New Radionuclides and Technological Advances in SPECT and PET Scanners
Abstract
:Simple Summary
Abstract
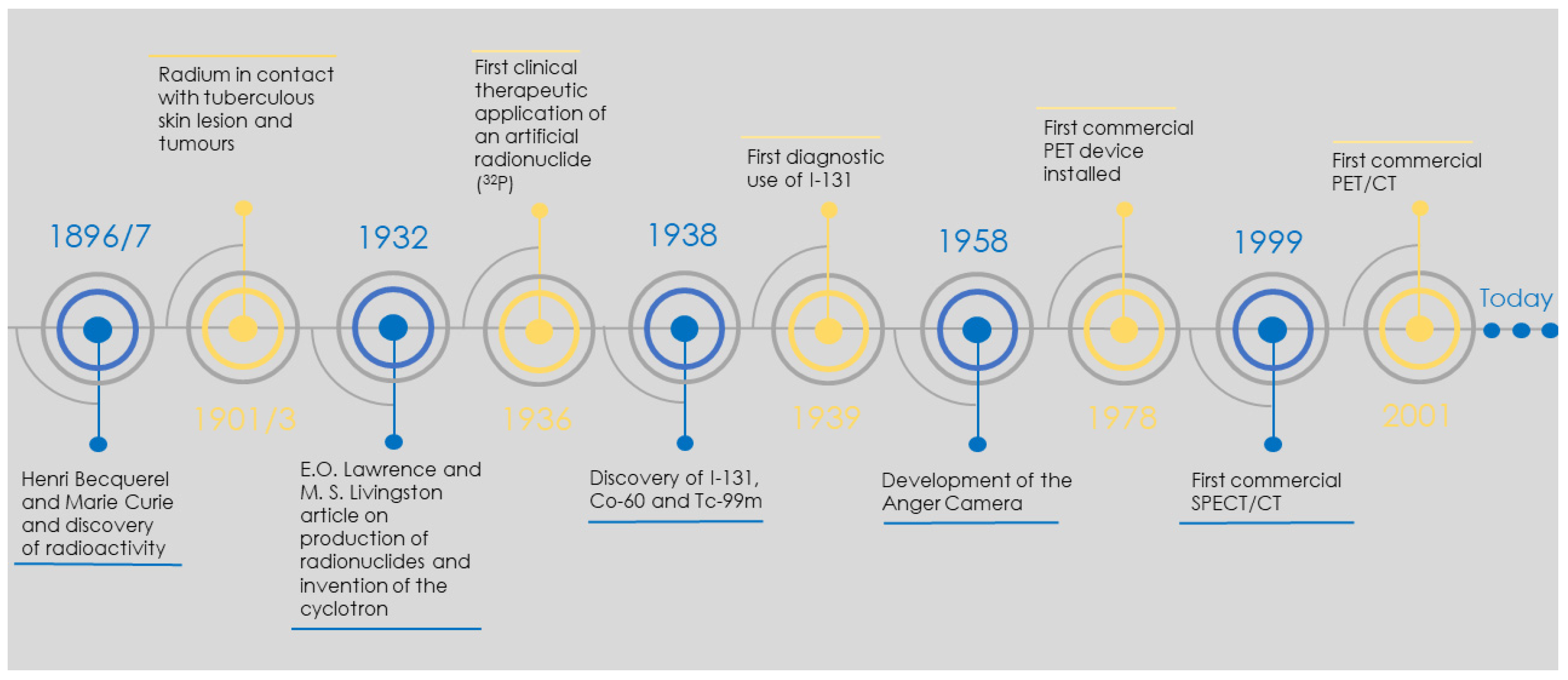
1. Introduction
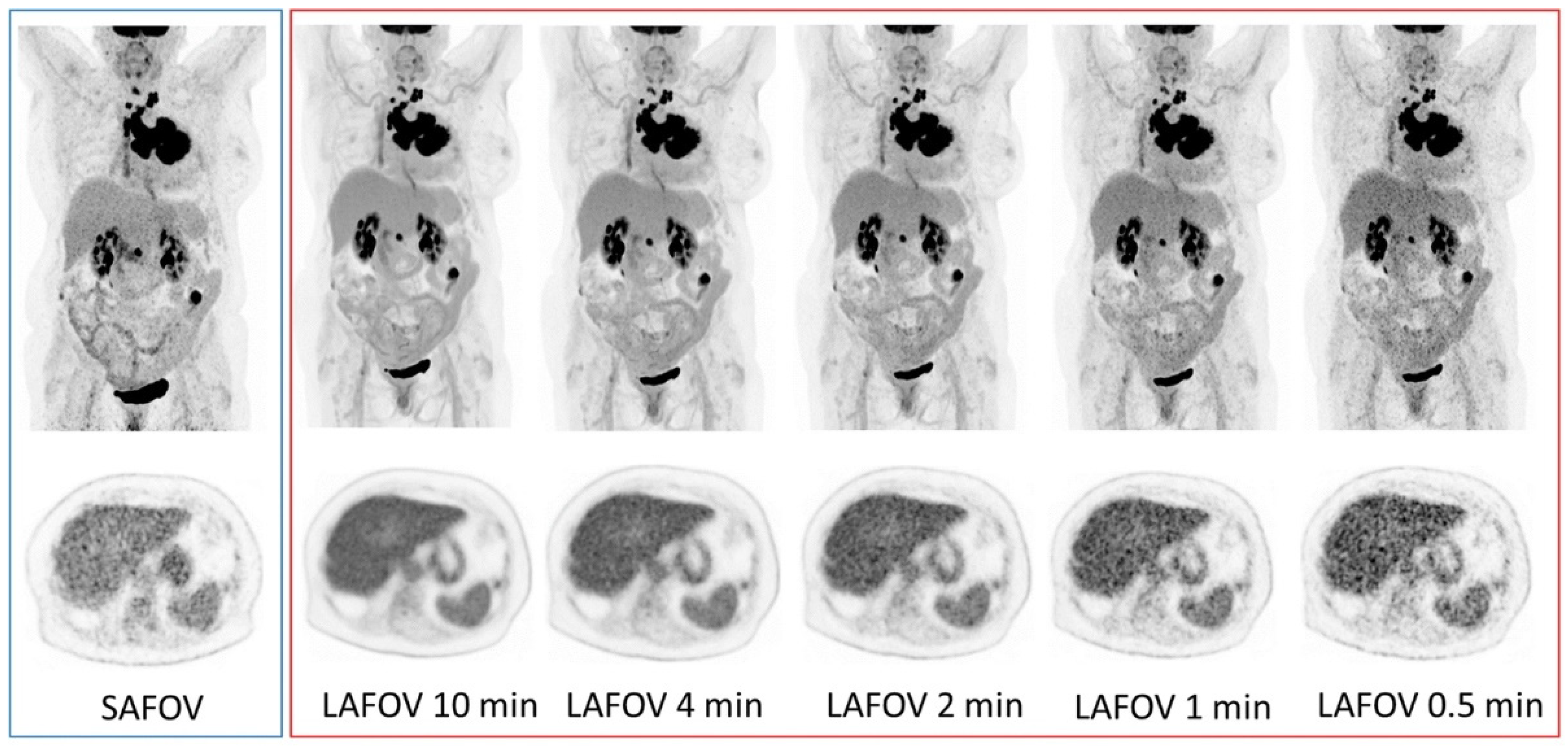
