0.870 (0.649–1.165) | 0.793 (0.586–1.072) | 1.227 (1.118–1.346) | 1.174 (1.059–1.302) |
| Pneumonectomy | 3.700 (2.582–5.304) | 2.939 (1.998–4.324) | 2.470 (2.125–2.871) | 2.145 (1.807–2.547) |
| Age | 1.046 (1.034–1.058) | 1.041 (1.028–1.054) | 1.012 (1.009–1.015) | 1.008 (1.004–1.012) |
| Men | 2.509 (1.971–3.194) | 1.670 (1.296–2.153) | 1.632 (1.526–1.744) | 1.320 (1.224–1.424) |
| Pulmonary disease | 5.873 ((4.725–7.301) | 4.091 (3.257–5.138) | 6.709 (6.263–7.187) | 5.911 (5.504–6.348) |
| Heart disease | 3.090 (2.518–3.791) | 1.496 (1.191–1.881) | 2.484 (2.296–2.688) | 1.690 (1.542–1.852) |
| Peripheral vascular disease | 3.079 (2.437–3.890) | 1.706 (1.323–2.202) | 2.393 (2.170–2.640) | 1.670 (1.491–1.871) |
| Neurological disease | 3.043 (2.227–4.157) | 1.846 (1.314–2.594) | 1.766 (1.531–2.038) | 1.261 (1.069–1.489) |
| Liver disease | 7.398 (4.672–11.713) | 4.990 (2.949–8.445) | 2.291 (1.688–3.110) | 1.727 (1.207–2.469) |
| Renal disease | 4.882 (3.567–6.681) | 2.581 (1.819–3.662) | 2.252 (1.899–2.671) | 1.509 (1.237–1.839) |
| Endocrine disease | 1.706 (1.331–2.185) | 0.978 (0.745–1.282) | 1.359 (1.242–1.488) | 1.033 (0.930–1.148) |
| Metabolic disease | 2.830 (2.283–3.508) | 1.543 (1.220–1.952) | 2.196 (2.018–2.389) | 1.437 (1.303–1.584) |
| Infectious disease | 12.223 (7.864–18.999) | 5.128 (3.082–8.532) | 23.997 (13.526–42.574) | 16.675 (9.056–30.704) |
| Hematological disease | 3.091 (2.329–4.103) | 1.386 (1.010–1.901) | 2.381 (2.099–2.701) | 1.407 (1.213–1.632) |
| Other malignant lesions | 2.059 (1.688–2.512) | 1.410 (1.141–1.742) | 1.493 (1.402–1.590) | 1.239 (1.153–1.331) |
| Other therapies ** | 1.848 (1.414–2.415) | - | 1.603 (1.451–1.772) | - |
| Charlson index (reference = 0) ** | ||||
| 1 | 2.156 (1.611–2.884) | - | 1.706 (1.573–1.851) | - |
| 2 | 3.748 (2.665–5.271) | - | 2.448 (2.182–2.747) | - |
| ≥3 | 3.734 (2.87–4.851) | - | 2.094 (1.933–2.267) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pages, P.-B.; Cottenet, J.; Bonniaud, P.; Tubert-Bitter, P.; Piroth, L.; Cadranel, J.; Bernard, A.; Quantin, C. Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study. Cancers 2021, 13, 6277. https://doi.org/10.3390/cancers13246277
Pages P-B, Cottenet J, Bonniaud P, Tubert-Bitter P, Piroth L, Cadranel J, Bernard A, Quantin C. Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study. Cancers. 2021; 13(24):6277. https://doi.org/10.3390/cancers13246277
Chicago/Turabian StylePages, Pierre-Benoit, Jonathan Cottenet, Philippe Bonniaud, Pascale Tubert-Bitter, Lionel Piroth, Jacques Cadranel, Alain Bernard, and Catherine Quantin. 2021. "Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study" Cancers 13, no. 24: 6277. https://doi.org/10.3390/cancers13246277
APA StylePages, P.-B., Cottenet, J., Bonniaud, P., Tubert-Bitter, P., Piroth, L., Cadranel, J., Bernard, A., & Quantin, C. (2021). Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study. Cancers, 13(24), 6277. https://doi.org/10.3390/cancers13246277







