Comparison of Overall Survival between Surgical Resection and Radiofrequency Ablation for Hepatitis B-Related Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
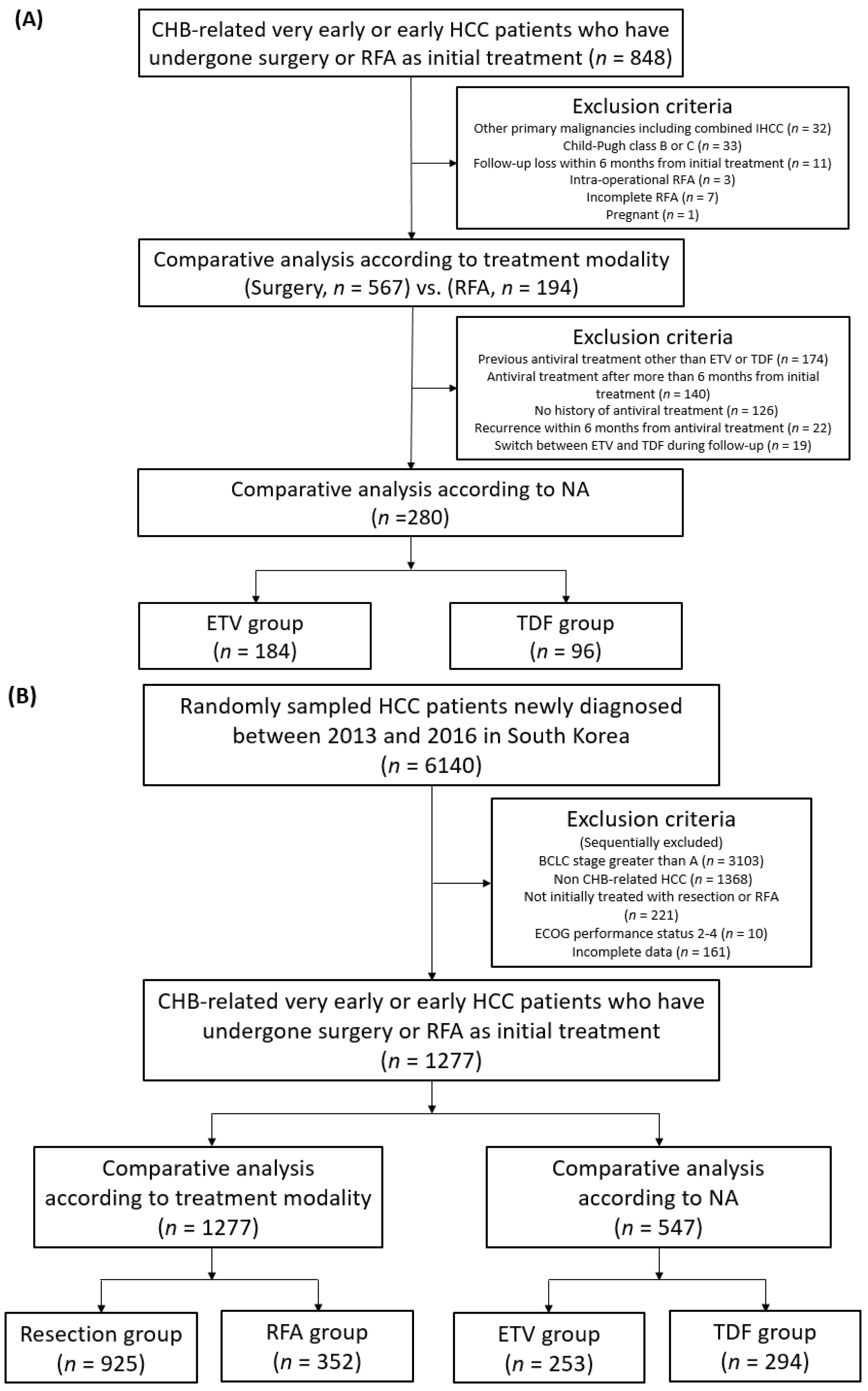
2. Materials and Methods
2.1. Study Objects
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Overall and Recurrence-Free Survivals in the Entire Hospital Cohort
3.3. Survival Outcomes in the Subgroup of Single HCC Smaller Than 3 cm
3.4. Overall Survival in the Nationwide Cohort
3.5. Safety Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abubakar, I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Kudo, M. The 2008 Okuda lecture: Management of hepatocellular carcinoma: From surveillance to molecular targeted therapy. J. Gastroenterol. Hepatol. 2010, 25, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569. [Google Scholar] [CrossRef] [PubMed]
- N’Kontchou, G.; Mahamoudi, A.; Aout, M.; Ganne-Carrié, N.; Grando, V.; Coderc, E.; Vicaut, E.; Trinchet, J.C.; Sellier, N.; Beaugrand, M. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009, 50, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, Y.J.; Han, J.K.; Choi, B. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014, 270, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Li, J.-Q.; Zheng, Y.; Guo, R.-P.; Liang, H.-H.; Zhang, Y.-Q.; Lin, X.-J.; Lau, W.Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006, 243, 321. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, Y.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Li, B.; Xu, D.; Yin, Z.; Xie, F.; Yang, J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010, 10, 1–7. [Google Scholar] [CrossRef]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wang, C.-C.; Hung, C.-H.; Chen, C.-L.; Lu, S.-N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012, 56, 412–418. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Ercolani, G.; Pinna, A.D. Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 4106. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kokudo, N.; Makuuchi, M.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O. Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J. Hepatol. 2013, 58, 724–729. [Google Scholar] [CrossRef]
- Pompili, M.; Saviano, A.; de Matthaeis, N.; Cucchetti, A.; Ardito, F.; Federico, B.; Brunello, F.; Pinna, A.D.; Giorgio, A.; Giulini, S.M. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J. Hepatol. 2013, 59, 89–97. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, W.; Liang, X.; Li, D.; Lou, H.; Chen, R.; Wang, K.; Pan, H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2014, 29, 193–200. [Google Scholar] [CrossRef]