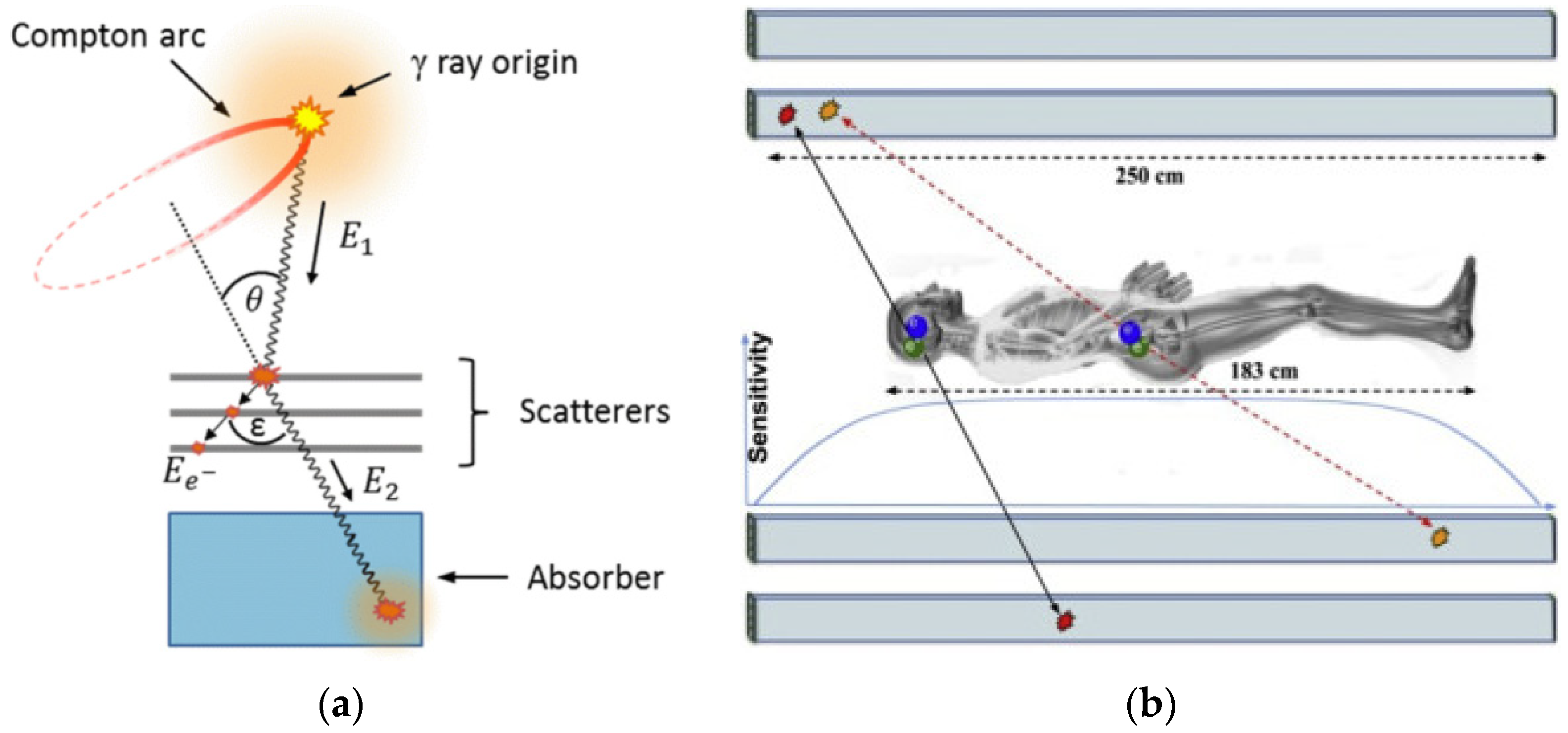
2. Instrumentation Developments
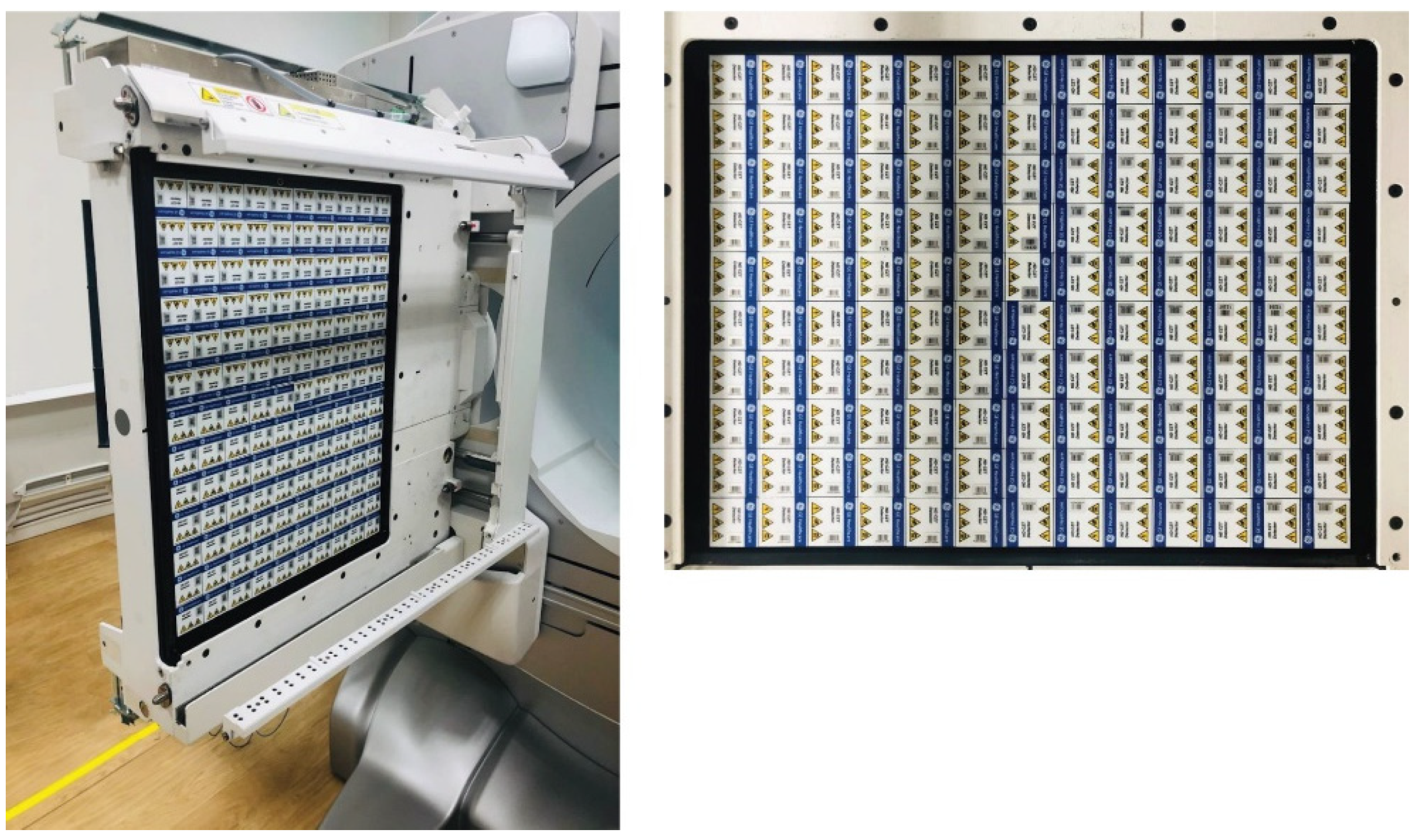
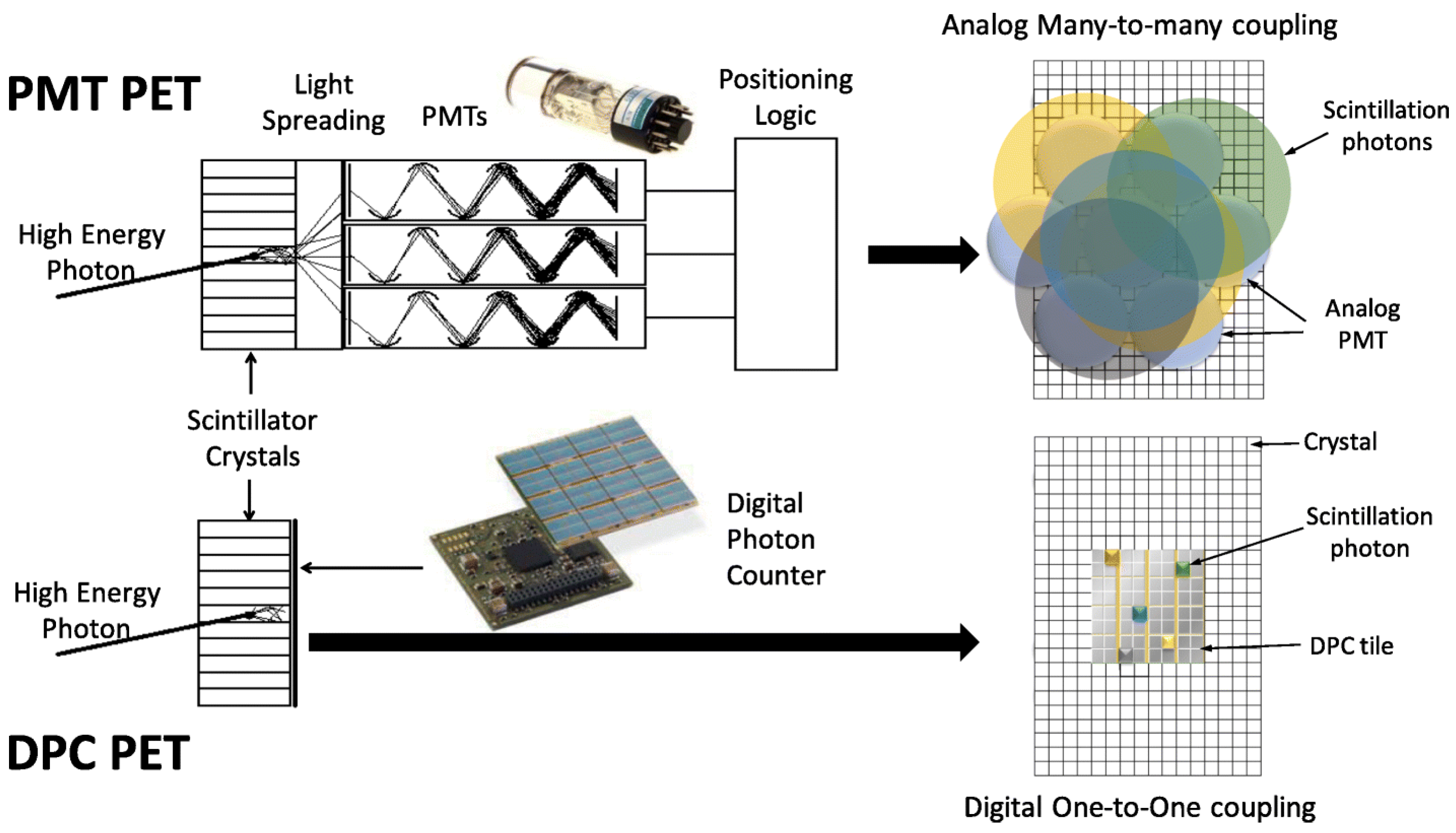
