The Cap-Binding Complex CBC and the Eukaryotic Translation Factor eIF4E: Co-Conspirators in Cap-Dependent RNA Maturation and Translation
Abstract
Simple Summary
Abstract
1. Overview
2. RNA Maturation, Nuclear Export, and Translation Focusing on the Roles of Cap-Chaperones
2.1. The Process of m7G Capping
2.2. RNA Splicing
2.3. Cleavage and Polyadenylation
2.4. Nuclear RNA Export
2.5. Translation
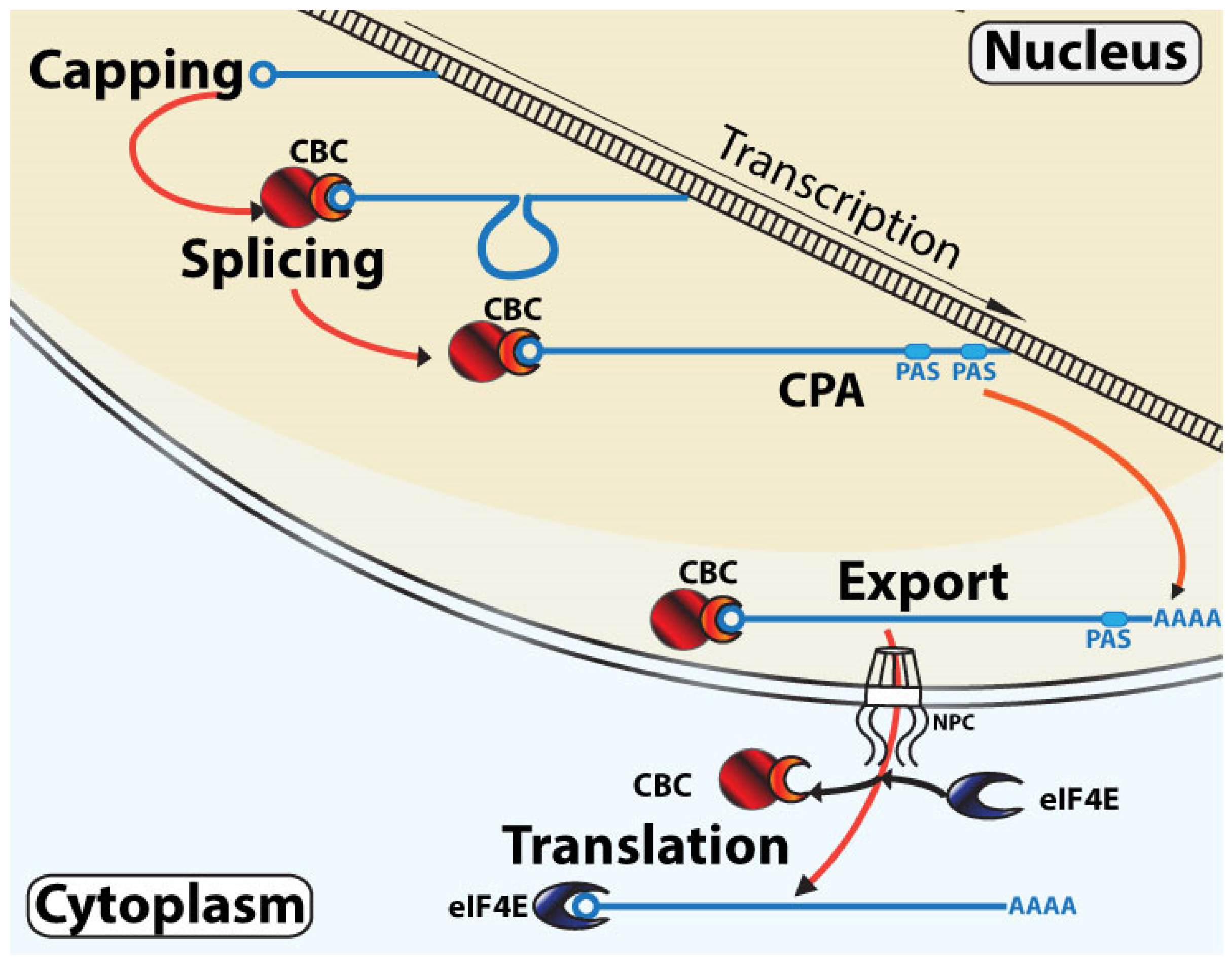
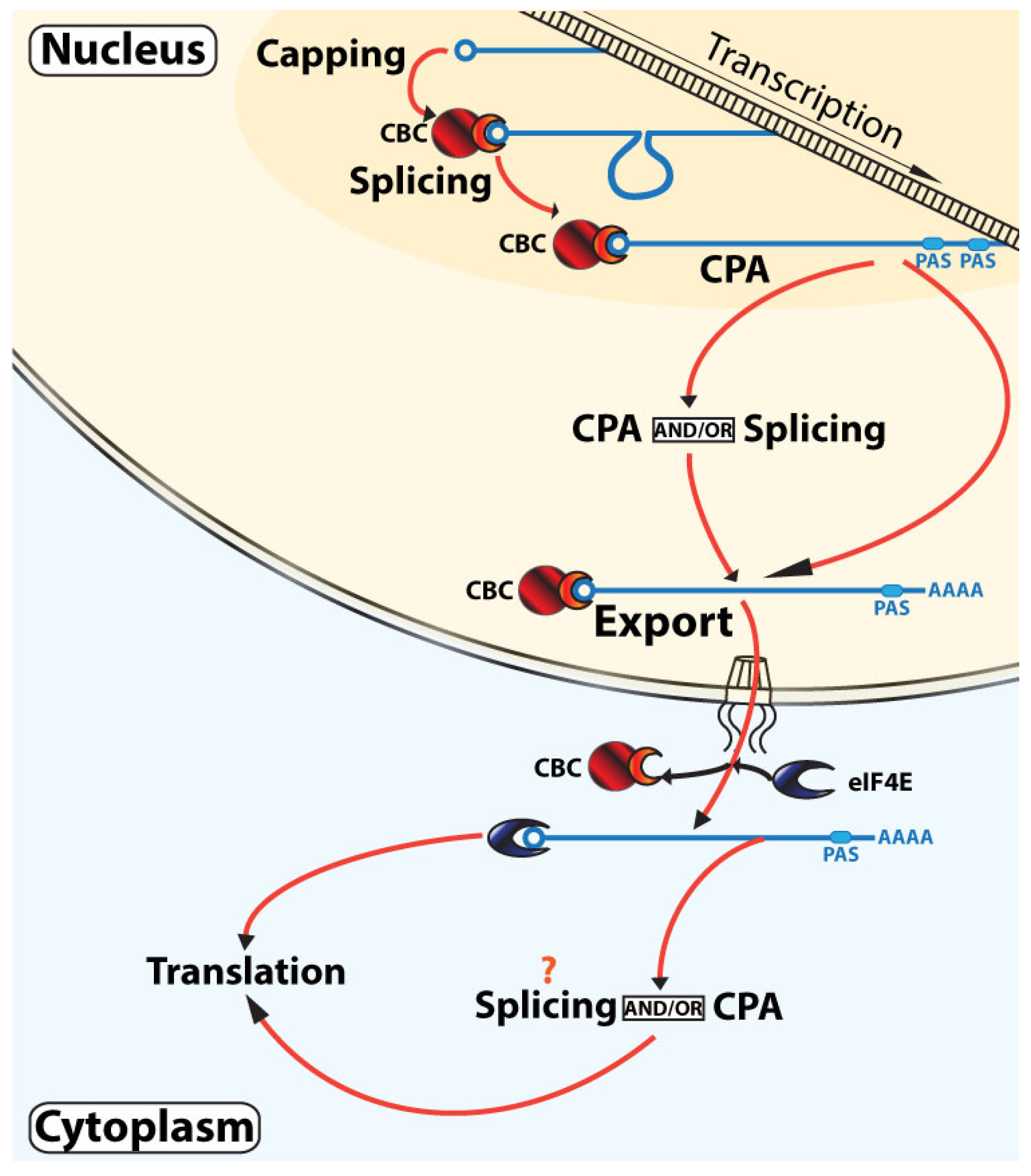
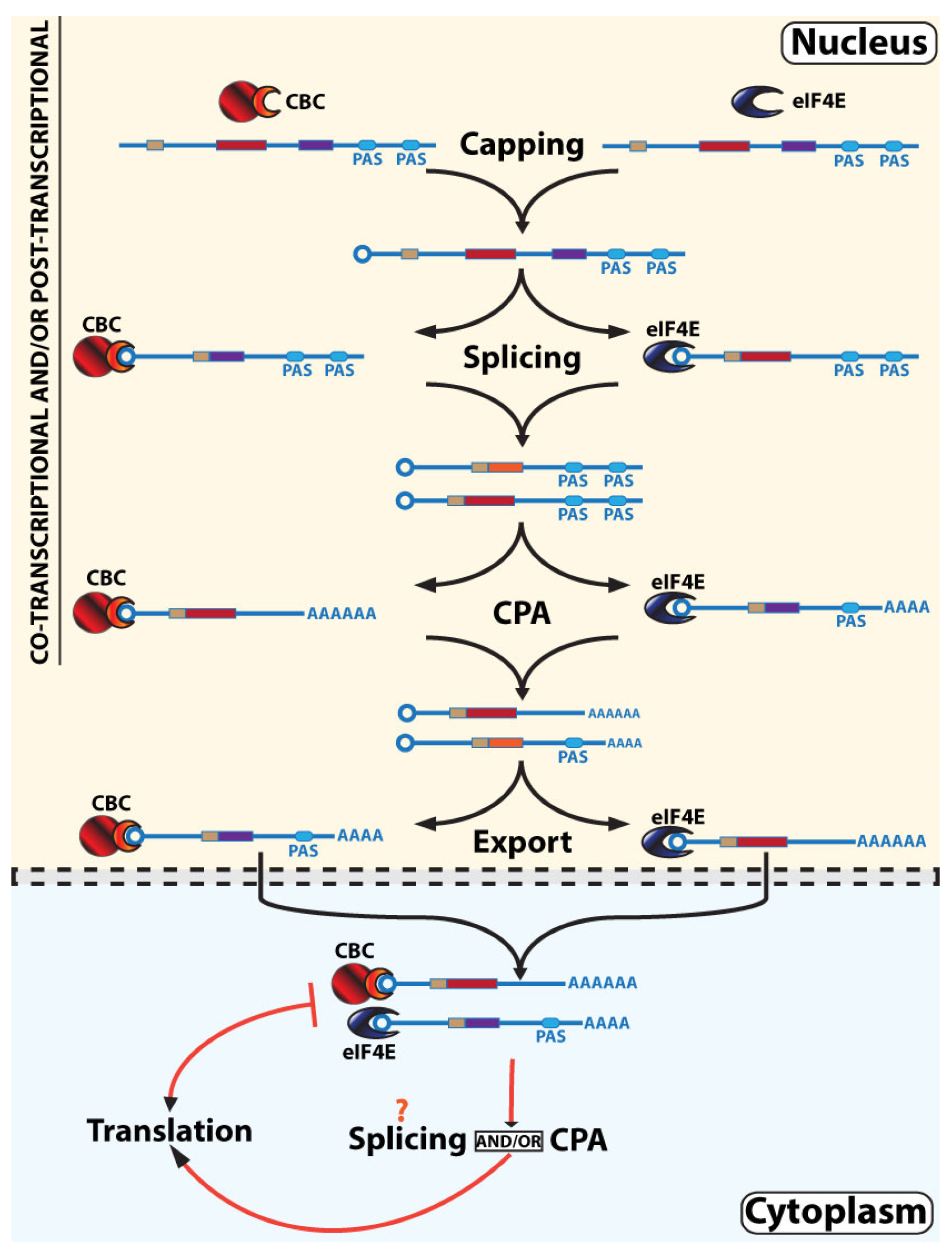
3. Models for RNA Maturation Based on These Observations and Their Interplay with Translation
3.1. Classical Linear RNA Maturation Model
3.2. Non-Linear RNA Maturation Model
3.3. Parallel, Non-Linear, Interleaved RNA Maturation Model
4. Dysregulation of Cap-Chaperones in Cancer
5. Open Questions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urbanski, L.M.; Leclair, N.; Anczukow, O. Alternative-splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip. Rev. RNA 2018, 9, e1476. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, N.K.; Shaji, F.; Koshre, G.R.; Laishram, R.S. Alternative polyadenylation: An enigma of transcript length variation in health and disease. Wiley Interdiscip. Rev. RNA 2021, e1692. [Google Scholar] [CrossRef]
- Stewart, M. Polyadenylation and nuclear export of mRNAs. J. Biol. Chem. 2019, 294, 2977–2987. [Google Scholar] [CrossRef]
- Borden, K.; Culjkovic-Kraljacic, B.; Cowling, V.H. To cap it all off, again: Dynamic capping and recapping of coding and non-coding RNAs to control transcript fate and biological activity. Cell Cycle 2021, 20, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Topisirovic, I.; Svitkin, Y.V.; Sonenberg, N.; Shatkin, A.J. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip. Rev. RNA 2011, 2, 277–298. [Google Scholar] [CrossRef]
- Gebhardt, A.; Habjan, M.; Benda, C.; Meiler, A.; Haas, D.A.; Hein, M.Y.; Mann, A.; Mann, M.; Habermann, B.; Pichlmair, A. mRNA export through an additional cap-binding complex consisting of NCBP1 and NCBP3. Nat. Commun. 2015, 6, 8192. [Google Scholar] [CrossRef]
- Lewis, J.D.; Izaurralde, E.; Jarmolowski, A.; McGuigan, C.; Mattaj, I.W. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5’ splice site. Genes Dev. 1996, 10, 1683–1698. [Google Scholar] [CrossRef] [PubMed]
- Pabis, M.; Neufeld, N.; Steiner, M.C.; Bojic, T.; Shav-Tal, Y.; Neugebauer, K.M. The nuclear cap-binding complex interacts with the U4/U6.U5 tri-snRNP and promotes spliceosome assembly in mammalian cells. RNA 2013, 19, 1054–1063. [Google Scholar] [CrossRef]
- Rambout, X.; Maquat, L.E. The nuclear cap-binding complex as choreographer of gene transcription and pre-mRNA processing. Genes Dev. 2020, 34, 1113–1127. [Google Scholar] [CrossRef]
- Visa, N.; Izaurralde, E.; Ferreira, J.; Daneholt, B.; Mattaj, I.W. A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J. Cell Biol. 1996, 133, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yilmaz, A.; Marsh, K.; Cochrane, A.; Boris-Lawrie, K. Thriving under stress: Selective translation of HIV-1 structural protein mRNA during Vpr-mediated impairment of eIF4E translation activity. PLoS Pathog. 2012, 8, e1002612. [Google Scholar] [CrossRef]
- Toro-Ascuy, D.; Rojas-Araya, B.; Garcia-de-Gracia, F.; Rojas-Fuentes, C.; Pereira-Montecinos, C.; Gaete-Argel, A.; Valiente-Echeverria, F.; Ohlmann, T.; Soto-Rifo, R. A Rev-CBP80-eIF4AI complex drives Gag synthesis from the HIV-1 unspliced mRNA. Nucleic Acids Res. 2018, 46, 11539–11552. [Google Scholar] [CrossRef]
- Ryu, I.; Kim, Y.K. Translation initiation mediated by nuclear cap-binding protein complex. BMB Rep. 2017, 50, 186–193. [Google Scholar] [CrossRef]
- Borden, K.L.B.; Volpon, L. The diversity, plasticity, and adaptability of cap-dependent translation initiation and the associated machinery. RNA Biol. 2020, 17, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic-Kraljacic, B.; Skrabanek, L.; Revuelta, M.V.; Gasiorek, J.; Cowling, V.H.; Cerchietti, L.; Borden, K.L.B. The eukaryotic translation initiation factor eIF4E elevates steady-state m(7)G capping of coding and noncoding transcripts. Proc. Natl. Acad. Sci. USA 2020, 117, 26773–26783. [Google Scholar] [CrossRef]
- Culjkovic-Kraljacic, B.; Baguet, A.; Volpon, L.; Amri, A.; Borden, K.L.B. The oncogene eIF4E reprograms the nuclear pore complext to promote mRNA export and oncogenic transformation. Cell Rep. 2012, 2, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic, B.; Topisirovic, I.; Skrabanek, L.; Ruiz-Gutierrez, M.; Borden, K.L. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3’UTR. J. Cell Biol. 2005, 169, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic, B.; Topisirovic, I.; Skrabanek, L.; Ruiz-Gutierrez, M.; Borden, K.L. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J. Cell Biol. 2006, 175, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Delaleau, M.; Borden, K.L.B. Nuclear eIF4E Stimulates 3’-End Cleavage of Target RNAs. Cell Rep. 2019, 27, 1397–1408 e1394. [Google Scholar] [CrossRef] [PubMed]
- Volpon, L.; Culjkovic-Kraljacic, B.; Sohn, H.S.; Blanchet-Cohen, A.; Osborne, M.J.; Borden, K.L.B. A biochemical framework for eIF4E-dependent mRNA export and nuclear recycling of the export machinery. RNA 2017, 23, 927–937. [Google Scholar] [CrossRef]