- Qi, X.; Tang, Y.; An, D.; Bai, M.; Shi, X.; Wang, J.; Han, G.; Fan, D. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: A meta-analysis of randomized controlled trials. J. Clin. Gastroenterol. 2014, 48, 450–457. [Google Scholar] [CrossRef]
- Xu, Q.; Kobayashi, S.; Ye, X.; Meng, X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: A meta-analysis of 16,103 patients. Sci. Rep. 2014, 4, 7252. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, D.; Ludwig, J.; Kim, H. Radiofrequency ablation versus resection for hepatocellular carcinoma in patients with Child–Pugh A liver cirrhosis: A meta-analysis. Clin. Radiol. 2017, 72, 1066–1075. [Google Scholar] [CrossRef]
- Ng, K.; Chok, K.; Chan, A.; Cheung, T.; Wong, T.; Fung, J.; Yuen, J.; Poon, R.; Fan, S.; Lo, C. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1775–1784. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, J.M.; Yoon, J.-H.; Kim, Y.J.; Park, J.-W.; Park, S.-J.; Kim, S.H.; Yi, N.-J.; Suh, K.-S. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann. Surg. Treat. Res. 2018, 94, 74. [Google Scholar] [CrossRef]
- Ito, T.; Tanaka, S.; Iwai, S.; Takemura, S.; Hagihara, A.; Uchida-Kobayashi, S.; Shinkawa, H.; Nishioka, T.; Kawada, N.; Kubo, S. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: A case-control study with propensity score matching. Hepatol. Res. 2016, 46, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Kim, J.M.; Rhim, H.; Lee, M.W.; Kim, Y.-s.; Lim, H.K.; Choi, D.; Song, K.D.; Kwon, C.H.D.; Joh, J.-W. Small hepatocellular carcinoma: Radiofrequency ablation versus nonanatomic resection—propensity score analyses of long-term outcomes. Radiology 2015, 275, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, T.W.; Cha, D.I.; Song, K.D.; Lee, M.W.; Rhim, H.; Lim, H.K.; Sinn, D.H.; Kim, J.M.; Kim, K. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyses of long-term outcomes. J. Hepatol. 2018, 69, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Lee, J.-H.; Lee, D.H.; Yu, S.J.; Kim, Y.J.; Yoon, J.-H.; Kim, H.-C.; Lee, J.M.; Chung, J.W.; Yi, N.-J. Small single-nodule hepatocellular carcinoma: Comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014, 271, 909–918. [Google Scholar] [CrossRef]
- Xu, X.-L.; Liu, X.-D.; Liang, M.; Luo, B.-M. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: Systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology 2018, 287, 461–472. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.W.; Lee, J.M.; Kim, J.M.; Lee, M.W.; Rhim, H.; Hur, Y.H.; Suh, K.-S. Laparoscopic Liver Resection versus Percutaneous Radiofrequency Ablation for Small Single Nodular Hepatocellular Carcinoma: Comparison of Treatment Outcomes. Liver Cancer 2021, 10, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Q.; Li, Y.; Deng, S.; Wei, S.; Li, X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: A meta-analysis of randomized and nonrandomized controlled trials. PLoS ONE 2014, 9, e84484. