A Novel and Effective Method for Human Primary Skin Melanocytes and Metastatic Melanoma Cell Isolation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Tissue Samples
2.2. Cell Culture Media and Reagents
2.3. Cell Lines
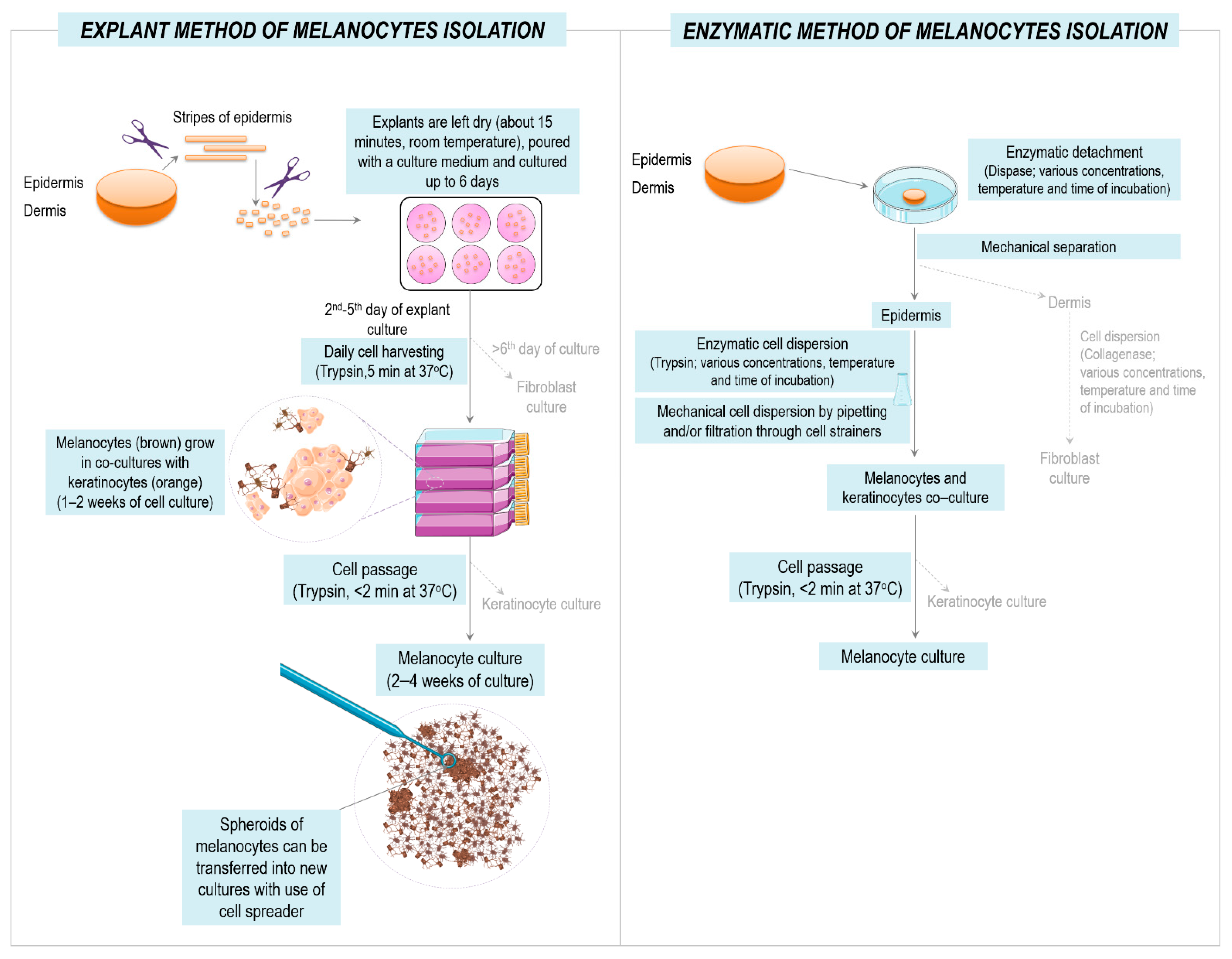
2.4. Isolation and Culture of Primary Melanocytes and Melanoma Cells
2.5. Calculation of Melanoma Cell Population Doubling Time
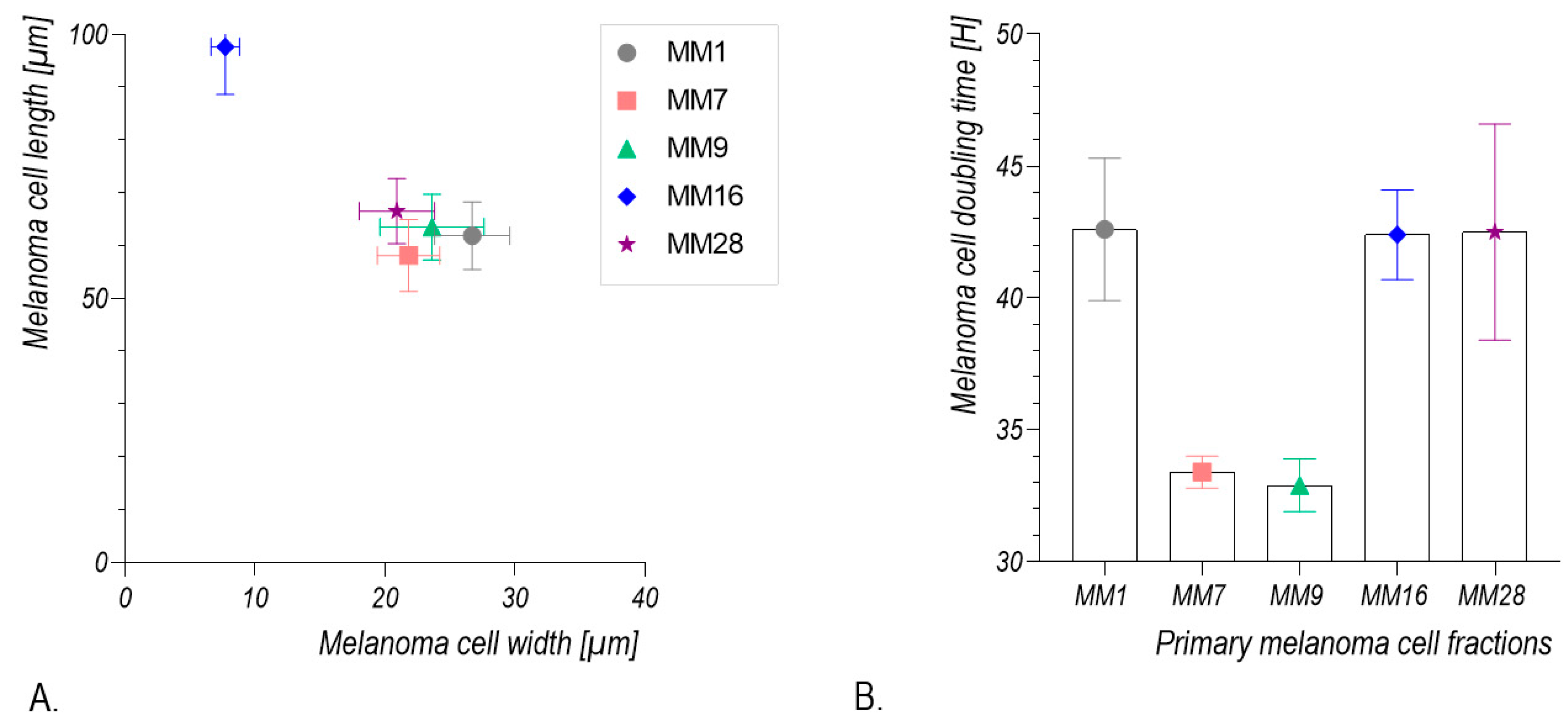
2.6. Morphometric Analysis of Melanoma Cells
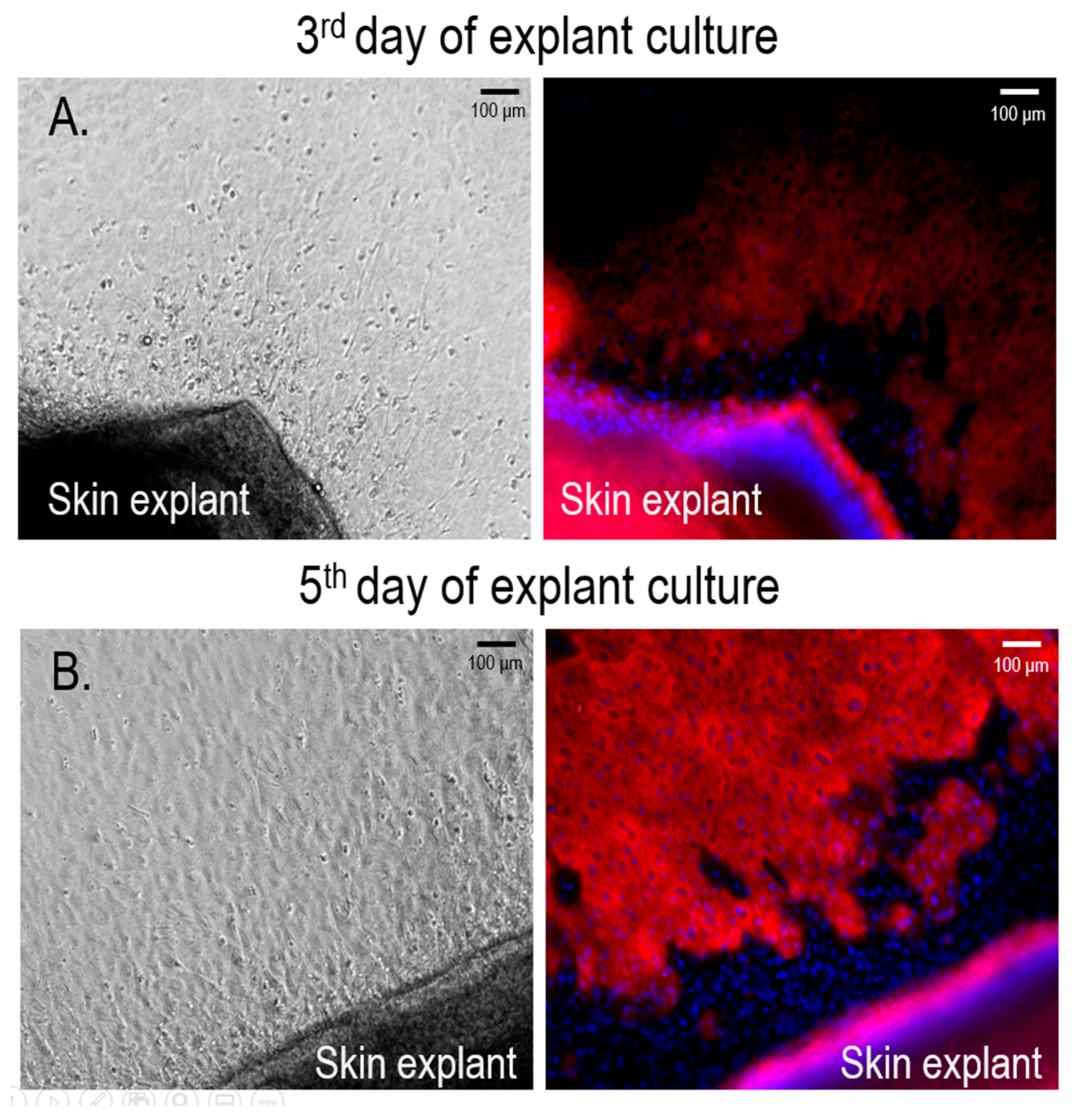
2.7. Immunofluorescence Staining
2.8. RNA Isolation and cDNA Synthesis
2.9. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
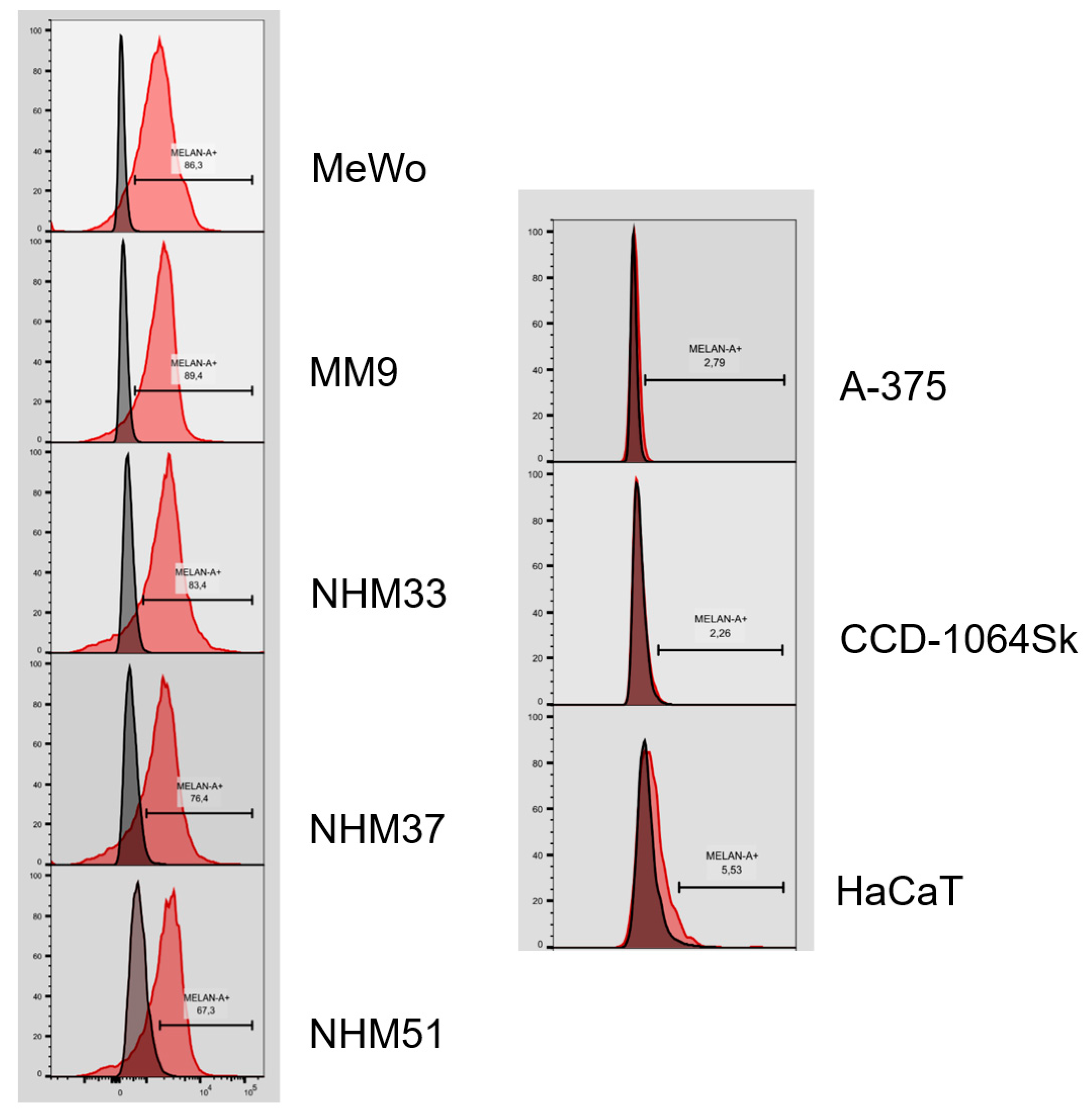
2.10. Flow Cytometry Analysis
3. Results
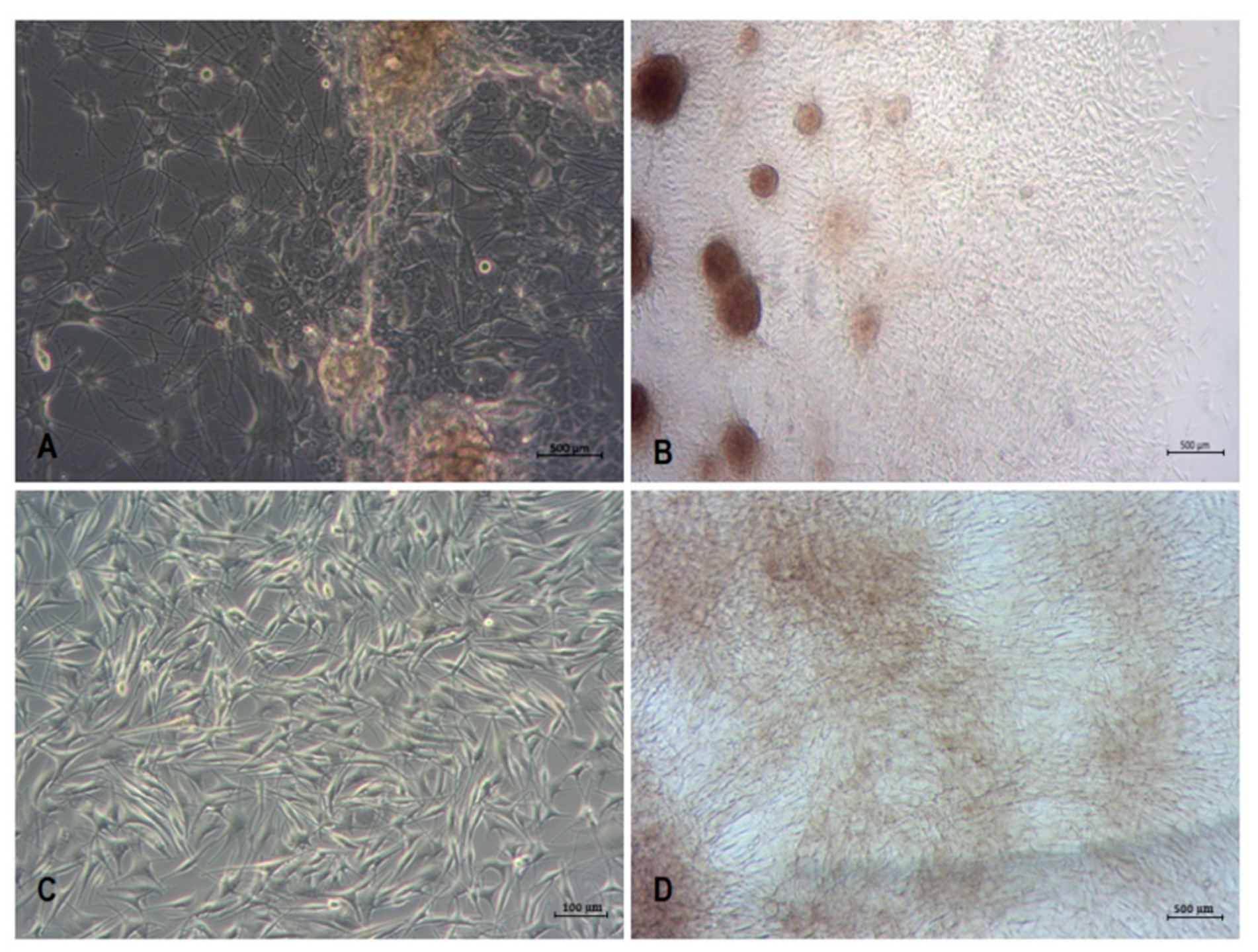
3.1. Isolation and Culture of Primary Human Melanocytes from Skin Explants
3.2. Isolation and Culture of Human Melanoma Cells from Explants with Melanoma Metastases
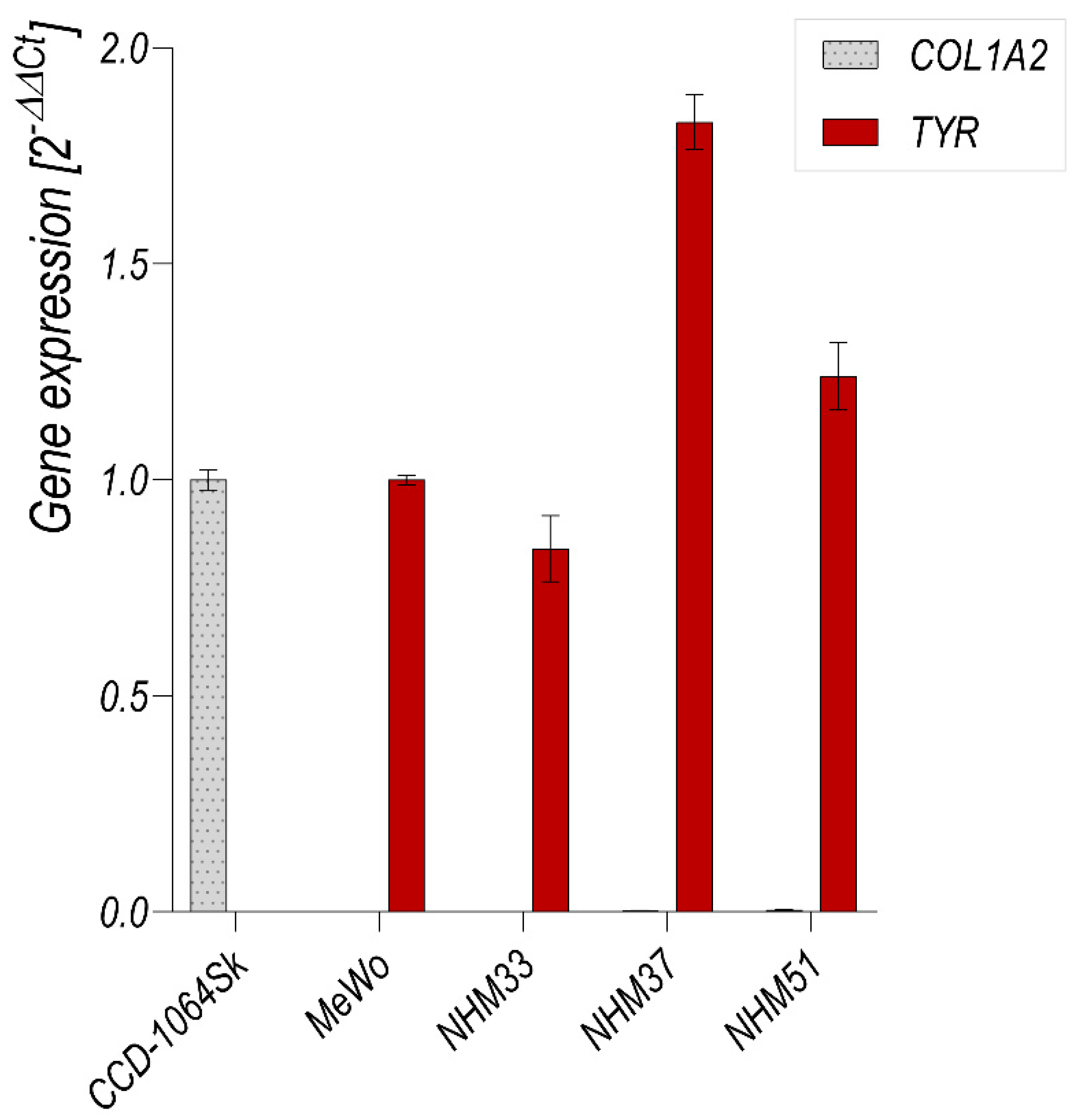