2.1. The Detector Revolution
2.2. New Arrangements
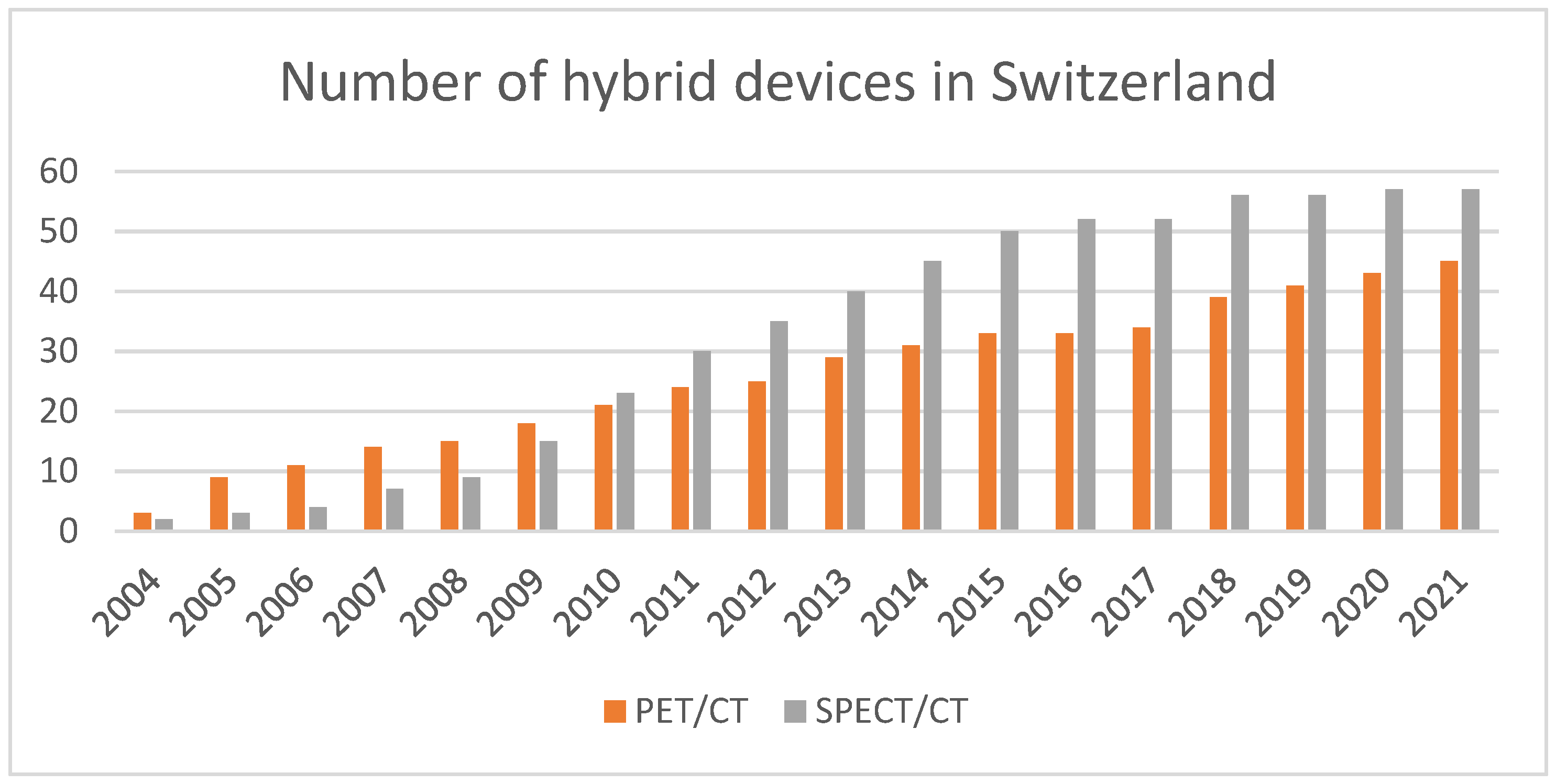
2.3. Modality Variation
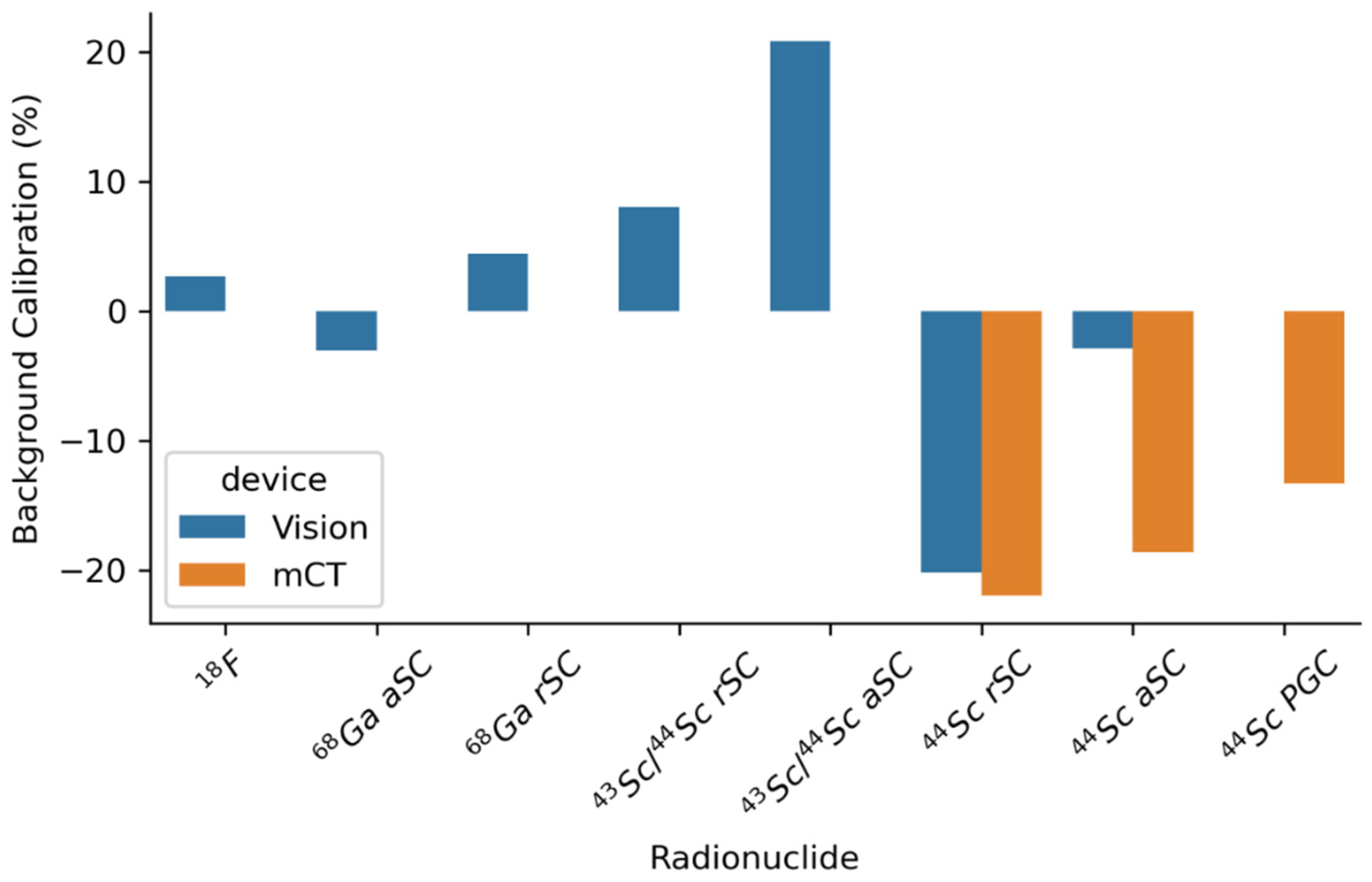
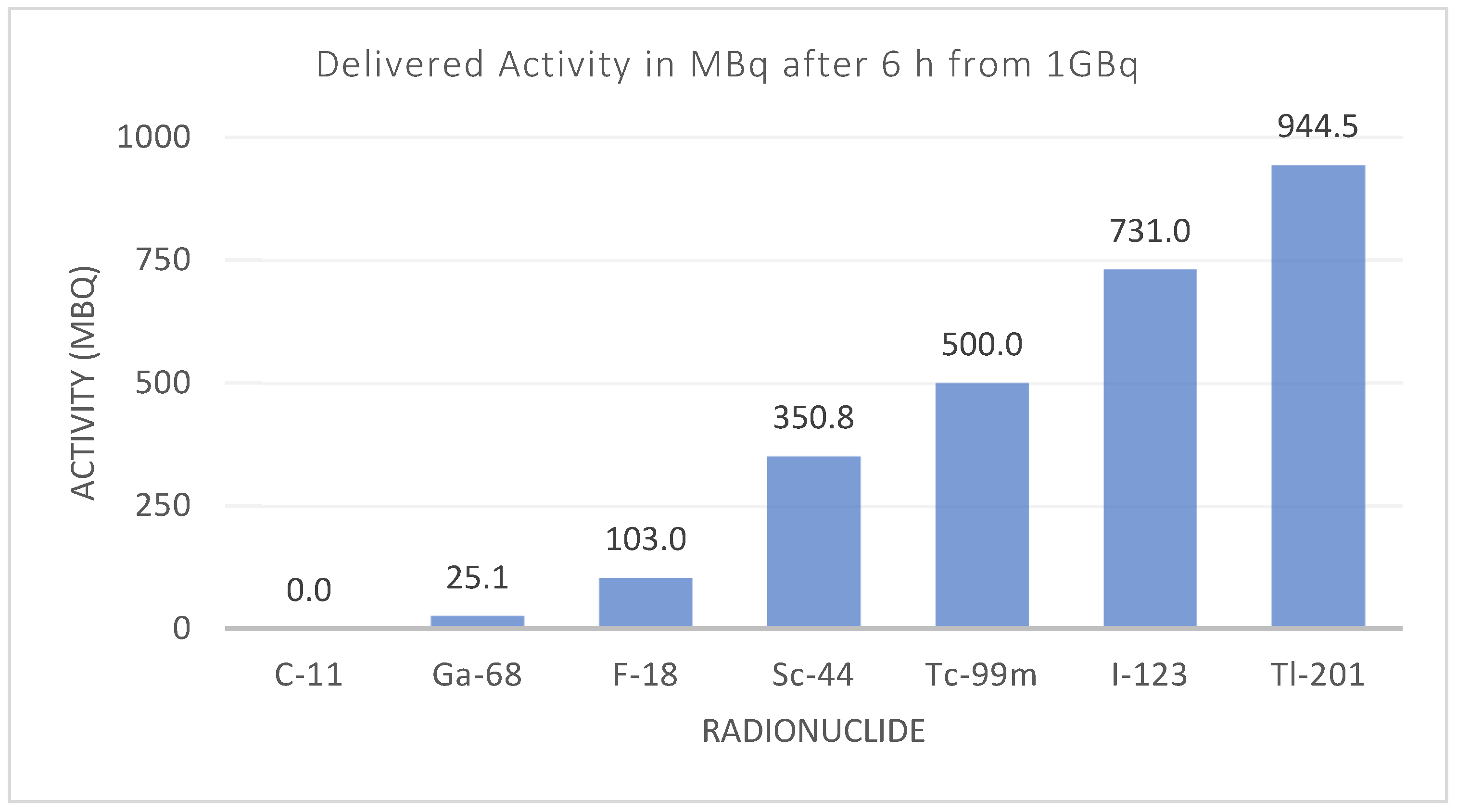
3. Radionuclide Development
3.1. Drive for Radionuclide Development
3.2. The Revolving World of the Radionuclide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SNMMI. Historical Timeline. Important Moments in the History of Nuclear Medicine. Available online: https://www.snmmi.org/AboutSNMMI/Content.aspx?ItemNumber=4175 (accessed on 2 October 2021).