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Chen, C.J.; Yang, H.I. Natural history of chronic hepatitis B REVEALed. J. Gastroenterol. Hepatol. 2011, 26, 628–638. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular Carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Hung, I.F.; Poon, R.T.; Lai, C.-L.; Fung, J.; Fan, S.-T.; Yuen, M.-F. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am. J. Gastroenterol. 2008, 103, 1663–1673. [Google Scholar] [CrossRef]
- Chuma, M.; Hige, S.; Kamiyama, T.; Meguro, T.; Nagasaka, A.; Nakanishi, K.; Yamamoto, Y.; Nakanishi, M.; Kohara, T.; Sho, T. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 2009, 44, 991–999. [Google Scholar] [CrossRef][Green Version]
- Liaw, Y.-F. Antiviral therapy of chronic hepatitis B: Opportunities and challenges in Asia. J. Hepatol. 2009, 51, 403–410. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Schall, R.A. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Liaw, Y.-F. Impact of hepatitis B therapy on the long-term outcome of liver disease. Liver Int. 2011, 31, 117–121. [Google Scholar] [CrossRef]
- Chang, T.T.; Liaw, Y.F.; Wu, S.S.; Schiff, E.; Han, K.H.; Lai, C.L.; Safadi, R.; Lee, S.S.; Halota, W.; Goodman, Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010, 52, 886–893. [Google Scholar] [CrossRef]
- Yin, J.; Li, N.; Han, Y.; Xue, J.; Deng, Y.; Shi, J.; Guo, W.; Zhang, H.; Wang, H.; Cheng, S. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: A two-stage longitudinal clinical study. J. Clin. Oncol. 2013, 31, 3647–3655. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-T.; Ho, H.J.; Su, C.-W.; Lee, T.-Y.; Wang, S.-Y.; Wu, C.; Wu, J.-C. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B—a nationwide cohort study. Gastroenterology 2014, 147, 143–151.e5. [Google Scholar] [CrossRef]
- Huang, G.; Lau, W.Y.; Wang, Z.-G.; Pan, Z.-Y.; Yuan, S.-X.; Shen, F.; Zhou, W.-P.; Wu, M.-C. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: A randomized controlled trial. Ann. Surg. 2015, 261, 56–66. [Google Scholar] [CrossRef]
- Wong, G.H.; Tse, Y.K.; Chan, H.Y.; Yip, T.F.; Tsoi, K.F.; Wong, V.S. Oral nucleos(t)ide analogues reduce recurrence and death in chronic hepatitis B-related hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2016, 43, 802–813. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Grieco, A.; Pompili, M.; Caminiti, G.; Miele, L.; Covino, M.; Alfei, B.; Rapaccini, G.L.; Gasbarrini, G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: Comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut 2005, 54, 411–418. [Google Scholar] [CrossRef]