3.3. Flow Cytometry Analysis of Isolated Melanocytes and Melanoma Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, M.-Y.; Li, L.; Herlyn, M. Cultivation of normal human epidermal melanocytes in the absence of phorbol esters. Methods Mol. Med. 2005, 107, 013–028. [Google Scholar] [CrossRef]
- Litwack, G. Chapter 13-Metabolism of amino Acids. In Human Biochemistry; Litwack, G., Ed.; Academic Press: Boston, MA, USA, 2018; pp. 359–394. [Google Scholar] [CrossRef]
- Zurina, I.; Gorkun, A.A.; Dzhussoeva, E.V.; Kolokoltsova, T.D.; Markov, D.D.; Kosheleva, N.V.; Morozov, S.G.; Saburina, I.N. Human melanocyte-derived spheroids: A precise test system for drug screening and a multicellular unit for tissue engineering. Front. Bioeng. Biotechnol. 2020, 8, 540. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and intertumor heterogeneity in melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef] [PubMed]
- Sobiepanek, A.; Paone, A.; Cutruzzolà, F.; Kobiela, T. Biophysical characterization of melanoma cell phenotype markers during metastatic progression. Eur. Biophys. J. 2021, 50, 523–542. [Google Scholar] [CrossRef]
- Raigani, S.; Cohen, S.; Boland, G.M. The role of surgery for melanoma in an era of effective systemic therapy. Curr. Oncol. Rep. 2017, 19, 17. [Google Scholar] [CrossRef]
- Hsiao, W.-C.; Young, T.-H. Characteristics of melanocyte spheroids formed through different biomaterial-induced processes. J. Formos. Med. Assoc. 2019, 118, 152–161. [Google Scholar] [CrossRef]
- Godwin, L.S.; Castle, J.T.; Kohli, J.; Goff, P.; Cairney, C.; Keith, N.; Sviderskaya, E.V.; Bennett, D.C. Isolation, culture, and transfection of melanocytes. Curr. Protoc. Cell Biol. 2014, 63, 1.8.1–1.8.20. [Google Scholar] [CrossRef]
- Cario, M.; Taieb, A. Isolation and culture of epidermal melanocytes. In Skin Tissue Engineering; Humana: New York, NY, USA, 2019; Volume 1993, pp. 33–46. [Google Scholar] [CrossRef]
