Clinical Studies on Cytokine-Induced Killer Cells: Lessons from Lymphoma Trials
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Immunologic Approaches in Lymphoma Therapy
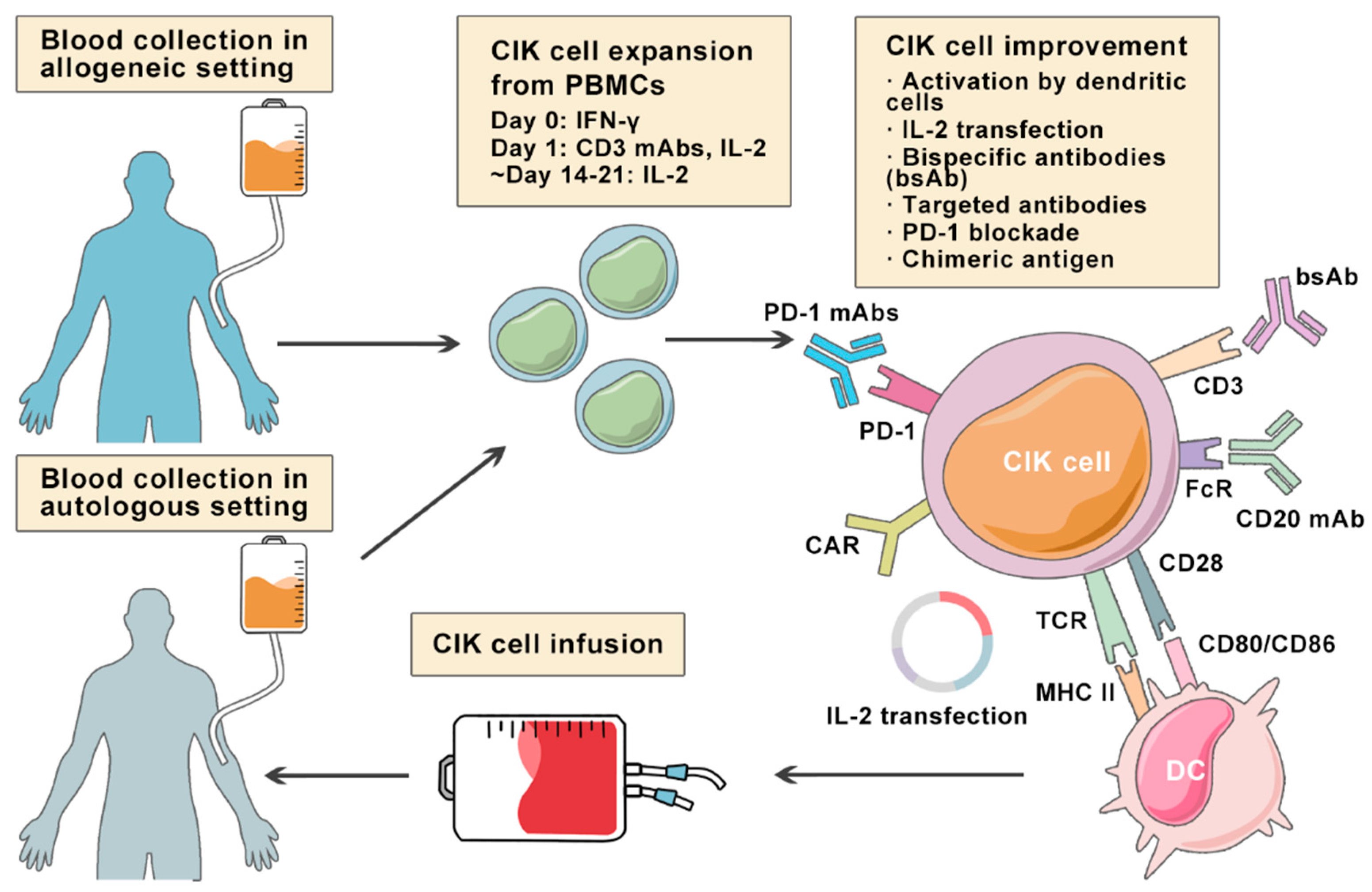
3. Cytokine-Induced Killer Cells
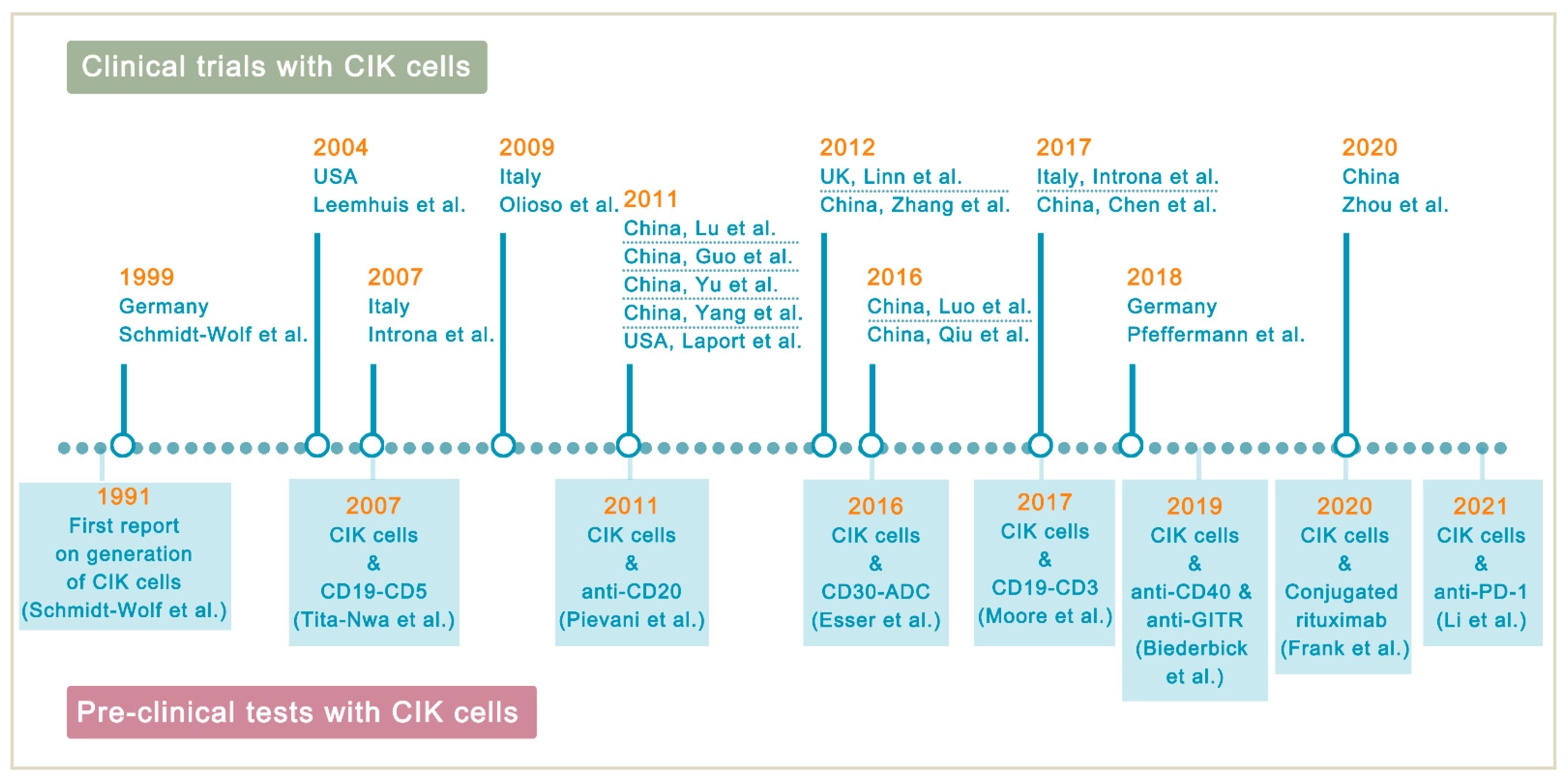
4. Clinical Studies of CIK Cells in Lymphoma Treatment
4.1. Clinical Studies on Autologous CIK Cells
4.2. Clinical Studies on DC-CIK Cells
4.3. Clinical Studies on Allogeneic CIK Cells after Allo-HSCT
5. Improving CIK Cell Therapy on Lymphoma
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA A Cancer J. Clin. 2017, 68, 116–132. [Google Scholar] [CrossRef]
- Tun, A.M.; Ansell, S.M. Immunotherapy in Hodgkin and non-Hodgkin lymphoma: Innate, adaptive and targeted immunological strategies. Cancer Treat. Rev. 2020, 88, 102042. [Google Scholar] [CrossRef]
- Hagemeister, F. Rituximab for the treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs 2010, 70, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Forstpointner, R.; Dreyling, M.; Repp, R.; Hermann, S.; Hänel, A.; Metzner, B.; Pott, C.; Hartmann, F.; Rothmann, F.; Rohrberg, R.; et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004, 104, 3064–3071. [Google Scholar]
- Grant, B.W.; Jung, S.-H.; Johnson, J.L.; Kostakoglu, L.; Hsi, E.; Byrd, J.C.; Jones, J.; Leonard, J.P.; Martin, S.E.; Cheson, B.D.; et al. A phase 2 trial of extended induction epratuzumab and rituximab for previously untreated follicular lymphoma: CALGB 50701. Cancer 2013, 119, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Czuczman, M.S.; Leonard, J.P.; Jung, S.; Johnson, J.L.; Hsi, E.D.; Byrd, J.C.; Cheson, B.D. Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness. Ann. Oncol. 2012, 23, 2356–2362. [Google Scholar] [CrossRef]
- Vitolo, U.; Trneny, M.; Belada, D.; Burke, J.M.; Carella, A.M.; Chua, N.; Abrisqueta, P.; Demeter, J.; Flinn, I.; Hong, X.; et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2017, 35, 3529–3537. [Google Scholar] [CrossRef]
- Sun, L.L.; Ellerman, D.; Mathieu, M.; Hristopoulos, M.; Chen, X.; Li, Y.; Yan, X.; Clark, R.; Reyes, A.; Stefanich, E.; et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci. Transl. Med. 2015, 7, 287ra270. [Google Scholar] [CrossRef]
- Viardot, A.; Goebeler, M.-E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T.; Mihalyova, J.; Kascak, M.; Duras, J.; Hajek, R. PD-1/PD-L1 inhibitors in haematological malignancies: Update 2017. Immunology 2017, 152, 357–371. [Google Scholar] [CrossRef]
- Wang, C.M.; Wu, Z.Q.; Wang, Y.; Guo, Y.L.; Dai, H.R.; Wang, X.H.; Li, X.; Zhang, Y.J.; Zhang, W.Y.; Chen, M.X.; et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin. Cancer Res. 2017, 23, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Gauthier, J.; Hay, K.A.; Voutsinas, J.M.; Wu, Q.; Pender, B.S.; Hawkins, R.M.; Vakil, A.; Steinmetz, R.N.; Riddell, S.R.; et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 2019, 134, 636–640. [Google Scholar] [CrossRef]