- Cillo, U.; Vitale, A.; Grigoletto, F.; Farinati, F.; Brolese, A.; Zanus, G.; Neri, D.; Boccagni, P.; Srsen, N.; D’Amico, F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J. Hepatol. 2006, 44, 723–731. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, J.-W. Epidemiology of liver cancer in South Korea. Clin. Mol. Hepatol. 2018, 24, 1. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Goh, E.L.; Chidambaram, S.; Ma, S. Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A meta-analysis of the long-term survival outcomes. Int. J. Surg. 2018, 50, 35–42. [Google Scholar] [CrossRef]
- Jin, B.; Chen, M.-t.; Fei, Y.-t.; Mao, Y.-l. Safety and efficacy for laparoscopic versus open hepatectomy: A meta-analysis. Surg. Oncol. 2018, 27, A26–A34. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Colecchia, A.; Ercolani, G.; Bolondi, L.; Pinna, A.D. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 2013, 59, 300–307. [Google Scholar] [CrossRef] [PubMed]

| Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Resection (n = 567) | RFA (n = 194) | p | Resection | RFA | p | SMD |
| Age, years | 55.2 ± 9.5 | 58.3 ± 8.4 | <0.01 | 56.1 ± 9.3 | 56.5 ± 9.5 | 0.76 | 0.06 |
| Sex, male (%) | 76.7 | 74.7 | 0.65 | 76.3 | 79.2 | 0.59 | 0.07 |
| Smoking (%) | 26.1 | 18.6 | 0.04 | 26.0 | 19.7 | 0.39 | 0.15 |
| Alcohol (%) | 34.4 | 25.8 | 0.03 | 32.9 | 36.7 | 0.62 | 0.08 |
| Diabetes mellitus (%) | 12.2 | 19.1 | 0.02 | 14.3 | 25.7 | 0.07 | 0.12 |
| Hypertension (%) | 27.0 | 26.3 | 0.92 | 27.8 | 32.3 | 0.53 | 0.10 |
| Child–Pugh Score | 0.01 | 0.55 | 0.06 | ||||
| 5 (%) | 90.3 | 82.5 | 88.4 | 90.1 | |||
| 6 (%) | 9.7 | 17.5 | 11.6 | 9.9 | |||
| BCLC stage | <0.01 | 0.20 | 0.15 | ||||
| 0 (%) | 16.0 | 58.8 | 26.9 | 33.9 | |||
| A (%) | 84.0 | 41.2 | 73.1 | 66.1 | |||
| Tumor size, cm | 3.9 ± 2.5 | 1.7 ± 0.6 | <0.01 | 3.4 ± 2.4 | 2.3 ± 0.9 | <0.01 | 0.57 |
| Number of nodules | <0.01 | 0.96 | 0.01 | ||||
| 1 (%) | 95.8 | 89.7 | 93.7 | 93.6 | |||
| 2 or 3 (%) | 4.2 | 10.3 | 6.3 | 6.4 | |||
| Tumor location | 0.03 | 0.36 | 0.13 | ||||
| Peripheral † (%) | 51.0 | 41.8 | 49.0 | 42.6 | |||
| Central †† (%) | 49.0 | 58.2 | 51.0 | 57.4 | |||
| Perivascular tumor * (%) | 28.2 | 21.6 | 0.09 | 26.8 | 18.6 | 0.09 | 0.20 |
| Peribiliary tumor ** (%) | 22.9 | 16.5 | 0.07 | 21.8 | 14.9 | 0.13 | 0.18 |
| Presence of varix (%) | 6.0 | 12.4 | 0.01 | 7.7 | 7.4 | 0.89 | 0.01 |
| Cirrhosis (%) | 63.1 | 85.6 | <0.01 | 68.3 | 70.6 | 0.77 | 0.05 |
| HBsAg-positive (%) | 94.9 | 95.9 | 0.72 | 95.6 | 97.9 | 0.08 | 0.13 |
| HBeAg-positive (%) | 13.8 | 22.7 | 0.01 | 13.6 | 24.0 | 0.07 | 0.27 |
| Antiviral treatment *** (%) | 67.2 | 66.0 | 0.82 | 71.1 | 63.6 | 0.27 | 0.16 |