- Liu, F.; Luo, X.-S.; Shen, H.-Y.; Dong, J.-S.; Yang, J. Using human hair follicle-derived keratinocytes and melanocytes for constructing pigmented tissue-engineered skin. Ski. Res. Technol. 2011, 17, 373–379. [Google Scholar] [CrossRef]
- Szabad, G.; Kormos, B.; Pivarcsi, A.; Széll, M.; Kis, K.; Szabó, A.K.; Dobozy, A.; Kemény, L.; Bata-Csörgő, Z. Human adult epidermal melanocytes cultured without chemical mitogens express the EGF receptor and respond to EGF. Arch. Dermatol. Res. 2007, 299, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Yarani, R.; Mansouri, K.; Mohammadi-Motlagh, H.R.; Bakhtiari, M.; Mostafaie, A. New procedure for epidermal cell isolation using kiwi fruit actinidin, and improved culture of melanocytes in the presence of leukaemia inhibitory factor and forskolin. Cell Prolif. 2013, 46, 348–355. [Google Scholar] [CrossRef]
- Ścieżyńska, A.; Nogowska, A.; Sikorska, M.; Konys, J.; Karpińska, A.; Komorowski, M.; Ołdak, M.; Malejczyk, J. Isolation and culture of human primary keratinocytes–A methods review. Exp. Dermatol. 