- Hirmas, N.; Leyh, C.; Sraieb, M.; Barbato, F.; Schaarschmidt, B.M.; Umutlu, L.; Nader, M.; Wedemeyer, H.; Ferdinandus, J.; Rischpler, C.; et al. 68 Ga-PSMA-11 PET/CT Improves Tumor Detection and Impacts Management in Patients with Hepatocellular Carcinoma. J. Nucl. Med. 2021, 62, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Weber, W.A.; Czernin, J.; Anderson, C.J.; Badawi, R.D.; Barthel, H.; Bengel, F.; Bodei, L.; Buvat, I.; DiCarli, M.; Graham, M.M.; et al. The future of nuclear medicine, molecular imaging, and theranostics. J. Nucl. Med. 2020, 61, 263S–272S. [Google Scholar] [CrossRef]
- Seo, Y.; Mari, C.; Hasegawa, B.H. Technological Development and Advances in Single-Photon Emission Computed Tomography/Computed Tomography. Semin. Nucl. Med. 2008, 38, 177–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, D.W. Combined Positron Emission Tomography-Computed Tomography: The Historical Perspective. Semin. Ultrasound CT MRI 2008, 29, 232–235. [Google Scholar] [CrossRef] [Green Version]
- Anger, H.O. Nuclear Medicine Pioneer, Hal O. Anger, 1920–2005. J. Nucl. Med. Technol. 2005, 33, 250–253. [Google Scholar]
- Aide, N.; Lasnon, C.; Kesner, A.; Levin, C.S.; Buvat, I.; Iagaru, A.; Hermann, K.; Badawi, R.D.; Cherry, S.R.; Bradley, K.M.; et al. New PET technologies—Embracing progress and pushing the limits. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2711–2726. [Google Scholar] [CrossRef]
- Rogasch, J.M.M.; Boellaard, R.; Pike, L.; Borchmann, P.; Johnson, P.; Wolf, J.; Barrington, S.F.; Kobe, C. Moving the goalposts while scoring—the dilemma posed by new PET technologies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2696–2710. [Google Scholar] [CrossRef] [PubMed]
- Del Sordo, S.; Abbene, L.; Caroli, E.; Mancini, A.M.; Zappettini, A.; Ubertini, P. Progress in the development of CdTe and CdZnTe semiconductor radiation detectors for astrophysical and medical applications. Sensors 2009, 9, 3491–3526. [Google Scholar] [CrossRef]
- Ito, T.; Matsusaka, Y.; Onoguchi, M.; Ichikawa, H.; Okuda, K.; Shibutani, T.; Shishido, M.; Sato, K. Experimental evaluation of the GE NM/CT 870 CZT clinical SPECT system equipped with WEHR and MEHRS collimator. J. Appl. Clin. Med. Phys. 2021, 22, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Eisen, Y.; Shor, A.; Mardor, I. CdTe and CdZnTe X-ray and gamma-ray detectors for imaging systems. IEEE Trans. Nucl. Sci. 2004, 51, 1191–1198. [Google Scholar] [CrossRef]
- Spartiotis, K.; Leppänen, A.; Pantsar, T.; Pyyhtiä, J.; Laukka, P.; Muukkonen, K.; Männistö, O.; Kinnari, J.; Schulman, T. A photon counting CdTe gamma- and X-ray camera. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 550, 267–277. [Google Scholar] [CrossRef]
- Ogawa, K.; Ohmura, N.; Iida, H.; Nakamura, K.; Nakahara, T.; Kubo, A. Development of an ultra-high resolution SPECT system with a CdTe semiconductor detector. Ann. Nucl. Med. 2009, 23, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.S.; Berman, D.S.; Ziffer, J.; Nagler, M.; Sandler, M.; Patton, J.; Hutton, B.; Sharir, T.; Haim, S.B.; Haim, S.B. A novel high-sensitivity rapid-acquisition single-photon cardiac imaging camera. J. Nucl. Med. 2009, 50, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.V.; Faber, T.L.; Esteves, F.P. Cardiac dedicated ultrafast SPECT cameras: New designs and clinical implications. J. Nucl. Med. 2011, 52, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Verger, A.; Imbert, L.; Yagdigul, Y.; Fay, R.; Djaballah, W.; Rouzet, F.; Fourquet, N.; Poussier, S.; Roch, V.; Le Guludec, D.; et al. Factors affecting the myocardial activity acquired during exercise SPECT with a high-sensitivity cardiac CZT camera as compared with conventional Anger camera. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 522–528. [Google Scholar] [CrossRef]
- Ben-Haim, S.; Kennedy, J.; Keidar, Z. Novel cadmium zinc telluride devices for myocardial perfusion imaging—Technological aspects and clinical applications. Semin. Nucl. Med. 2016, 46, 273–285. [Google Scholar] [CrossRef]
- Niimi, T.; Nanasato, M.; Sugimoto, M.; Maeda, H. Evaluation of Cadmium-Zinc-Telluride Detector-based Single-Photon Emission Computed Tomography for Nuclear Cardiology: A Comparison with Conventional Anger Single-Photon Emission Computed Tomography. Nucl. Med. Mol. Imaging 2017, 51, 331–337. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakano, S.; Gatate, Y.; Okano, N.; Muramatsu, T.; Nishimura, S.; Kuji, I.; Fukushima, K.; Matsunari, I. Feasibility of simultaneous 99mTc-tetrofosmin and 123I-BMIPP dual-tracer imaging with cadmium-zinc-telluride detectors in patients undergoing primary coronary intervention for acute myocardial infarction. J. Nucl. Cardiol. 2021, 28, 187–195. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakano, S.; Gatate, Y.; Sugi, K.; Okano, N.; Muramatsu, T.; Nishimura, S.; Kuji, I.; Fukushima, K.; Matsunari, I. Shortened acquisition time in simultaneous 99mTc-tetrofosmin and 123I-β-methyl-p-iodophenyl pentadecanoic acid dual-tracer imaging with cadmium–zinc–telluride detectors in patients undergoing primary coronary intervention for acute myocardial infarction. Nucl. Med. Commun. 2019, 40, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Gnesin, S.; Kieffer, C.; Zeimpekis, K.; Papazyan, J.P.; Guignard, R.; Prior, J.O.; Verdun, F.R.; Lima, T.V.M. Phantom-based image quality assessment of clinical 18F-FDG protocols in digital PET/CT and comparison to conventional PMT-based PET/CT. EJNMMI Phys. 2020, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Maniawski, P.; Knopp, M. V Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res. 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.V.M.; Gnesin, S.; Strobel, K.; Pérez, M.D.S.; Roos, J.E.; Müller, C.; van der Meulen, N.P. Fifty shades of scandium: Comparative study of pet capabilities using sc-43 and sc-44 with respect to conventional clinical radionuclides. Diagnostics 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Wacholz, C.; Hruska, C.; OConnor, M. Veriton Multi-CZT Detector SPECT/CT System Acceptance Testing. J. Nucl. Med. 2020, 61, 3003. [Google Scholar]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First human imaging studies with the explorer total-body PET scanner. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Hünermund, J.N.; Prenosil, G.; Mingels, C.; Bohn, K.P.; Viscione, M.; Sari, H.; Vollnberg, B.; Shi, K.; Afshar-Oromieh, A.; et al. Clinical performance of long axial field of view PET/CT: A head-to-head intra-individual comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2395–2404. [Google Scholar] [CrossRef]
- Siegel, S.B.; Member, S.; Aykac, M.; Bal, H.; Bendriem, B.; Bharkhada, D.; Cabello, J.; Eriksson, L.A.; Panin, V.; Rothfuss, H.; et al. Preliminary Performance of a Prototype, One- Meter Long PET Tomograph. In Proceedings of the IEEE NSS-MIC, Boston, MA, USA, 31 October–7 November 2020. [Google Scholar]
- Siemens Healthineers Biograph Vision. Available online: https://www.siemens-healthineers.com/molecular-imaging/pet-ct/biograph-vision (accessed on 2 October 2021).