| Platelets, ×1000/mm3 | 161.3 ± 54.0 | 128.8 ± 47.1 | <0.01 | 156.0 ± 51.6 | 147.9 ± 43.6 | 0.09 | 0.17 |
| Total bilirubin, mg/dL | 0.9 ± 0.4 | 0.8 ± 0.4 | <0.02 | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.07 | 0.13 |
| ALT, U/L | 39.1 ± 24.1 | 39.3 ± 37.5 | 0.95 | 38.3 ± 23.8 | 40.2 ± 30.7 | 0.64 | 0.07 |
| Albumin, g/dL | 4.1 ± 0.3 | 4.0 ± 0.4 | 0.02 | 4.1 ± 0.4 | 4.1 ± 0.4 | 0.46 | 0.11 |
| PT, INR | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.02 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.46 | 0.08 |
| Serum creatinine, mg/dL | 0.9 ± 0.4 | 0.9 ± 0.5 | 0.58 | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.58 | 0.06 |
| AFP, ng/mL | 19.7 (4.8–317.1) | 7.8 (3.7–40.8) | <0.01 | 17.6 (4.6–250.8) | 9.9 (3.9–33.0) | 0.01 | 0.17 |
| PIVKA, mAU/mL | 56.0 (26.0–371.5) | 24.0 (17.0–35.0) | <0.01 | 41.0 (23.0–196.0) | 27.0 (20.0–58.9) | 0.06 | 0.03 |
| HBV DNA, log10 IU/mL | 2.9 ± 2.4 | 2.0 ± 2.5 | <0.01 | 2.7 ± 2.4 | 2.5 ± 2.6 | 0.56 | 0.09 |
| OS in the Entire Hospital Cohort | ||||
| Variables | HR | p | aHR | p |
| Total bilirubin | 1.847 (1.045–3.265) | 0.03 | ||
| Albumin | 0.364 (0.162–0.822) | 0.02 | 0.387 (0.160–0.939) | 0.04 |
| Serum creatinine | 1.652 (1.499–1.819) | <0.01 | 1.318 (1.058–1.640) | 0.01 |
| Tumor size | 1.159 (1.084–1.240) | <0.01 | 1.114 (1.038–1.195) | <0.01 |
| Presence of varix | 3.037 (1.633–5.649) | <0.01 | 2.734 (1.334–5.610) | 0.01 |
| Antiviral treatment † | 0.504 (0.271–0.936) | 0.03 | 0.444 (0.251–0.786) | 0.01 |
| RFA compared to resection | 0.774 (0.368–1.630) | 0.50 | 0.870 (0.400–1.897) | 0.73 |
| RFS in the Entire Hospital Cohort | ||||
| Variables | HR | p | aHR | p |
| Platelets | 0.996 (0.993–0.998) | <0.01 | 0.996 (0.994–0.999) | 0.01 |
| Albumin | 0.638 (0.431–0.945) | 0.03 | ||
| Serum creatinine | 1.224 (1.028–1.458) | 0.02 | ||
| Tumor size | 1.051 (1.006–1.098) | 0.03 | 1.131 (1.067–1.198) | <0.01 |
| Number of nodules †† | 1.574 (1.142–2.169) | 0.01 | 1.726 (1.229–2.423) | <0.01 |
| HBeAg-positive | 1.759 (1.100–2.813) | 0.02 | 1.606 (1.039–2.480) | 0.03 |
| Cirrhosis | 1.597 (1.054–2.420) | 0.03 | 1.578 (1.020–2.440) | 0.04 |
| Presence of varix | 1.491 (1.029–2.160) | 0.03 | ||
| Antiviral treatment † | 0.555 (0.390–0.788) | <0.01 | 0.544 (0.391–0.757) | <0.01 |
| RFA compared to resection | 1.491 (1.034–2.149) | 0.03 | 1.562 (1.099–2.219) | 0.01 |
| OS in the Subgroup of the Hospital Cohort with a Single HCC Smaller than 3 cm | ||||
| Variables | HR | p | aHR | p |
| Total bilirubin | 2.296 (1.159–4.550) | 0.02 | ||
| Albumin | 0.310 (0.111–0.865) | 0.03 | ||
| Serum creatinine | 1.915 (1.676–2.189) | <0.01 | 2.364 (1.917–2.915) | <0.01 |
| log10(HBV DNA) | 1.122 (0.960–1.311) | 0.15 | ||