- Ghram, M.; Morris, G.; Culjkovic-Kraljacic, B.; Gendron, P.; Skrabanek, L.; Revuelta, M.V.; Cercheitti, L.; Guzman, M.L.; Borden, K.L.B. The eukaryotic translation initiation factor eIF4E reprogrammes the splicing machinery and drives alternative splicing. biorXiv 2021. [Google Scholar] [CrossRef]
- Lejbkowicz, F.; Goyer, C.; Darveau, A.; Neron, S.; Lemieux, R.; Sonenberg, N. A fraction of the mRNA 5’ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc. Natl. Acad. Sci. USA 1992, 89, 9612–9616. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Sharma, M.; Kentsis, A.; Perez, J.M.; Strudwick, S.; Borden, K.L. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J. 2001, 20, 4547–4559. [Google Scholar] [CrossRef] [PubMed]
- Volpon, L.; Culjkovic-Kraljacic, B.; Osborne, M.J.; Ramteke, A.; Sun, Q.; Niesman, A.; Chook, Y.M.; Borden, K.L. Importin 8 mediates m7G cap-sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. Proc. Natl. Acad. Sci. USA 2016, 113, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, J.; Hwang, H.J.; Kim, L.; Jeong, K.; Song, H.K.; Rufener, S.C.; Muhlemann, O.; Kim, Y.K. Translation mediated by the nuclear cap-binding complex is confined to the perinuclear region via a CTIF-DDX19B interaction. Nucleic Acids Res. 2021, 49, 8261–8276. [Google Scholar] [CrossRef]
- Proudfoot, N.J.; Furger, A.; Dye, M.J. Integrating mRNA processing with transcription. Cell 2002, 108, 501–512. [Google Scholar] [CrossRef]
- Culjkovic-Kraljacic, B.; Borden, K.L. Aiding and abetting cancer: mRNA export and the nuclear pore. Trends Cell Biol. 2013, 23, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Natalizio, B.J.; Wente, S.R. Postage for the messenger: Designating routes for nuclear mRNA export. Trends Cell Biol. 2013, 23, 365–373. [Google Scholar] [CrossRef]
- Grasso, L.; Suska, O.; Davidson, L.; Gonatopoulos-Pournatzis, T.; Williamson, R.; Wasmus, L.; Wiedlich, S.; Peggie, M.; Stavridis, M.P.; Cowling, V.H. mRNA Cap Methylation in Pluripotency and Differentiation. Cell Rep. 2016, 16, 1352–1365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galloway, A.; Cowling, V.H. mRNA cap regulation in mammalian cell function and fate. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 270–279. [Google Scholar] [CrossRef]
- Stevens, A. An exoribonuclease from Saccharomyces cerevisiae: Effect of modifications of 5’ end groups on the hydrolysis of substrates to 5’ mononucleotides. Biochem. Biophys. Res. Commun. 1978, 81, 656–661. [Google Scholar] [CrossRef]
- Ling, S.H.; Qamra, R.; Song, H. Structural and functional insights into eukaryotic mRNA decapping. Wiley Interdiscip. Rev. RNA 2011, 2, 193–208. [Google Scholar] [CrossRef]
- Pillutla, R.C.; Shimamoto, A.; Furuichi, Y.; Shatkin, A.J. Human mRNA capping enzyme (RNGTT) and cap methyltransferase (RNMT) map to 6q16 and 18p11.22-p11.23, respectively. Genomics 1998, 54, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Shatkin, A.J. Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes. Mol. Cell. Biol. 2008, 28, 5829–5836. [Google Scholar] [CrossRef][Green Version]
- Mao, X.; Schwer, B.; Shuman, S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol. Cell. Biol. 1995, 15, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Shafer, B.; Chu, C.; Shatkin, A.J. Human mRNA cap methyltransferase: Alternative nuclear localization signal motifs ensure nuclear localization required for viability. Mol. Cell. Biol. 2005, 25, 2644–2649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srinivasan, P.; Piano, F.; Shatkin, A.J. mRNA capping enzyme requirement for Caenorhabditis elegans viability. J. Biol. Chem. 2003, 278, 14168–14173. [Google Scholar] [CrossRef] [PubMed]
- Cowling, V.H. Regulation of mRNA cap methylation. Biochem. J. 2010, 425, 295–302. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Shibagaki, Y.; Murakoshi, T.; Suzuki, M.; Nakamura, A.; Gotoh, H.; Mizumoto, K. Cloning and characterization of two human cDNAs encoding the mRNA capping enzyme. Biochem. Biophys. Res. Commun. 1998, 243, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Okabe, T.; Doi, R.; Shimmi, O.; Arisawa, M.; Yamada-Okabe, H. Isolation and characterization of a human cDNA for mRNA 5’-capping enzyme. Nucleic Acids Res. 1998, 26, 1700–1706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yue, Z.; Maldonado, E.; Pillutla, R.; Cho, H.; Reinberg, D.; Shatkin, A.J. Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc. Natl. Acad. Sci. USA 1997, 94, 12898–12903. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Das, K.; Tyminski, J.R.; Bauman, J.D.; Guan, R.; Qiu, W.; Montelione, G.T.; Arnold, E.; Shatkin, A.J. Structure of the guanylyltransferase domain of human mRNA capping enzyme. Proc. Natl. Acad. Sci. USA 2011, 108, 10104–10108. [Google Scholar] [CrossRef] [PubMed]
- Gonatopoulos-Pournatzis, T.; Dunn, S.; Bounds, R.; Cowling, V.H. RAM/Fam103a1 is required for mRNA cap methylation. Mol. Cell 2011, 44, 585–596. [Google Scholar] [CrossRef]
- Fabrega, C.; Hausmann, S.; Shen, V.; Shuman, S.; Lima, C.D. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol. Cell 2004, 13, 77–89. [Google Scholar] [CrossRef]