- Dreger, P. Allogeneic stem cell transplant in non-Hodgkin lymphomas: Still an indication? Hematol. Oncol. 2021, 39 (Suppl. S1), 100–103. [Google Scholar] [CrossRef]
- Chu, Y.; Zhou, X.; Wang, X. Antibody-drug conjugates for the treatment of lymphoma: Clinical advances and latest progress. J. Hematol. Oncol. 2021, 14, 1–20. [Google Scholar] [CrossRef]
- De Claro, R.A.; McGinn, K.; Kwitkowski, V.; Bullock, J.; Khandelwal, A.; Habtemariam, B.; Ouyang, Y.; Saber, H.; Lee, K.; Koti, K.; et al. U.S. Food and Drug Administration Approval Summary: Brentuximab Vedotin for the Treatment of Relapsed Hodgkin Lymphoma or Relapsed Systemic Anaplastic Large-Cell Lymphoma. Clin. Cancer Res. 2012, 18, 5845–5849. [Google Scholar] [CrossRef]
- Introna, M. CIK as therapeutic agents against tumors. J. Autoimmun. 2017, 85, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Verneris, M.R.; Karami, M.; Baker, J.; Jayaswal, A.; Negrin, R.S. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 2004, 103, 3065–3072. [Google Scholar] [CrossRef]
- Karimi, M.; Cao, T.M.; Baker, J.A.; Verneris, M.R.; Soares, L.; Negrin, R.S. Silencing Human NKG2D, DAP10, and DAP12 Reduces Cytotoxicity of Activated CD8+T Cells and NK Cells. J. Immunol. 2005, 175, 7819–7828. [Google Scholar] [CrossRef]
- Frigault, M.J.; Maus, M.V. State of the art in CAR T cell therapy for CD19+ B cell malignancies. J. Clin. Investig. 2020, 130, 1586–1594. [Google Scholar] [CrossRef]
- Goebeler, M.-E.; Bargou, R.C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020, 17, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, A.; Jamieson, A.; Liu, S.D.; Shastri, N.; Raulet, D. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000, 1, 119–126. [Google Scholar] [CrossRef]
- Ramos, C.A.; Heslop, H.E.; Brenner, M.K. CAR-T Cell Therapy for Lymphoma. Annu. Rev. Med. 2016, 67, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schmidt-Wolf, I.G.H. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020, 235, 9291–93033. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Abramson, M.J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, D.M.A.; Wang, M.L.; Arnason, J.E.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, M.C.; et al. Pivotal Safety and Efficacy Results from Transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed/Refractory (R/R) Large B Cell Lymphomas. Blood 2019, 134, 241. [Google Scholar] [CrossRef]
- Bachanova, V.; Westin, J.; Tam, C.; Borchmann, P.; Jaeger, U.; McGuirk, J.; Holte, H.; Waller, E.; Jaglowski, S.; Bishop, M.; et al. Correlative Analyses of Cytokine Release Syndrome and Neurological Events in Tisagenlecleucel-Treated Relapsed/Refractory Diffuse Large B-Cell Lymphoma Patients. Clin. Lymphoma Myeloma Leuk. 2019, 19, S251–S252. [Google Scholar] [CrossRef]