| Presence of varix | 6.806 (3.242–14.290) | <0.01 | 6.842 (3.030–15.447) | <0.01 |
| Antiviral treatment † | 0.517 (0.239–1.121) | 0.09 | 0.289 (0.141–0.585) | <0.01 |
| RFA compared to resection | 0.735 (0.336–1.610) | 0.44 | 0.509 (0.213–1.215) | 0.13 |
| RFS in the Subgroup of the Hospital Cohort with a Single HCC Smaller than 3 cm | ||||
| Variables | HR | p | aHR | p |
| Platelets | 0.995 (0.991–0.999) | 0.01 | 0.995 (0.991–0.998) | <0.01 |
| Total bilirubin | 1.535 (1.045–2.255) | 0.03 | ||
| HBeAg-positive | 2.035 (1.199–3.456) | 0.01 | 1.827 (1.142–2.290) | 0.01 |
| Tumor size | 1.571 (1.009–2.446) | 0.045 | 1.734 (1.152–2.611) | 0.01 |
| Presence of varix | 1.650 (1.005–2.709) | 0.048 | ||
| Antiviral treatment † | 0.480 (0.306–0.755) | <0.01 | 0.538 (0.372–0.776) | <0.01 |
| RFA compared to resection | 1.628 (1.114–2.379) | 0.01 | 1.539 (1.057–2.242) | 0.02 |
| OS in the Entire Nationwide Cohort | ||||
| Variables | HR | p | aHR | p |
| Age | 1.024 (1.008–1.040) | <0.01 | 1.022 (1.004–1.039) | 0.01 |
| Albumin | 0.423 (0.301–0.594) | <0.01 | 0.511 (0.324–0.804) | <0.01 |
| Child–Pugh score ††† | 2.067 (1.414–3.021) | <0.01 | ||
| Tumor size | 1.150 (1.106–1.196) | <0.01 | 1.148 (1.078–1.223) | <0.01 |
| Antiviral treatment † | 0.620 (0.457–0.840) | <0.01 | 0.655 (0.451–0.952) | 0.03 |
| RFA compared to resection | 0.805 (0.593–1.092) | 0.16 | 0.981 (0.661–1.456) | 0.92 |
| Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Resection (n = 253) | RFA (n = 167) | p | Resection | RFA | p | SMD |
| Age, years | 54.4 ± 9.5 | 58.0 ± 8.3 | <0.01 | 55.7 ± 9.2 | 55.1 ± 9.2 | 0.75 | 0.06 |
| Sex, male (%) | 74.7 | 73.7 | 0.90 | 74.7 | 79.2 | 0.38 | 0.11 |
| Smoking (%) | 28.9 | 19.2 | 0.03 | 28.5 | 25.1 | 0.67 | 0.08 |
| Alcohol (%) | 31.2 | 26.3 | 0.33 | 30.8 | 35.6 | 0.52 | 0.10 |
| Diabetes mellitus (%) | 13.0 | 16.8 | 0.36 | 14.4 | 15.6 | 0.79 | 0.03 |
| Hypertension (%) | 26.1 | 25.7 | 1.00 | 27.6 | 23.0 | 0.41 | 0.11 |
| Child–Pugh Score | <0.01 | 0.84 | 0.02 | ||||
| 5 (%) | 92.5 | 82.6 | 88.5 | 89.2 | |||
| 6 (%) | 7.5 | 17.4 | 11.5 | 10.8 | |||
| BCLC stage | <0.01 | 0.68 | 0.06 | ||||
| 0 (%) | 36.0 | 68.3 | 49.1 | 46.2 | |||
| A (%) | 64.0 | 31.7 | 50.9 | 53.8 | |||
| Tumor size, cm | 2.1 ± 0.6 | 1.6 ± 0.5 | <0.01 | 2.0 ± 0.6 | 2.0 ± 0.7 | 0.94 | 0.01 |
| Tumor location | 0.28 | 0.92 | 0.01 | ||||
| Peripheral † (%) | 50.2 | 44.3 | 48.2 | 47.5 | |||
| Central †† (%) | 49.8 | 55.7 | 51.8 | 52.5 | |||
| Perivascular tumor * (%) | 17.4 | 21.0 | 0.43 | 18.1 | 20.8 | 0.60 | 0.07 |
| Peribiliary tumor ** (%) | 13.0 | 16.2 | 0.45 | 13.9 | 16.5 | 0.59 | 0.07 |
| Presence of varix (%) | 7.9 | 13.2 | 0.11 | 7.4 | 7.7 | 0.90 | 0.01 |
| Cirrhosis (%) | 72.7 | 85.6 | <0.01 | 76.6 | 77.5 | 0.88 | 0.02 |