- Trotman, J.B.; Giltmier, A.J.; Mukherjee, C.; Schoenberg, D.R. RNA guanine-7 methyltransferase catalyzes the methylation of cytoplasmically recapped RNAs. Nucleic Acids Res. 2017, 45, 10726–10739. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Kedersha, N.L.; Schoenberg, D.R. Identification of a cytoplasmic complex that adds a cap onto 5’-monophosphate RNA. Mol. Cell. Biol. 2009, 29, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, D.R.; Maquat, L.E. Re-capping the message. Trends Biochem. Sci. 2009, 34, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Aregger, M.; Kaskar, A.; Varshney, D.; Fernandez-Sanchez, M.E.; Inesta-Vaquera, F.A.; Weidlich, S.; Cowling, V.H. CDK1-Cyclin B1 Activates RNMT, Coordinating mRNA Cap Methylation with G1 Phase Transcription. Mol. Cell 2016, 61, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Grudzien-Nogalska, E.; Kiledjian, M. New insights into decapping enzymes and selective mRNA decay. Wiley Interdiscip. Rev. RNA 2017, 8, e1379. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Patil, D.P.; Kennedy, B.A.; Bakthavachalu, B.; Bundschuh, R.; Schoenberg, D.R. Identification of cytoplasmic capping targets reveals a role for cap homeostasis in translation and mRNA stability. Cell Rep. 2012, 2, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Boutz, P.L.; Bhutkar, A.; Sharp, P.A. Detained introns are a novel, widespread class of post-transcriptionally spliced introns. Genes Dev. 2015, 29, 63–80. [Google Scholar] [CrossRef]
- Girard, C.; Will, C.L.; Peng, J.; Makarov, E.M.; Kastner, B.; Lemm, I.; Urlaub, H.; Hartmuth, K.; Luhrmann, R. Post-transcriptional spliceosomes are retained in nuclear speckles until splicing completion. Nat. Commun. 2012, 3, 994. [Google Scholar] [CrossRef]
- Prasanth, K.V.; Prasanth, S.G.; Xuan, Z.; Hearn, S.; Freier, S.M.; Bennett, C.F.; Zhang, M.Q.; Spector, D.L. Regulating gene expression through RNA nuclear retention. Cell 2005, 123, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Gonatopoulos-Pournatzis, T.; Cowling, V.H. Cap-binding complex (CBC). Biochem. J. 2014, 457, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Riechmann, J.L.; Meyerowitz, E.M. Transcriptome-wide analysis of uncapped mRNAs in Arabidopsis reveals regulation of mRNA degradation. Plant. Cell 2008, 20, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Cowling, V.H. Enhanced mRNA cap methylation increases cyclin D1 expression and promotes cell transformation. Oncogene 2010, 29, 930–936. [Google Scholar] [CrossRef]
- Osborne, M.; Volpon, L.; Memarpooryazdi, M.; Thambipillai, A.; Czarnota, S.; Culjkovic-Kraljacic, B.; Tahran, C.; Oeffinger, M.; Cowling, V.H.; Borden, K.L.B. Identification and characterization of the interaction between the methyl-7-guanosine cap maturation enzyme RNMT and the cap-binding protein eIF4E. 2021; under review. [Google Scholar]
- Wahl, M.C.; Will, C.L.; Luhrmann, R. The spliceosome: Design principles of a dynamic RNP machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef]
- Effenberger, K.A.; Urabe, V.K.; Jurica, M.S. Modulating splicing with small molecular inhibitors of the spliceosome. Wiley Interdiscip. Rev. RNA 2017, 8, e1381. [Google Scholar] [CrossRef]
- Vargas, D.Y.; Shah, K.; Batish, M.; Levandoski, M.; Sinha, S.; Marras, S.A.; Schedl, P.; Tyagi, S. Single-molecule imaging of transcriptionally coupled and uncoupled splicing. Cell 2011, 147, 1054–1065. [Google Scholar] [CrossRef]
- Pandya-Jones, A.; Black, D.L. Co-transcriptional splicing of constitutive and alternative exons. RNA 2009, 15, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Turunen, J.J.; Niemela, E.H.; Verma, B.; Frilander, M.J. The significant other: Splicing by the minor spliceosome. Wiley Interdiscip. Rev. RNA 2013, 4, 61–76. [Google Scholar] [CrossRef]
- Bell, T.J.; Miyashiro, K.Y.; Sul, J.Y.; Buckley, P.T.; Lee, M.T.; McCullough, R.; Jochems, J.; Kim, J.; Cantor, C.R.; Parsons, T.D.; et al. Intron retention facilitates splice variant diversity in calcium-activated big potassium channel populations. Proc. Natl. Acad. Sci. USA 2010, 107, 21152–21157. [Google Scholar] [CrossRef]
- Gornemann, J.; Kotovic, K.M.; Hujer, K.; Neugebauer, K.M. Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol. Cell 2005, 19, 53–63. [Google Scholar] [CrossRef]
- O’Mullane, L.; Eperon, I.C. The pre-mRNA 5’ cap determines whether U6 small nuclear RNA succeeds U1 small nuclear ribonucleoprotein particle at 5’ splice sites. Mol. Cell. Biol. 1998, 18, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- Lenasi, T.; Peterlin, B.M.; Barboric, M. Cap-binding protein complex links pre-mRNA capping to transcription elongation and alternative splicing through positive transcription elongation factor b (P-TEFb). J. Biol. Chem. 2011, 286, 22758–22768. [Google Scholar] [CrossRef]
- Anczukow, O.; Akerman, M.; Clery, A.; Wu, J.; Shen, C.; Shirole, N.H.; Raimer, A.; Sun, S.; Jensen, M.A.; Hua, Y.; et al. SRSF1-Regulated Alternative Splicing in Breast Cancer. Mol. Cell 2015, 60, 105–117. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Harvey, S.E.; Hu, X.; Cheng, C. RNA G-quadruplex secondary structure promotes alternative splicing via the RNA-binding protein hnRNPF. Genes Dev. 2017, 31, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Izaurralde, E.; Lewis, J.; Gamberi, C.; Jarmolowski, A.; McGuigan, C.; Mattaj, I.W. A cap-binding protein complex mediating U snRNA export. Nature 1995, 376, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Segref, A.; Bachi, A.; Wilm, M.; Mattaj, I.W. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 2000, 101, 187–198. [Google Scholar] [CrossRef]