- Goebeler, M.-E.; Knop, S.; Viardot, A.; Kufer, P.; Topp, M.S.; Einsele, H.; Noppeney, R.; Hess, G.; Kallert, S.; Mackensen, A.; et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients with Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results from a Phase I Study. J. Clin. Oncol. 2016, 34, 1104–1111. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.H.; Finke, S.; Trojaneck, B.; Denkena, A.; Lefterova, P.; Schwella, N.; Heuft, H.-G.; Prange, G.; Korte, M.; Takeya, M.; et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br. J. Cancer 1999, 81, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, T.; Wells, S.; Scheffold, C.; Edinger, M.; Negrin, R.S. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol. Blood Marrow Transplant. 2005, 11, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Olioso, P.; Giancola, R.; Di Riti, M.; Contento, A.; Accorsi, P.; Iacone, A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: A pilot clinical trial. Hematol. Oncol. 2009, 27, 130–139. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; He, X.-P.; Tan, X.-H.; Zhou, Y.; Chen, X.; Shi, Y.-J.; Liu, X.-D.; Chen, H.-R. A clinical study of cytokine-induced killer cells for the treatment of refractory lymphoma. Oncol. Lett. 2011, 2, 531–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, X.-C.; Yang, B.; Yu, R.-L.; Chi, X.-H.; Tuo, S.; Tuo, C.-W.; Zhu, H.-L.; Wang, Y.; Jiang, C.-G.; Fu, X.-B.; et al. Clinical Study of Autologous Cytokine-Induced Killer Cells for the Treatment of Elderly Patients with Diffuse Large B-Cell Lymphoma. Cell Biophys. 2012, 62, 257–265. [Google Scholar] [CrossRef]
- Yang, B.; Lu, X.-C.; Yu, R.-L.; Chi, X.-H.; Liu, Y.; Wang, Y.; Dai, H.-R.; Zhu, H.-L.; Cai, L.-L.; Han, W.-D. Repeated transfusions of autologous cytokine-induced killer cells for treatment of haematological malignancies in elderly patients: A pilot clinical trial. Hematol. Oncol. 2012, 30, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, J.; Li, C.-P.; Xu, J.-Y.; Chen, B. Autologous Cytokine-Induced Killer Cell Immunotherapy for Patients with High-Risk Diffuse Large B Cell Lymphoma After the First Complete Remission. OncoTargets Ther. 2020, 13, 5879–5885. [Google Scholar] [CrossRef]
- Introna, M.; Borleri, G.; Conti, E.; Franceschetti, M.; Barbui, A.M.; Broady, R.; Dander, E.; Gaipa, G.; D’Amico, G.; Biagi, E.; et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: A phase I study. Haematologica 2007, 92, 952–959. [Google Scholar] [CrossRef]
- Yu, J.; Ren, X.; Li, H.; Cao, S.; Han, Y.; Enoki, T.; Kato, I.; Cao, C.; Hao, X. Synergistic Effect of CH-296 and Interferon Gamma on Cytokine-Induced Killer Cells Expansion for Patients with Advanced-Stage Malignant Solid Tumors. Cancer Biother. Radiopharm. 2011, 26, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Laport, G.G.; Sheehan, K.; Baker, J.; Armstrong, R.; Wong, R.M.; Lowsky, R.; Johnston, L.J.; Shizuru, J.A.; Miklos, D.; Arai, S.; et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 1679–1687. [Google Scholar] [CrossRef]