- Aldawood, S.; Thirolf, P.G.; Miani, A.; Böhmer, M.; Dedes, G.; Gernhäuser, R.; Lang, C.; Liprandi, S.; Maier, L.; Marin, T.; et al. Development of a Compton camera for prompt-gamma medical imaging. Radiat. Phys. Chem. 2017, 140, 190–197. [Google Scholar] [CrossRef]
- Moskal, P.; Stępień, E. Prospects and Clinical Perspectives of Total-Body PET Imaging Using Plastic Scintillators. PET Clin. 2020, 15, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Bodei, L.; Chiesa, C.; Flux, G.; Forrer, F.; Kraeber-Bodere, F.; Brans, B.; Lambert, B.; Konijnenberg, M.; Borson-Chazot, F.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Sposito, C.; Mazzaferro, V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: Reasons for a failure, and perspectives for the future. HepatoBiliary Surg. Nutr. 2018, 7, 487–489. [Google Scholar] [CrossRef]
- Kubik, A.; Budzyńska, A.; Kacperski, K.; MacIak, M.; Kuć, M.; Piasecki, P.; Wiliński, M.; Konior, M.; Dziuk, M.; Iller, E. Evaluation of qualitative and quantitative data of Y-90 imaging in SPECT/CT and PET/CT phantom studies. PLoS ONE 2021, 16, e0246848. [Google Scholar] [CrossRef]
- Vouche, M.; Vanderlinden, B.; Delatte, P.; Lemort, M.; Hendlisz, A. New Imaging Techniques for 90Y Microsphere Radioembolization. J. Nucl. Med. Radiat. Ther. 2011, 2, 2. [Google Scholar] [CrossRef]
- Fabbri, C.; Sarti, G.; Cremonesi, M.; Ferrari, M.; Di Dia, A.; Agostini, M.; Botta, F.; Paganelli, G. Quantitative analysis of 90Y Bremsstrahlung SPECT-CT images for application to 3D patient-specific dosimetry. Cancer Biother. Radiopharm. 2009, 24, 145–154. [Google Scholar] [CrossRef]
- Minarik, D.; Sjögreen Gleisner, K.; Ljungberg, M. Evaluation of quantitative (90)Y SPECT based on experimental phantom studies. Phys. Med. Biol. 2008, 53, 5689–5703. [Google Scholar] [CrossRef]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonné, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Mausner, L.F.; Kolsky, K.L.; Joshi, V.; Srivastava, S.C. Radionuclide development at BNL for nuclear medicine therapy. Appl. Radiat. Isot. 1998, 49, 285–294. [Google Scholar] [CrossRef]
- Grundler, P.V.; Eichler, R.; Talip, Z.; Schubiger, P.A.; Schibli, R.; van der Meulen, N.P. The metamorphosis of radionuclide production and development at paul scherrer institute. Chimia 2020, 74, 968–975. [Google Scholar] [CrossRef]
- Sainz-Esteban, A.; Prasad, V.; Schuchardt, C.; Zachert, C.; Carril, J.M.; Baum, R.P. Comparison of sequential planar 177Lu-DOTA-TATE dosimetry scans with 68Ga-DOTA-TATE PET/CT images in patients with metastasized neuroendocrine tumours undergoing peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 501–511. [Google Scholar] [CrossRef]
- Domnanich, K.A.; Eichler, R.; Müller, C.; Jordi, S.; Yakusheva, V.; Braccini, S.; Behe, M.; Schibli, R.; Türler, A.; van der Meulen, N.P. Production and separation of 43Sc for radiopharmaceutical purposes. EJNMMI Radiopharm. Chem. 2017, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—Preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; Van Der Meulen, N.P. Scandium and terbium radionuclides for radiotheranostics: Current state of development towards clinical application. Br. J. Radiol. 2018, 91, 20180074. [Google Scholar] [CrossRef] [PubMed]
- Huclier-Markai, S.; Alliot, C.; Kerdjoudj, R.; Mougin-Degraef, M.; Chouin, N.; Haddad, F. Promising Scandium Radionuclides for Nuclear Medicine: A Review on the Production and Chemistry up to In Vivo Proofs of Concept. Cancer Biother. Radiopharm. 2018, 33, 316–329. [Google Scholar] [CrossRef]
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; Van Der Meulen, N.; Türler, A.; Schibli, R. Promising prospects for 44Sc-/47Sc-based theragnostics: Application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef]
- Lima, T.V.M.; Gnesin, S.; Nitzsche, E.; Ortega, P.G.; Müller, C.; van der Meulen, N.P. First Phantom-Based Quantitative Assessment of Scandium-44 Using a Commercial PET Device. Front. Phys. 2020, 8, 241. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Bilewicz, A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J. Inorg. Biochem. 2011, 105, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; Van Der Walt, N.T.; Türler, A.; Schibli, R. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for α- and β--radionuclide therapy: An in vivo proof-of-concept study with a new receptor-targeted folate derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef] [Green Version]
- Champion, C.; Quinto, M.A.; Morgat, C.; Zanotti-Fregonara, P.; Hindié, E. Comparison between three promising β-emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics 2016, 6, 1611–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, P.; Benjegård, S.A.; Kölby, L.; Johanson, V.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Dosimetric comparison of radionuclides for therapy of somatostatin receptor-expressing tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 514–524. [Google Scholar] [CrossRef]