- Segref, A.; Mattaj, I.W.; Ohno, M. The evolutionarily conserved region of the U snRNA export mediator PHAX is a novel RNA-binding domain that is essential for U snRNA export. RNA 2001, 7, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kuzuoglu-Ozturk, D.; Hu, Z.; Rama, M.; Devericks, E.; Weiss, J.; Chiang, G.G.; Worland, S.T.; Brenner, S.E.; Goodarzi, H.; Gilbert, L.A.; et al. Revealing molecular pathways for cancer cell fitness through a genetic screen of the cancer translatome. Cell Rep. 2021, 35, 109321. [Google Scholar] [CrossRef]
- Proudfoot, N.J. Ending the message: Poly(A) signals then and now. Genes Dev. 2011, 25, 1770–1782. [Google Scholar] [CrossRef]
- Curinha, A.; Oliveira Braz, S.; Pereira-Castro, I.; Cruz, A.; Moreira, A. Implications of polyadenylation in health and disease. Nucleus 2014, 5, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.R.; Martin, G.; Keller, W.; Zavolan, M. Means to an end: Mechanisms of alternative polyadenylation of messenger RNA precursors. Wiley Interdiscip. Rev. RNA 2014, 5, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Manley, J.L. Alternative polyadenylation of mRNA precursors. Nat. Rev. Mol. Cell Biol. 2017, 18, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Malka, Y.; Steiman-Shimony, A.; Rosenthal, E.; Argaman, L.; Cohen-Daniel, L.; Arbib, E.; Margalit, H.; Kaplan, T.; Berger, M. Post-transcriptional 3-UTR cleavage of mRNA transcripts generates thousands of stable uncapped autonomous RNA fragments. Nat. Commun. 2017, 8, 2029. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.; Miura, P. Emerging Roles for 3’ UTRs in Neurons. Int. J. Mol. Sci. 2020, 21, 3413. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, C.; Luisier, R.; Crerar, H.; Darsinou, M.; Blokzijl-Franke, S.; Lenn, T.; Luscombe, N.M.; Cuda, G.; Gaspari, M.; Saiardi, A.; et al. Cytoplasmic cleavage of IMPA1 3’ UTR is necessary for maintaining axon integrity. Cell Rep. 2021, 34, 108778. [Google Scholar] [CrossRef]
- Narita, T.; Yung, T.M.; Yamamoto, J.; Tsuboi, Y.; Tanabe, H.; Tanaka, K.; Yamaguchi, Y.; Handa, H. NELF interacts with CBC and participates in 3’ end processing of replication-dependent histone mRNAs. Mol. Cell 2007, 26, 349–365. [Google Scholar] [CrossRef]
- Gruber, J.J.; Olejniczak, S.H.; Yong, J.; La Rocca, G.; Dreyfuss, G.; Thompson, C.B. Ars2 promotes proper replication-dependent histone mRNA 3’ end formation. Mol. Cell 2012, 45, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hallais, M.; Pontvianne, F.; Andersen, P.R.; Clerici, M.; Lener, D.; Benbahouche Nel, H.; Gostan, T.; Vandermoere, F.; Robert, M.C.; Cusack, S.; et al. CBC-ARS2 stimulates 3’-end maturation of multiple RNA families and favors cap-proximal processing. Nat. Struct. Mol. Biol. 2013, 20, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Christie, J.; Pienaar, M.; Gambling, J.; Nickerson, P.E.; Alford, S.C.; Chow, R.L.; Howard, P.L. Mutagenesis of ARS2 Domains to Assess Possible Roles in Cell Cycle Progression and MicroRNA and Replication-Dependent Histone mRNA Biogenesis. Mol. Cell. Biol. 2015, 35, 3753–3767. [Google Scholar] [CrossRef] [PubMed]
- So, B.R.; Di, C.; Cai, Z.; Venters, C.C.; Guo, J.; Oh, J.M.; Arai, C.; Dreyfuss, G. A Complex of U1 snRNP with Cleavage and Polyadenylation Factors Controls Telescripting, Regulating mRNA Transcription in Human Cells. Mol. Cell 2019, 76, 590–599.e4. [Google Scholar] [CrossRef] [PubMed]
- Jarmolowski, A.; Boelens, W.C.; Izaurralde, E.; Mattaj, I.W. Nuclear export of different classes of RNA is mediated by specific factors. J. Cell Biol. 1994, 124, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Fornerod, M.; Ohno, M.; Yoshida, M.; Mattaj, I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 1997, 90, 1051–1060. [Google Scholar] [CrossRef]
- Reed, R.; Hurt, E. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 2002, 108, 523–531. [Google Scholar] [CrossRef]
- Katahira, J. mRNA export and the TREX complex. Biochim. Biophys. Acta 2012, 1819, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, B.M.; Dales, S.; Blobel, G.; Zhong, H. The nucleoporin Nup98 associates with the intranuclear filamentous protein network of TPR. Proc. Natl. Acad. Sci. USA 2001, 98, 3208–3213. [Google Scholar] [CrossRef]
- Enninga, J.; Levy, D.E.; Blobel, G.; Fontoura, B.M. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 2002, 295, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Seo, H.S.; Blobel, G.; Hoelz, A. Structural and functional analysis of the interaction between the nucleoporin Nup98 and the mRNA export factor Rae1. Proc. Natl. Acad. Sci. USA 2010, 107, 10406–10411. [Google Scholar] [CrossRef]
- Dufu, K.; Livingstone, M.J.; Seebacher, J.; Gygi, S.P.; Wilson, S.A.; Reed, R. ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex. Genes Dev. 2010, 24, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Wu, X.; He, Z.; Wang, L.; Yin, S.; Tian, B.; Li, G.; Cheng, H. ALYREF mainly binds to the 5’ and the 3’ regions of the mRNA in vivo. Nucleic Acids Res. 2017, 45, 9640–9653. [Google Scholar] [CrossRef] [PubMed]