- Linn, Y.C.; Niam, M.; Chu, S.; Choong, A.; Yong, H.X.; Heng, K.K.; Hwang, W.; Loh, Y.; Goh, Y.T.; Suck, G.; et al. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant. 2012, 47, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.; Wei, J.; Liu, L.; Yin, Y.; Gu, Y.; Shu, Y. The effects of cytokine-induced killer cells for the treatment of patients with solid tumors: A clinical retrospective study. J. Cancer Res. Clin. Oncol. 2012, 138, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zeng, H.-Q.; Shen, Y.; Zhang, P.; Lou, S.-F.; Chen, L.; Deng, J.-C. Allogeneic hematopoietic stem cell transplantation following donor CIK cell infusion: A phase I study in patients with relapsed/refractory hematologic malignancies. Leuk. Res. 2016, 48, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yun, M.M.; Dong, X.; Xu, M.; Zhao, R.; Han, X.; Zhou, E.; Yun, F.; Su, W.; Liu, C.; et al. Combination of cytokine-induced killer and dendritic cells pulsed with antigenic α-1,3-galactosyl epitope-enhanced lymphoma cell membrane for effective B-cell lymphoma immunotherapy. Cytotherapy 2016, 18, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, M.; Song, Q.; Wu, J.; Wang, X.; Zhou, X.; Yuan, Y.; Song, Y.; Jiang, N.; Zhao, Y.; et al. Enhanced antitumor effects and improved immune status of dendritic cell and cytokine-induced killer cell infusion in advanced cancer patients. Mol. Clin. Oncol. 2017, 7, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Lussana, F.; Algarotti, A.; Gotti, E.; Valgardsdottir, R.; Micò, C.; Grassi, A.; Pavoni, C.; Ferrari, M.L.; Delaini, F.; et al. Phase II Study of Sequential Infusion of Donor Lymphocyte Infusion and Cytokine-Induced Killer Cells for Patients Relapsed after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 2070–2078. [Google Scholar] [CrossRef]
- Pfeffermann, L.-M.; Pfirrmann, V.; Huenecke, S.; Bremm, M.; Bonig, H.; Kvasnicka, H.-M.; Klingebiel, T.; Bader, P.; Rettinger, E. Epstein-Barr virus–specific cytokine-induced killer cells for treatment of Epstein-Barr virus–related malignant lymphoma. Cytotherapy 2018, 20, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Zhou, X.; Lyerly, H.; Morse, M.A.; Ren, J. DC-CIK as a widely applicable cancer immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-J.; Wang, H.; Pan, K.; Li, Y.-Q.; Huang, L.-X.; Chen, S.-P.; He, J.; Ke, M.-L.; Zhao, J.-J.; Li, J.-J.; et al. Comparative study on anti-tumor immune response of autologous cytokine-induced killer (CIK) cells, dendritic cells-CIK (DC-CIK), and semi-allogeneic DC-CIK. Chin. J. Cancer 2010, 29, 641–648. [Google Scholar] [CrossRef]
- Nishimura, R.; Baker, J.; Beilhack, A.; Zeiser, R.; Olson, J.A.; Sega, E.I.; Karimi, M.; Negrin, R.S. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood 2008, 112, 2563–2574. [Google Scholar] [CrossRef]
- Lee, S.; Chong, S.; Lee, J.; Kim, S.; Min, Y.H.; Hahn, J.; Ko, Y. Difference in the expression of Fas/Fas-ligand and the lymphocyte subset reconstitution according to the occurrence of acute GVHD. Bone Marrow Transplant. 1997, 20, 883–888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sangiolo, D.; Martinuzzi, E.; Todorovic, M.; Vitaggio, K.; Vallario, A.; Jordaney, N.; Carnevale-Schianca, F.; Capaldi, A.; Geuna, M.; Casorzo, L.; et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: Implications for their infusion across major HLA barriers. Int. Immunol. 2008, 20, 841–848. [Google Scholar] [CrossRef]