| HBsAg-positive (%) | 97.6 | 95.8 | 0.44 | 97.7 | 97.6 | 0.94 | 0.01 |
| HBeAg-positive (%) | 16.6 | 23.4 | 0.11 | 19.5 | 23.4 | 0.54 | 0.10 |
| Antiviral treatment *** (%) | 77.1 | 64.7 | 0.01 | 79.5 | 60.4 | <0.01 | 0.43 |
| Platelets, ×1000/mm3 | 151.0 ± 47.8 | 126.4 ± 48.4 | <0.01 | 145.7 ± 46.6 | 145.0 ± 49.4 | 0.90 | 0.02 |
| Total bilirubin, mg/dL | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.10 | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.25 | 0.13 |
| ALT, U/L | 37.0 ± 23.4 | 36.3 ± 28.0 | 0.78 | 36.1 ± 22.9 | 41.3 ± 28.4 | 0.23 | 0.20 |
| Albumin, g/dL | 4.1 ± 0.3 | 4.1 ± 0.4 | 0.03 | 4.1 ± 0.4 | 4.1 ± 0.4 | 0.36 | 0.11 |
| PT, INR | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.71 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.39 | 0.11 |
| Serum creatinine, mg/dL | 0.9 ± 0.2 | 0.9 ± 0.5 | 0.85 | 0.9 ± 0.2 | 0.9 ± 0.4 | 0.42 | 0.08 |
| AFP, ng/mL | 16.6 (4.8–163.9) | 7.1 (3.4–33.0) | <0.01 | 14.7 (3.9–153.2) | 10.5 (3.8–89.4) | 0.98 | 0.45 |
| PIVKA, mAU/mL | 32.0 (21.0–58.0) | 23.0 (17.0–35.0) | <0.01 | 29.0 (4.8–317.1) | 25.0 (19.0–50.1) | 0.77 | 0.09 |
| HBV DNA, log10 IU/mL | 2.7 ± 2.4 | 1.8 ± 2.4 | <0.01 | 2.4 ± 2.4 | 2.5 ± 2.6 | 0.84 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, M.H.; Lee, J.-H.; Kim, J.Y.; Hong, J.H.; Park, M.K.; Cho, H.J.; Choi, N.R.; Kim, J.; Kim, M.A.; Nam, J.Y.; et al. Comparison of Overall Survival between Surgical Resection and Radiofrequency Ablation for Hepatitis B-Related Hepatocellular Carcinoma. Cancers 2021, 13, 6009. https://doi.org/10.3390/cancers13236009
Hur MH, Lee J-H, Kim JY, Hong JH, Park MK, Cho HJ, Choi NR, Kim J, Kim MA, Nam JY, et al. Comparison of Overall Survival between Surgical Resection and Radiofrequency Ablation for Hepatitis B-Related Hepatocellular Carcinoma. Cancers. 2021; 13(23):6009. https://doi.org/10.3390/cancers13236009
Chicago/Turabian StyleHur, Moon Haeng, Jeong-Hoon Lee, Ju Yeon Kim, Ji Hoon Hong, Min Kyung Park, Hee Jin Cho, Na Ryung Choi, Jihye Kim, Minseok Albert Kim, Joon Yeul Nam, and et al. 2021. "Comparison of Overall Survival between Surgical Resection and Radiofrequency Ablation for Hepatitis B-Related Hepatocellular Carcinoma" Cancers 13, no. 23: 6009. https://doi.org/10.3390/cancers13236009
APA StyleHur, M. H., Lee, J.-H., Kim, J. Y., Hong, J. H., Park, M. K., Cho, H. J., Choi, N. R., Kim, J., Kim, M. A., Nam, J. Y., Lee, Y. B., Cho, E. J., Yu, S. J., Kim, Y. J., Lee, D. H., Lee, J. M., Hong, S. K., Yi, N.-J., Lee, K.-W., ... Yoon, J.-H. (2021). Comparison of Overall Survival between Surgical Resection and Radiofrequency Ablation for Hepatitis B-Related Hepatocellular Carcinoma. Cancers, 13(23), 6009. https://doi.org/10.3390/cancers13236009