- Rufener, S.C.; Muhlemann, O. eIF4E-bound mRNPs are substrates for nonsense-mediated mRNA decay in mammalian cells. Nat. Struct. Mol. Biol. 2013, 20, 710–717. [Google Scholar] [CrossRef]
- Calero, G.; Wilson, K.F.; Ly, T.; Rios-Steiner, J.L.; Clardy, J.C.; Cerione, R.A. Structural basis of m7GpppG binding to the nuclear cap-binding protein complex. Nat. Struct. Biol. 2002, 9, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Dufu, K.; Lee, C.S.; Hsu, J.L.; Dias, A.; Reed, R. Human mRNA export machinery recruited to the 5’ end of mRNA. Cell 2006, 127, 1389–1400. [Google Scholar] [CrossRef]
- Dou, Y.; Barbosa, I.; Jiang, H.; Iasillo, C.; Molloy, K.R.; Schulze, W.M.; Cusack, S.; Schmid, M.; Le Hir, H.; LaCava, J.; et al. NCBP3 positively impacts mRNA biogenesis. Nucleic Acids Res. 2020, 48, 10413–10427. [Google Scholar] [CrossRef] [PubMed]
- Rambout, X.; Maquat, L.E. NCBP3: A Multifaceted Adaptive Regulator of Gene Expression. Trends Biochem. Sci. 2021, 46, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.M.; Cusack, S. Structural basis for mutually exclusive co-transcriptional nuclear cap-binding complexes with either NELF-E or ARS2. Nat. Commun. 2017, 8, 1302. [Google Scholar] [CrossRef] [PubMed]
- Topisirovic, I.; Siddiqui, N.; Lapointe, V.L.; Trost, M.; Thibault, P.; Bangeranye, C.; Pinol-Roma, S.; Borden, K.L. Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export-competent RNP. EMBO J. 2009, 28, 1087–1098. [Google Scholar] [CrossRef]
- Kudo, N.; Matsumori, N.; Taoka, H.; Fujiwara, D.; Schreiner, E.P.; Wolff, B.; Yoshida, M.; Horinouchi, S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 1999, 96, 9112–9117. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Topisirovic, I. Signaling Pathways Involved in the Regulation of mRNA Translation. Mol. Cell. Biol. 2018, 38, e00070-18. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Dinman, J.D.; Green, R. Translation Elongation and Recoding in Eukaryotes. Cold Spring Harb. Perspect. Biol 2018, 10. [Google Scholar] [CrossRef]
- Borden, K.L. The eukaryotic translation initiation factor eIF4E wears a “cap” for many occasions. Translation 2016, 4, e1220899. [Google Scholar] [CrossRef]
- Smith, R.C.L.; Kanellos, G.; Vlahov, N.; Alexandrou, C.; Willis, A.E.; Knight, J.R.P.; Sansom, O.J. Translation initiation in cancer at a glance. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef] [PubMed]
- Kapp, L.D.; Lorsch, J.R. The molecular mechanics of eukaryotic translation. Annu. Rev. Biochem. 2004, 73, 657–704. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014, 83, 779–812. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Aashaq, S.; Andrabi, K.I. Eukaryotic initiation factor 4E (eIF4E): A recap of the cap-binding protein. J. Cell Biochem. 2019, 120, 14201–14212. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Roman, A.R.; Wente, S.R. Inositol polyphosphates: A new frontier for regulating gene expression. Chromosoma 2008, 117, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.; Siepmann, A.; Sturm, D.; Windgassen, M.; Scarcelli, J.J.; Seedorf, M.; Cole, C.N.; Krebber, H. The DEAD-box RNA helicase Dbp5 functions in translation termination. Science 2007, 315, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Li, X.; Serin, G.; Maquat, L.E. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 2001, 106, 607–617. [Google Scholar] [CrossRef]
- Maquat, L.E.; Tarn, W.Y.; Isken, O. The pioneer round of translation: Features and functions. Cell 2010, 142, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, J.; Kim, Y.K. Crosstalk between translation and the aggresome-autophagy pathway. Autophagy 2018, 14, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Koritzinsky, M.; Magagnin, M.G.; van den Beucken, T.; Seigneuric, R.; Savelkouls, K.; Dostie, J.; Pyronnet, S.; Kaufman, R.J.; Weppler, S.A.; Voncken, J.W.; et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006, 25, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Uniacke, J.; Perera, J.K.; Lachance, G.; Francisco, C.B.; Lee, S. Cancer cells exploit eIF4E2-directed synthesis of hypoxia response proteins to drive tumor progression. Cancer Res. 2014, 74, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Kim, K.M.; Cho, H.; Choe, J.; Kim, Y.K. Pioneer round of translation occurs during serum starvation. Biochem. Biophys. Res. Commun. 2007, 362, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Allen, G.E.; Kloehn, J.; Abid, K.; Jaquier-Gubler, P.; Curran, J.A. eIF4E3 forms an active eIF4F complex during stresses (eIF4FS) targeting mTOR and re-programs the translatome. Nucleic Acids Res. 2021, 49, 5159–5176. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Ryu, I.; Park, J.; Hwang, H.J.; Ha, H.; Park, Y.; Oh, S.T.; Kim, Y.K. Staufen1 and UPF1 exert opposite actions on the replacement of the nuclear cap-binding complex by eIF4E at the 5’ end of mRNAs. Nucleic Acids Res. 2019, 47, 9313–9328. [Google Scholar] [CrossRef] [PubMed]