- Ansell, S.M. Fundamentals of immunology for understanding immunotherapy for lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Tita-Nwa, F.; Moldenhauer, G.; Herbst, M.; Kleist, C.; Ho, A.D.; Kornacker, M. Cytokine-induced killer cells targeted by the novel bispecific antibody CD19xCD5 (HD37xT5.16) efficiently lyse B-lymphoma cells. Cancer Immunol. Immunother. 2007, 56, 1911–1920. [Google Scholar] [CrossRef]
- Moore, P.A.; Zhang, W.; Rainey, G.J.; Burke, S.; Li, H.; Huang, L.; Gorlatov, S.; Veri, M.C.; Aggarwal, S.; Yang, Y.; et al. Ap-plication of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 2011, 117, 4542–4551. [Google Scholar] [CrossRef]
- Circosta, P.; Elia, A.R.; Landra, I.; Machiorlatti, R.; Todaro, M.; Aliberti, S.; Brusa, D.; Deaglio, S.; Chiaretti, S.; Bruna, R.; et al. Tailoring CD19xCD3-DART exposure enhances T-cells to eradication of B-cell neoplasms. OncoImmunology 2018, 7, e1341032. [Google Scholar] [CrossRef]
- Pievani, A.; Belussi, C.; Klein, C.; Rambaldi, A.; Golay, J.T.; Introna, M. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood 2011, 117, 510–518. [Google Scholar] [CrossRef]
- Esser, L.; Weiher, H.; Schmidt-Wolf, I. Increased Efficacy of Brentuximab Vedotin (SGN-35) in Combination with Cytokine-Induced Killer Cells in Lymphoma. Int. J. Mol. Sci. 2016, 17, 1056. [Google Scholar] [CrossRef]
- Frank, M.J.; Olsson, N.; Huang, A.; Tang, S.-W.; Negrin, R.S.; Elias, J.E.; Meyer, E.H. A novel antibody-cell conjugation method to enhance and characterize cytokine-induced killer cells. Cytotherapy 2020, 22, 135–143. [Google Scholar] [CrossRef]
- Biederbick, K.D.; Schmidt-Wolf, I.G. Efficacy of cytokine-induced killer cells targeting CD40 and GITR. Oncol. Lett. 2019, 17, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sharma, A.; Bloemendal, M.; Schmidt-Wolf, R.; Kornek, M.; Schmidt-Wolf, I.G.H. PD-1 blockade enhances cytokine-induced killer cell-mediated cytotoxicity in B-cell non-Hodgkin lymphoma cell lines. Oncol. Lett. 2021, 22, 613. [Google Scholar] [CrossRef] [PubMed]
- Hontscha, C.; Borck, Y.; Zhou, H.; Messmer, D.; Schmidt-Wolf, I.G.H. Clinical trials on CIK cells: First report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2010, 137, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Schmeel, F.; Coch, C.; Schmidt-Wolf, I.G.H. Cytokine-induced killer (CIK) cells in cancer immunotherapy: Report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2015, 141, 839–849. [Google Scholar] [CrossRef]
| CIK Cells | CAR-T Cells | BiTEs | |
|---|---|---|---|
| Manufacturing Process | PBMCs sequentially activated with 1000 IU/m IFN-γ on day 0, and 50 ng/mL anti-CD3 mAb and 600 IU/mL IL-2 on the following day; IL-2 supplemented every 2–3 days | T cells with CAR gene transduction primarily by lentiviruses; CARs consist of an scFv based ectodomain for antigen-binding, a transmembrane domain, and an endodomain containing TCR CD3ζ chain with or without costimulatory signaling components | Antibodies designed to bind to a selected TAA and CD3 on T cells simultaneously; produced in bioreactors by mammalian cell lines as secreted polypeptides |