- Lehenberger, S.; Barkhausen, C.; Cohrs, S.; Fischer, E.; Grünberg, J.; Hohn, A.; Köster, U.; Schibli, R.; Türler, A.; Zhernosekov, K. The low-energy β—And electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl. Med. Biol. 2011, 38, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, G.-J.; Čomor, J.J.; Daković, M.; Soloviev, D.; Tamburella, C.; Hagebø, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G.; Starodub, G.Y.; et al. Production routes of the alpha emitting 149 Tb for medical application Medical radionuclide production/Therapeutic radionuclides/Spallation production/Heavy ion induced nuclear reaction/149 Tb. Radiochim. Acta 2002, 90, 247–252. [Google Scholar] [CrossRef]
- Bailey, D.L.; Willowson, K.P. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J. Nucl. Med. 2013, 54, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einstein, A.J. Breaking America’s Dependence on Imported Molybdenum. JACC Cardiovasc. Imaging 2009, 2, 369–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilsizian, V.; Narula, J. Seeking Remedy for Molly’s Woe. Time for a Thallium Pill? JACC Cardiovasc. Imaging 2009, 2, 375–377. [Google Scholar] [CrossRef] [Green Version]
- Werner, P.; Neumann, C.; Eiber, M.; Wester, H.J.; Schottelius, M. [99cmTc]Tc-PSMA-I&S-SPECT/CT: Experience in prostate cancer imaging in an outpatient center. EJNMMI Res. 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. Pet imaging with a [68ga]gallium-labelled psma ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kübler, H.; Beer, A.J.; et al. Diagnostic efficacy of 68Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, P.J.; Emmett, L.; Ho, B.; Delprado, W.; Ting, F.; Nguyen, Q.; Stricker, P.D. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017, 119, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herlemann, A.; Wenter, V.; Kretschmer, A.; Thierfelder, K.M.; Bartenstein, P.; Faber, C.; Gildehaus, F.J.; Stief, C.G.; Gratzke, C.; Fendler, W.P. 68Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur. Urol. 2016, 70, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Öbek, C.; Doğanca, T.; Demirci, E.; Ocak, M.; Kural, A.R.; Yıldırım, A.; Yücetaş, U.; Demirdağ, Ç.; Erdoğan, S.M.; Kabasakal, L. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.P.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morigi, J.J.; Stricker, P.D.; Van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Uprimny, C. 68 Ga-PSMA-11 PET/CT: The rising star of nuclear medicine in prostate cancer imaging? Wien. Med. Wochenschr. 2019, 169, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Kesch, C.; Kratochwil, C.; Mier, W.; Kopka, K.; Giesel, F.L. 68Ga or 18F for prostate cancer imaging? J. Nucl. Med. 2017, 58, 687–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, M.; Mirzoyan, L.; Horn, T.; Peeken, J.C.; Wurzer, A.; Wester, H.J.; Makowski, M.; Weber, W.A.; Eiber, M.; Rauscher, I. Matched-Pair Comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in Patients with Primary and Biochemical Recurrence of Prostate Cancer: Frequency of Non-Tumor-Related Uptake and Tumor Positivity. J. Nucl. Med. 2021, 62, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilsizian, V.; Taillefer, R. Journey in evolution of nuclear cardiology: Will there be another quantum leap with the f-18-labeled myocardial perfusion tracers? JACC Cardiovasc. Imaging 2012, 5, 1269–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokop, E.K.; Strauss, H.W.; Shaw, J.; Pitt, B.; Wagner, H.N. Comparison of regional myocardial perfusion determined by ionic potassium 43 to that determined by microspheres. Circulation 1974, 50, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Pohost, G.M.; Zir, L.M.; Moore, R.H.; McKusick, K.A.; Guiney, T.E.; Beller, G.A. Differentiation of transiently ischemic from infarcted myocardium by serial imaging after a single dose of Thallium 201. Circulation 1977, 55, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciagrà, R.; Lubberink, M.; Hyafil, F.; Saraste, A.; Slart, R.H.J.A.; Agostini, D.; Nappi, C.; Georgoulias, P.; Bucerius, J.; Rischpler, C.; et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1040–1069. [Google Scholar] [CrossRef]
- Harnett, D.T.; Hazra, S.; Maze, R.; Mc Ardle, B.A.; Alenazy, A.; Simard, T.; Henry, E.; Dwivedi, G.; Glover, C.; deKemp, R.A.; et al. Clinical performance of Rb-82 myocardial perfusion PET and Tc-99m-based SPECT in patients with extreme obesity. J. Nucl. Cardiol. 2019, 26, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, I.S.; Memmott, M.J.; Tonge, C.M.; Arumugam, P. The impact of prompt gamma compensation on myocardial blood flow measurements with rubidium-82 dynamic PET. J. Nucl. Cardiol. 2018, 25, 596–605. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Meulen, N.P.; Strobel, K.; Lima, T.V.M. New Radionuclides and Technological Advances in SPECT and PET Scanners. Cancers 2021, 13, 6183. https://doi.org/10.3390/cancers13246183
van der Meulen NP, Strobel K, Lima TVM. New Radionuclides and Technological Advances in SPECT and PET Scanners. Cancers. 2021; 13(24):6183. https://doi.org/10.3390/cancers13246183
Chicago/Turabian Stylevan der Meulen, Nicholas P., Klaus Strobel, and Thiago Viana Miranda Lima. 2021. "New Radionuclides and Technological Advances in SPECT and PET Scanners" Cancers 13, no. 24: 6183. https://doi.org/10.3390/cancers13246183
APA Stylevan der Meulen, N. P., Strobel, K., & Lima, T. V. M. (2021). New Radionuclides and Technological Advances in SPECT and PET Scanners. Cancers, 13(24), 6183. https://doi.org/10.3390/cancers13246183