- Garre, E.; Romero-Santacreu, L.; De Clercq, N.; Blasco-Angulo, N.; Sunnerhagen, P.; Alepuz, P. Yeast mRNA cap-binding protein Cbc1/Sto1 is necessary for the rapid reprogramming of translation after hyperosmotic shock. Mol. Biol. Cell 2012, 23, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Kim, K.M.; Park, S.; Lee, Y.K.; Song, O.K.; Kim, M.K.; Lee, B.G.; Song, H.K.; Kim, Y.K. Rapid degradation of replication-dependent histone mRNAs largely occurs on mRNAs bound by nuclear cap-binding proteins 80 and 20. Nucleic Acids Res. 2013, 41, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Ryu, I.; Park, O.H.; Park, J.; Cho, H.; Yoo, J.S.; Chi, S.W.; Kim, M.K.; Song, H.K.; Kim, Y.K. eIF4AIII enhances translation of nuclear cap-binding complex-bound mRNAs by promoting disruption of secondary structures in 5’UTR. Proc. Natl. Acad. Sci. USA 2014, 111, E4577–E4586. [Google Scholar] [CrossRef]
- Kim, K.M.; Cho, H.; Choi, K.; Kim, J.; Kim, B.W.; Ko, Y.G.; Jang, S.K.; Kim, Y.K. A new MIF4G domain-containing protein, CTIF, directs nuclear cap-binding protein CBP80/20-dependent translation. Genes Dev. 2009, 23, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Su, D.; Scheliga, J.S.; Pluskal, T.; Boronat, S.; Motamedchaboki, K.; Campos, A.R.; Qi, F.; Hidalgo, E.; Yanagida, M.; et al. A Transcript-Specific eIF3 Complex Mediates Global Translational Control of Energy Metabolism. Cell Rep. 2016, 16, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Kranzusch, P.J.; Doudna, J.A.; Cate, J.H. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature 2016, 536, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Guo, Y. Knockdown of eIF3D inhibits breast cancer cell proliferation and invasion through suppressing the Wnt/beta-catenin signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 10420–10427. [Google Scholar]
- de la Parra, C.; Ernlund, A.; Alard, A.; Ruggles, K.; Ueberheide, B.; Schneider, R.J. A widespread alternate form of cap-dependent mRNA translation initiation. Nat. Commun. 2018, 9, 3068. [Google Scholar] [CrossRef] [PubMed]
- Lahr, R.M.; Fonseca, B.D.; Ciotti, G.E.; Al-Ashtal, H.A.; Jia, J.J.; Niklaus, M.R.; Blagden, S.P.; Alain, T.; Berman, A.J. La-related protein 1 (LARP1) binds the mRNA cap, blocking eIF4F assembly on TOP mRNAs. Elife 2017, 6, e24146. [Google Scholar] [CrossRef]
- Izaurralde, E.; Lewis, J.; McGuigan, C.; Jankowska, M.; Darzynkiewicz, E.; Mattaj, I.W. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell 1994, 78, 657–668. [Google Scholar] [CrossRef]
- Lewis, J.D.; Izaurralde, E. The role of the cap structure in RNA processing and nuclear export. Eur. J. Biochem. 1997, 247, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Dostie, J.; Lejbkowicz, F.; Sonenberg, N. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J. Cell Biol. 2000, 148, 239–247. [Google Scholar] [CrossRef]
- Topisirovic, I.; Guzman, M.L.; McConnell, M.J.; Licht, J.D.; Culjkovic, B.; Neering, S.J.; Jordan, C.T.; Borden, K.L. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol. Cell. Biol. 2003, 23, 8992–9002. [Google Scholar] [CrossRef] [PubMed]
- Topisirovic, I.; Culjkovic, B.; Cohen, N.; Perez, J.M.; Skrabanek, L.; Borden, K.L. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J. 2003, 22, 689–703. [Google Scholar] [CrossRef]
- Lejeune, F.; Ishigaki, Y.; Li, X.; Maquat, L.E. The exon junction complex is detected on CBP80-bound but not eIF4E-bound mRNA in mammalian cells: Dynamics of mRNP remodeling. EMBO J. 2002, 21, 3536–3545. [Google Scholar] [CrossRef]
- Matsuda, D.; Hosoda, N.; Kim, Y.K.; Maquat, L.E. Failsafe nonsense-mediated mRNA decay does not detectably target eIF4E-bound mRNA. Nat. Struct. Mol. Biol. 2007, 14, 974–979. [Google Scholar] [CrossRef]
- Bisogno, L.S.; Keene, J.D. RNA regulons in cancer and inflammation. Curr. Opin. Genet. Dev. 2018, 48, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Keene, J.D. RNA regulons: Coordination of post-transcriptional events. Nat. Rev. Genet. 2007, 8, 533–543. [Google Scholar] [CrossRef]
- Keene, J.D.; Lager, P.J. Post-transcriptional operons and regulons co-ordinating gene expression. Chromosome Res. 2005, 13, 327–337. [Google Scholar] [CrossRef]
- Coutinho de Oliveira, L.; Volpon, L.; Rahardjo, A.K.; Osborne, M.J.; Culjkovic-Kraljacic, B.; Trahan, C.; Oeffinger, M.; Kwok, B.H.; Borden, K.L.B. Structural studies of the eIF4E-VPg complex reveal a direct competition for capped RNA: Implications for translation. Proc. Natl. Acad. Sci. USA 2019, 116, 24056–24065. [Google Scholar] [CrossRef]
- Wang, J.; Alvin Chew, B.L.; Lai, Y.; Dong, H.; Xu, L.; Balamkundu, S.; Cai, W.M.; Cui, L.; Liu, C.F.; Fu, X.Y.; et al. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res. 2019, 47, e130. [Google Scholar] [CrossRef]
- Carroll, M.; Borden, K.L. The oncogene eIF4E: Using biochemical insights to target cancer. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2013, 33, 227–238. [Google Scholar] [CrossRef]
- Culjkovic, B.; Borden, K.L. Understanding and Targeting the Eukaryotic Translation Initiation Factor eIF4E in Head and Neck Cancer. J. Oncol. 2009, 2009, 981679. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.A.; Culjkovic-Kraljacic, B.; Emond, A.; Pettersson, F.; Midura, R.; Lauer, M.; Del Rincon, S.; Cali, V.; Assouline, S.; Miller, W.H.; et al. The eukaryotic translation initiation factor eIF4E harnesses hyaluronan production to drive its malignant activity. Elife 2017, 6, e29830. [Google Scholar] [CrossRef]