2018, 28, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tissot, M.; Rolin, G.; Muret, P.; Robin, S.; Berthon, J.-Y.; He, L.; Humbert, P.; Viennet, C. Development and validation of a simple method for the extraction of human skin melanocytes. Cytotechnology 2018, 70, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Le Poole, I.C.; Wijngaard, R.M.; Westerhof, W.; Dormans, J.A.; Berg, F.M.; Verkruisen, R.P.; Dingemans, K.P.; Das, P.K. Organotypic culture of human skin to study melanocyte migration. Pigment. Cell Res. 1994, 7, 33–43. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Kasraee, B.; Pataky, M.; Nikolic, D.S.; Carraux, P.; Piguet, V.; Salomon, D.; Sorg, O.; Saurat, J.-H. A new spectrophotometric method for simple quantification of melanosomal transfer from melanocytes to keratinocytes. Exp. Dermatol. 2011, 20, 938–942. [Google Scholar] [CrossRef]
- Guo, A.; Jahoda, C.A.B. An improved method of human keratinocyte culture from skin explants: Cell expansion is linked to markers of activated progenitor cells. Exp. Dermatol. 2009, 18, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Norris, D.A.; Zekman, T.; Morelli, J.G. Effective elimination of fibroblasts in cultures of melanocytes by lowering calcium concentration in TPA depleted medium following geneticin treatment. Pigment. Cell Res. 1996, 9, 58–62. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Li, D.; Shi, H.-X.; Yang, Y.-H.; Tian, T.; Wang, L. Melanocyte spheroids are formed by repetitive long-term trypsinization. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 258–265. [Google Scholar] [CrossRef]
- Chung, S.; Lim, G.J.; Lee, J.Y. Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci. Rep. 2019, 9, 780. [Google Scholar] [CrossRef]
- Du, J.; Miller, A.J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Fisher, D.E. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol. 2003, 163, 333–343. [Google Scholar] [CrossRef]