| Effector Cells | CD3+CD56+ cells | Mostly αβ-TCR+ T cells | Endogenous CD8+ or CD4+ T cells |
| Cell Source | Autologous/allogeneic | Autologous/allogeneic | Autologous |
| Target Antigen | MIC A/B and ULBP1–4 | CD19, CD20, CD22, CD30, BCMA, etc. | CD19 |
| MHC Restriction | Dual-functional capability (non-MHC-restricted and TCR-mediated lysis) | TAA recognition by CARs is MHC-unrestricted | MHC-unrestricted |
| Mechanism | Release of perforin and granzyme B from CIK cells | Release of perforin and granzyme B from CAR-T cells | Activating T cells to release perforin and granzyme B by linking TAAs to CD3 on T cells |
| Toxicities and Side effects | Low-grade toxicities including fever, chills, fatigue, headache, and skin rash; grade 3 and 4 toxicities are rare; limited GVHD response in the allogeneic setting | CRS, ICANS, and MAS/HLH; potentially life-threatening | CRS and ICANS; severe toxicities are one of the major concerns |
| Clinical Efficacy | Varied due to heterogeneity of expension method and study design | Axicabtagene ciloleucel: 83% ORR, 58% CR; Tisagenlecleucel: 54% ORR, 40% CR; Lisocabtagene maraleucel: 73% ORR, 53% CR [27,28,29] | Blinatumomab: 36% to 69% ORR in relapsed/refractory NHL [11,30] |
| Study | Phase | Number of Patients | Therapeutic Approach | Clinical Response | Adverse Event | Conclusion |
|---|---|---|---|---|---|---|
| Schmidt-Wolf et al. (1999) [31] | Phase I | 10 (2 FL) | IL-2-transfected + untransfected auto-CIKs | 1 CR (1 FL), 6 PD; increased alkaline phosphatase and CRP | Grade 2 fever but resolved the next day with or without addition of antibiotics | CIK cells transfected with IL-2 gene can be administered without major side effects |
| Leemhuis et al. (2004) [32] | Phase I | 9 (7 HL, 2 NHL) | Auto-CIKs | 2 PR, 2 SD | 1 case of asymptomatic mild hypotension that quickly resolved; fever (38 °C); 1 patient with thrombocytopenia (thought to be related to disease progression) | CIK cells may have utility for the treatment of high-risk patients with evidence of minimal residual disease after autologous transplantation |
| Introna et al. (2007) [38] | Phase I | 11 (3 HL) | Allo-CIKs | 3 CR (1 HL), 1 SD (1 HL), 6 PD or death | Acute grade I and II GVHD in 4 patients | Allogeneic CIK cells are well tolerated and may contribute to clinical responses in patients relapsing after allogeneic HSCT |
| Olioso et al. (2009) [33] | Pilot | 12 (1 FL, 1 B-cell NHL, 1 DLBCL, 1 HL, 1 centroblastic-centrocytic NHL) | Auto-CIKs | 3 CR (1 NHL), 2 SD | Graded 2 fever and/or chills during the first cycle of infusions (4.3%) but promptly resolved without antibiotic treatment | CIK cell therapy is a safe treatment with some suggestion of efficacy that significantly enhances immune functions increasing absolute numbers of effector cells |
| Lu et al. (2011) [35] | NA | 9 (9 DLBCL) | Auto-CIKs + rhIL-2 subcutaneously administered | 9 CR; serum levels of β2-microglobulin and LDH were significantly decreased; higher Karnofsky score | 2 patients developed mild fatigue and low-grade fever at the initialadministration of rhIL-2 | Autologous CIK cell therapy is safe and efficacious for the treatment of elderly patients with DLBCL |