- Truitt, M.L.; Conn, C.S.; Shi, Z.; Pang, X.; Tokuyasu, T.; Coady, A.M.; Seo, Y.; Barna, M.; Ruggero, D. Differential Requirements for eIF4E Dose in Normal Development and Cancer. Cell 2015, 162, 59–71. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, A.; Harris, A.L. eIF4E expression in tumors: Its possible role in progression and malignacies. Int. J. Biochem. Cell Biol. 1999, 31, 59–72. [Google Scholar] [CrossRef]
- Graff, J.R.; Boghaert, E.R.; De Benedetti, A.; Tudor, D.L.; Zimmer, C.C.; Chan, S.K.; Zimmer, S.G. Reduction of translation initiation factor 4E decreases the malignancy of ras-transformed cloned rat embryo fibroblasts. Int. J. Cancer 1995, 60, 255–263. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Zhao, H.; Deng, J.; Long, Y.; Shuai, M.T.; Li, Q.; Gu, H.; Chen, Y.Q.; Leng, A.M. eIF4E promotes tumorigenesis and modulates chemosensitivity to cisplatin in esophageal squamous cell carcinoma. Oncotarget 2016, 7, 66851–66864. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, J.; Lu, C.; Mapara, M.Y.; Raza, S.; Hengst, U.; Lentzsch, S. Elevated Translation Initiation Factor eIF4E Is an Attractive Therapeutic Target in Multiple Myeloma. Mol. Cancer Ther. 2016, 15, 711–719. [Google Scholar] [CrossRef]
- Ruggero, D.; Montanaro, L.; Ma, L.; Xu, W.; Londei, P.; Cordon-Cardo, C.; Pandolfi, P.P. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat. Med. 2004, 10, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Wendel, H.G.; Silva, R.L.; Malina, A.; Mills, J.R.; Zhu, H.; Ueda, T.; Watanabe-Fukunaga, R.; Fukunaga, R.; Teruya-Feldstein, J.; Pelletier, J.; et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007, 21, 3232–3237. [Google Scholar] [CrossRef]
- Sonenberg, N.; Gingras, A.C. The mRNA 5’ cap-binding protein eIF4E and control of cell growth. Curr. Opin. Cell Biol. 1998, 10, 268–275. [Google Scholar] [CrossRef]
- Kentsis, A.; Dwyer, E.C.; Perez, J.M.; Sharma, M.; Chen, A.; Pan, Z.Q.; Borden, K.L. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J. Mol. Biol. 2001, 312, 609–623. [Google Scholar] [CrossRef]
- Rousseau, D.; Kaspar, R.; Rosenwald, I.; Gehrke, L.; Sonenberg, N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc. Natl. Acad. Sci. USA 1996, 93, 1065–1070. [Google Scholar]
- Culjkovic-Kraljacic, B.; Fernando, T.M.; Marullo, R.; Calvo-Vidal, N.; Verma, A.; Yang, S.; Tabbo, F.; Gaudiano, M.; Zahreddine, H.; Goldstein, R.L.; et al. Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B-cell lymphomas. Blood 2016, 127, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Culjkovic, B.; Cocolakis, E.; Rousseau, C.; Beslu, N.; Amri, A.; Caplan, S.; Leber, B.; Roy, D.C.; Miller, W.H., Jr.; et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): A proof-of-principle clinical trial with ribavirin. Blood 2009, 114, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R.; Konicek, B.W.; Vincent, T.M.; Lynch, R.L.; Monteith, D.; Weir, S.N.; Schwier, P.; Capen, A.; Goode, R.L.; Dowless, M.S.; et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Investig. 2007, 117, 2638–2648. [Google Scholar] [CrossRef]
- Pettersson, F.; Yau, C.; Dobocan, M.C.; Culjkovic-Kraljacic, B.; Retrouvey, H.; Puckett, R.; Flores, L.M.; Krop, I.E.; Rousseau, C.; Cocolakis, E.; et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2874–2884. [Google Scholar] [CrossRef]
- Urtishak, K.A.; Wang, L.S.; Culjkovic-Kraljacic, B.; Davenport, J.W.; Porazzi, P.; Vincent, T.L.; Teachey, D.T.; Tasian, S.K.; Moore, J.S.; Seif, A.E.; et al. Targeting EIF4E signaling with ribavirin in infant acute lymphoblastic leukemia. Oncogene 2019, 38, 2241–2262. [Google Scholar] [CrossRef]
- Kraljacic, B.C.; Arguello, M.; Amri, A.; Cormack, G.; Borden, K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia 2011, 25, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Culjkovic-Kraljacic, B.; Bergeron, J.; Caplan, S.; Cocolakis, E.; Lambert, C.; Lau, C.J.; Zahreddine, H.A.; Miller, W.H., Jr.; Borden, K.L. A phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica 2015, 100, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Cocolakis, E.; Borden, K.L. The Development of Novel Therapies for the Treatment of Acute Myeloid Leukemia (AML). Cancers 2012, 4, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.A.; Fury, M.G.; Sherman, E.J.; Ho, A.A.; Katabi, N.; Haque, S.S.; Pfister, D.G. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck 2017, 40, 233–241. [Google Scholar] [CrossRef]
- Zismanov, V.; Attar-Schneider, O.; Lishner, M.; Heffez Aizenfeld, R.; Tartakover Matalon, S.; Drucker, L. Multiple myeloma proteostasis can be targeted via translation initiation factor eIF4E. Int. J. Oncol. 2015, 46, 860–870. [Google Scholar] [CrossRef]
- Attar-Schneider, O.; Pasmanik-Chor, M.; Tartakover-Matalon, S.; Drucker, L.; Lishner, M. eIF4E and eIF4GI have distinct and differential imprints on multiple myeloma’s proteome and signaling. Oncotarget 2015, 6, 4315–4329. [Google Scholar] [CrossRef]
- Attar-Schneider, O.; Drucker, L.; Gottfried, M. Migration and epithelial-to-mesenchymal transition of lung cancer can be targeted via translation initiation factors eIF4E and eIF4GI. Lab. Investig. 2016, 96, 1004–1015. [Google Scholar] [CrossRef][Green Version]