- Perng, Y.-P.; Lin, C.-C.; Perng, I.-M.; Shen, Y.-C.; Chuang, C.-K.; Liao, S.-K. Culture medium induced morphological changes of melanoma cells associated with change in sensitivity to lysis by lymphokine-activated killer cells. Cancer Biother. Radiopharm. 1997, 12, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Benga, G. Basic studies on gene therapy of human malignant melanoma by use of the human interferon beta gene entrapped in cationic multilamellar liposomes. 1. Morphology and growth rate of six melanoma cell lines used in transfection experiments with the human interferon beta gene. J. Cell. Mol. Med. 2001, 5, 402–408. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścieżyńska, A.; Sobiepanek, A.; Kowalska, P.D.; Soszyńska, M.; Łuszczyński, K.; Grzywa, T.M.; Krześniak, N.; Góźdź, A.; Włodarski, P.K.; Galus, R.; et al. A Novel and Effective Method for Human Primary Skin Melanocytes and Metastatic Melanoma Cell Isolation. Cancers 2021, 13, 6244. https://doi.org/10.3390/cancers13246244
Ścieżyńska A, Sobiepanek A, Kowalska PD, Soszyńska M, Łuszczyński K, Grzywa TM, Krześniak N, Góźdź A, Włodarski PK, Galus R, et al. A Novel and Effective Method for Human Primary Skin Melanocytes and Metastatic Melanoma Cell Isolation. Cancers. 2021; 13(24):6244. https://doi.org/10.3390/cancers13246244
Chicago/Turabian StyleŚcieżyńska, Aneta, Anna Sobiepanek, Patrycja D. Kowalska, Marta Soszyńska, Krzysztof Łuszczyński, Tomasz M. Grzywa, Natalia Krześniak, Agata Góźdź, Paweł K. Włodarski, Ryszard Galus, and et al. 2021. "A Novel and Effective Method for Human Primary Skin Melanocytes and Metastatic Melanoma Cell Isolation" Cancers 13, no. 24: 6244. https://doi.org/10.3390/cancers13246244
APA StyleŚcieżyńska, A., Sobiepanek, A., Kowalska, P. D., Soszyńska, M., Łuszczyński, K., Grzywa, T. M., Krześniak, N., Góźdź, A., Włodarski, P. K., Galus, R., Kobiela, T., & Malejczyk, J. (2021). A Novel and Effective Method for Human Primary Skin Melanocytes and Metastatic Melanoma Cell Isolation. Cancers, 13(24), 6244. https://doi.org/10.3390/cancers13246244