| Guo et al. (2011) [34] | NA | 8 (2 HL, 6 NHL) | Auto-CIKs | 1 CR, 7 PR | Fever (38 °C) resolved with or without treatment, headache, nausea, vomiting, irritation disappearing after treatment withdrawal | CIK cell treatment of patients with refractory lymphoma achieves effective clinical responses with few side effects |
| Yu et al. (2011) [39] | NA | 20 (1 NHL) | Auto-CIKs + CH-296 | 12 SD, 8 PD (1 NHL) or death; 1-year survival rate 65%; mean OS 16.95 ± 6.10 months | Transient fever | CH-296 and IFN-γ synergistically promote anti-tumor efficiency of CIKs in advanced-stage malignant tumors |
| Yang et al. (2011) [36] | Pilot | 20 (1 LPL, 1 HL, 2 gastric MALT lymphoma, 5 DLBCL, 3CLL) | Auto-CIKs + subcutaneous injection of rhIL-2 | 11 CR (1 LPL, 1 HL, 2 Gastric MALT lymphoma, 2 DLBCL, 2 CLL), 7 PR (3 DLBCL, 1 CLL), 2 SD; mean survival time 20 months; increased Karnofsky score | Mild malaise and low-grade fever | Autologous CIK cells plus rhIL-2 treatment is safe and effective for treating haematological malignancies in elderly patients |
| Laport et al. (2011) [40] | NA | 18 (7 NHL, 1 HL) | Allo-CIKs | Mean OS 28 months; mean event-free survival 4 months; 12 CR (5 NHL, 1 HL), 3 PR (2 CLL), 3 PD | 2 patients with acute GVHD resolved with corticosteroids; 1 patient with chronic GVHD; transient grade 3 ventricular arrhythmia; transient rise in hepatic transaminase levels | CIK cell treatment is well tolerated and induces a low incidence of GVHD in patients with relapsed hematologic malignancies after allogeneic HSCT |
| Linn et al. (2012) [41] | Phase I/II | 16 (3 HL, 1 NHL) | Allo-CIKs | 5 patients achieved response attributable to CIK cell infusion (1 HL) | Acute GVHD occurred in three but easily treatable | This study provides some evidence suggestive of the efficacy of allogeneic CIK cells even after failure of DLI |
| Zhang et al. (2012) [42] | Retrospective | 40 (1 DLBCL) | Auto-CIKs | The 6-month, 1-year and 3-year OS rates were 70.0, 60.0 and 57.5%, respectively; The median OS was 34.9 months | Fever and poor appetite relieved quickly by simple treatment | CIKs immunotherapy can be an effective adjuvant instrument of the routine therapy of malignancy |
| Luo et al. (2016) [43] | Phase I | 15 (1 SLL, 1 ALCL, 2 DLBCL) | Allo-CIKs | All patients achieved engraftment and CR; 9 patients had died and 6 patients were surviving at a median follow-up of 1513 days | Two patients developed GVHD (grade I and III) but were controlled by additional immunosuppressant drugs; grade IV neutropenia in the transplant phase | Allogeneic HSCT combined with sequential infusion of donor CIK cells is well tolerated in salvage relapsed/refractory hematologic malignancy patients |