- Pettersson, F.; del Rincon, S.V.; Emond, A.; Huor, B.; Ngan, E.; Ng, J.; Dobocan, M.C.; Siegel, P.M.; Miller, W.H. Genetic and pharmacolgic inhibition of eIF4E reduces breast cancer cell migration, invasion and metastasis. Cancer Res. 2015, 75, 1102–1112. [Google Scholar] [CrossRef]
- Kentsis, A.; Topisirovic, I.; Culjkovic, B.; Shao, L.; Borden, K.L. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc. Natl. Acad. Sci. USA 2004, 101, 18105–18110. [Google Scholar] [CrossRef]
- Yi, T.; Kabha, E.; Papadopoulos, E.; Wagner, G. 4EGI-1 targets breast cancer stem cells by selective inhibition of translation that persists in CSC maintenance, proliferation and metastasis. Oncotarget 2014, 5, 6028–6037. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Jacobson, B.A.; Peterson, M.S.; Jay-Dixon, J.; Kratzke, M.G.; Sadiq, A.A.; Patel, M.R.; Kratzke, R.A. 4EGI-1 represses cap-dependent translation and regulates genome-wide translation in malignant pleural mesothelioma. Investig. New Drugs 2018, 36, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Volpon, L.; Osborne, M.J.; Zahreddine, H.; Romeo, A.A.; Borden, K.L. Conformational changes induced in the eukaryotic translation initiation factor eIF4E by a clinically relevant inhibitor, ribavirin triphosphate. Biochem. Biophys. Res. Commun. 2013, 434, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Kentsis, A.; Volpon, L.; Topisirovic, I.; Soll, C.E.; Culjkovic, B.; Shao, L.; Borden, K.L. Further evidence that ribavirin interacts with eIF4E. RNA 2005, 11, 1762–1766. [Google Scholar] [CrossRef]
- Zahreddine, H.A.; Culjkovic-Kraljacic, B.; Assouline, S.; Gendron, P.; Romeo, A.A.; Morris, S.J.; Cormack, G.; Jaquith, J.B.; Cerchietti, L.; Cocolakis, E.; et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 2014, 511, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Maeda, T.; Shinojima, T.; Nagata, H.; Ryuchi, R.; Oya, M. A clinical study to evaluate the efficacy and safety of docetaxal with ribavirin in patients with progressive castration resistant prostate cancer who have previously received docetaxol alone. J. Clin. Oncol. 2017. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Oh, Y.; Wheler, J.; Naing, A.; Brail, L.; Callies, S.; Andre, V.; Kadam, S.K.; Nasir, A.; et al. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6582–6591. [Google Scholar] [CrossRef]
- Topisirovic, I.; Ruiz-Gutierrez, M.; Borden, K.L. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004, 64, 8639–8642. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Blaydes, J.P. MNK1 and EIF4E are downstream effectors of MEKs in the regulation of the nuclear export of HDM2 mRNA. Oncogene 2008, 27, 1645–1649. [Google Scholar] [CrossRef]
- Dinner, S.; Platanias, L.C. Targeting the mTOR Pathway in Leukemia. J. Cell. Biochem. 2016, 117, 1745–1752. [Google Scholar] [CrossRef]
- Joshi, S.; Platanias, L.C. Mnk kinases in cytokine signaling and regulation of cytokine responses. Biomol. Concepts 2015, 6, 85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, S.; Platanias, L.C. Mnk kinase pathway: Cellular functions and biological outcomes. World J. Biol. Chem. 2014, 5, 321–333. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, W.; Guan, X.; Xie, X.; Zhang, Y. CPSF3 is a promising prognostic biomarker and predicts recurrence of non-small cell lung cancer. Oncol. Lett. 2019, 18, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Ross, N.T.; Lohmann, F.; Carbonneau, S.; Fazal, A.; Weihofen, W.A.; Gleim, S.; Salcius, M.; Sigoillot, F.; Henault, M.; Carl, S.H.; et al. CPSF3-dependent pre-mRNA processing as a druggable node in AML and Ewing’s sarcoma. Nat. Chem. Biol. 2020, 16, 50–59. [Google Scholar] [CrossRef]
- Kakegawa, J.; Sakane, N.; Suzuki, K.; Yoshida, T. JTE-607, a multiple cytokine production inhibitor, targets CPSF3 and inhibits pre-mRNA processing. Biochem. Biophys. Res. Commun. 2019, 518, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, A.; Tan, Y.; Wang, S.; Ma, Q.; Chen, X.; He, Z. NCBP1 promotes the development of lung adenocarcinoma through up-regulation of CUL4B. J. Cell. Mol. Med. 2019, 23, 6965–6977. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mars, J.-C.; Ghram, M.; Culjkovic-Kraljacic, B.; Borden, K.L.B. The Cap-Binding Complex CBC and the Eukaryotic Translation Factor eIF4E: Co-Conspirators in Cap-Dependent RNA Maturation and Translation. Cancers 2021, 13, 6185. https://doi.org/10.3390/cancers13246185
Mars J-C, Ghram M, Culjkovic-Kraljacic B, Borden KLB. The Cap-Binding Complex CBC and the Eukaryotic Translation Factor eIF4E: Co-Conspirators in Cap-Dependent RNA Maturation and Translation. Cancers. 2021; 13(24):6185. https://doi.org/10.3390/cancers13246185
Chicago/Turabian StyleMars, Jean-Clement, Mehdi Ghram, Biljana Culjkovic-Kraljacic, and Katherine L. B. Borden. 2021. "The Cap-Binding Complex CBC and the Eukaryotic Translation Factor eIF4E: Co-Conspirators in Cap-Dependent RNA Maturation and Translation" Cancers 13, no. 24: 6185. https://doi.org/10.3390/cancers13246185
APA StyleMars, J.-C., Ghram, M., Culjkovic-Kraljacic, B., & Borden, K. L. B. (2021). The Cap-Binding Complex CBC and the Eukaryotic Translation Factor eIF4E: Co-Conspirators in Cap-Dependent RNA Maturation and Translation. Cancers, 13(24), 6185. https://doi.org/10.3390/cancers13246185