| Qiu et al. (2016) [44] | NA | 14 (4 FL, 7 DLBCL, 1 NMZL, 1 ALCL, 1 LBL) | Auto-CIKs + dendritic cells pulsed with antigenic α-1,3-galactosyl epitope | 4 CR, 3 PR, 2 PD; Karnofsky score improved dramatically in 12 of 14 patients | Fever (37.5–38.0 °C), skin rash, transient hypotension | Combination of CIK and dendritic cells pulsed with antigenic α-1,3-galactosyl epitope is safe and has great potential for the treatment of refractory B-cell lymphoma |
| Chen et al. (2017) [45] | NA | 90 (8 lymphoma) | Group 1: Auto-CIKs + dendritic cells Group 2: Best supportive care alone | OS of patients in CIK and dendritic cell group was significantly higher than best supportive care group (14 months vs. 11 months) | Fever (<38.5 °C) spontaneously relieved | CIK and dendritic cell therapy can improve the imbalance of immune system and prolong the OS in patients with advanced cancer |
| Introna et al. (2017) [46] | Phase IIA | 74 (3 HL, 2 NHL) | DLI + Allo-CIKs | 19 CR, 3 PR, 8 SD, 41 PD, 2 death; 1-year and 3-year PFS was 31% and 29%; 1-year and 3-year was 51% and 40% | 12 patients (16%) developed acute GVHD (grade I–II in 7 cases and grade III–IV in 5); 11 patients (15%) developed chronic GVHD | A low incidence of GVHD is observed after the sequential administration of DLI and CIK cells and disease control can be achieved mostly after a cytogenetic or molecular relapse |
| Pfeffermann et al. (2018) [47] | NA | 1 PTLD | Allo-CIKs with EBV-specificity | Long-term clearance of plasma EBV DNA and rapid and sustained disappearance of large DLBCL nodes within 7 days | Chronic GVHD of the skin 6 months after CIK cell treatment improved with immunosuppressive medication | EBV peptide-induced CIK cells might be considered a therapy for EBV-related PTLD |
| Zhou et al. (2020) [37] | NA | 40 (40 DLBCL) | Group 1: Auto-CIKs Group 2: No transfusion | Significantly improved 5-year DFS of CIK group (79.3 ± 9.2% vs. 45.0 ± 11.1%) and 5-year OS of CIK group (90 ± 6.7% vs. 55 ± 11.1%) | Mild flu-like symptoms that was quickly relieved | Autologous CIK cell immunotherapy is a safe and efficacious option to improve the prognosis of patients with high-risk DLBCL after the first CR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Sharma, A.; Weiher, H.; Schmid, M.; Kristiansen, G.; Schmidt-Wolf, I.G.H. Clinical Studies on Cytokine-Induced Killer Cells: Lessons from Lymphoma Trials. Cancers 2021, 13, 6007. https://doi.org/10.3390/cancers13236007
Zhang Y, Sharma A, Weiher H, Schmid M, Kristiansen G, Schmidt-Wolf IGH. Clinical Studies on Cytokine-Induced Killer Cells: Lessons from Lymphoma Trials. Cancers. 2021; 13(23):6007. https://doi.org/10.3390/cancers13236007
Chicago/Turabian StyleZhang, Ying, Amit Sharma, Hans Weiher, Matthias Schmid, Glen Kristiansen, and Ingo G. H. Schmidt-Wolf. 2021. "Clinical Studies on Cytokine-Induced Killer Cells: Lessons from Lymphoma Trials" Cancers 13, no. 23: 6007. https://doi.org/10.3390/cancers13236007
APA StyleZhang, Y., Sharma, A., Weiher, H., Schmid, M., Kristiansen, G., & Schmidt-Wolf, I. G. H. (2021). Clinical Studies on Cytokine-Induced Killer Cells: Lessons from Lymphoma Trials. Cancers, 13(23), 6007. https://doi.org/10.3390/cancers13236007







